Abstract
Assessing respiratory effort is essential for optimizing ventilatory support. While diaphragm thickening fraction (DTF) has been proposed as a bedside parameter, the role of intercostal muscle thickening fraction (ITF) remains unclear, especially during noninvasive ventilation. This study investigates the relationship between DTF, ITF and tidal oesophageal pressure swing (ΔPoes) as surrogate parameters for respiratory effort under different exercise loads. We conducted a physiological study in healthy volunteers with no contraindications to noninvasive ventilation. Participants completed three exercise sets on a semi-recumbent cycle ergometer. Each set consisted of five randomised phases with different ventilatory settings. ΔPoes was measured using a nasogastric balloon catheter. Diaphragmatic and parasternal intercostal muscle thickening fractions (DTF and ITF) were assessed by ultrasound in B-mode. Repeated measures correlation and Friedman’s test were used to analyse associations and differences between parameters. 38 individuals were included in the study. Repeated measures correlation analysis revealed a moderate correlation between DTF and ΔPoes (ρ = 0.419, p < 0.001), whereas ITF did not correlate with either parameter. Both DTF and ΔPoes increased with exercise load and allowed discrimination between different exercise loads, although ΔPoes provided a more accurate separation. In contrast, ITF did not discriminate between exercise phases. These findings suggest that DTF could be a useful indicator of respiratory effort during noninvasive ventilation. ITF may only be informative in the presence of diaphragmatic dysfunction. Further investigations in clinical populations are needed to test the hypotheses derived from this study.
Similar content being viewed by others
Introduction
Noninvasive ventilation (NIV) is widely used in patients with acute respiratory failure to avoid invasive mechanical ventilation. By providing inspiratory pressure support (ΔPinsp), NIV can both enhance oxygen delivery and compensate for the patient’s increased respiratory effort1. An assessment of respiratory effort is clinically relevant because increased respiratory effort seems to be a key factor in the development of acute respiratory failure and allows for the precise prediction of NIV success2, as both insufficient support and excessive pressures can have negative consequences. Excessive inspiratory pressures have been demonstrated to result in lung injury and diffuse alveolar damage3. Furthermore, high ΔPinsp have been shown to cause air leaks at the patient-mask interface, which in turn have been demonstrated to negatively affect patient-ventilator interaction. Dys- or asynchronies between the patient and the ventilator, such as ineffective triggering, can cause patient discomfort and can lead to NIV failure4. Therefore, estimating respiratory effort can help to individualise ΔPinsp settings, as optimal therapy should aim to maintain respiratory effort close to the level of resting spontaneous breathing5.
While the degree of ventilator support can usually be read from the inspiratory-expiratory pressure difference, it remains a challenge to assess the patient’s remaining effort and determine their actual need for support6. It has been established that the tidal oesophageal pressure swing (ΔPoes) is a reliable surrogate parameter for respiratory effort7,8. Preliminary studies have indicated that the diaphragm thickening fraction (DTF), determined by ultrasound, may also be a valuable parameter in this regard9. While the diaphragm is the primary respiratory muscle at rest3, accessory respiratory muscles become increasingly important as respiratory effort increases10. Inspiratory thickening of accessory respiratory muscles, such as the parasternal intercostals, might thus be a valuable addition to diaphragm ultrasound in cases of high respiratory effort11. Ultrasonographic measurement of in- and expiratory thickness of the parasternal intercostals has been described as a reliable, reproducible, and feasible bedside method11. Although several groups have proposed using ultrasound to assess ITF as an indicator of respiratory effort, the available evidence remains inconsistent across different study populations and experimental settings12,13,14,15.
However, to our knowledge, this noninvasive approach has not yet been evaluated during NIV, neither in the context of de-novo acute respiratory failure nor in patients receiving NIV after extubation. This differentiation is clinically relevant, as patients undergoing weaning from invasive mechanical ventilation often exhibit diaphragmatic atrophy or dysfunction due to prolonged mechanical ventilation16, whereas patients with de novo acute respiratory failure are more likely to have intact diaphragmatic and intercostal muscle function. As our study focused on healthy volunteers, we assumed that their respiratory muscle function was intact.
The primary aim of this study was to examine whether ITF provides superior discrimination between different levels of respiratory effort in healthy subjects undergoing NIV, compared to DTF. The secondary aim was to confirm our previous findings on the correlation of DTF and ΔPoes in a randomized and blinded experimental setting.
Methods
Study design
This is a physiological study in healthy volunteers and was not prospectively registered.
Outcome measures
Outcome measures for this study were the correlation of ITF, DTF and ΔPoes, as well as the ability of those index tests to differentiate between different exercise loads.
Subjects
Healthy individuals aged 18 years or older, capable of providing written informed consent, were recruited for this study. Individuals were excluded from participation if they had a history of bleeding disorders, recurrent bleeding, or current anticoagulant therapy; if they had allergies to ultrasound gel or external materials containing plastic, such as NIV masks or gastric tubes; or if they refused to participate or had medical contraindications to NIV. The participants were seated on a semi-recumbent bicycle ergometer with a 45-degree torso incline.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Workload determination
Exercise intensity was controlled using a cycle ergometer, allowing independent adjustment of workload and pedalling cadence. Effort perception was assessed using the Borg rating of perceived exertion (RPE) scale. A score of 10 on the Borg RPE scale corresponds to a light effort, while 15 indicates a demanding exertion17. Prior to the experiment, individual target workloads were determined in every participant by gradually increasing the wattage at 40 rpm until the predefined Borg RPE levels of 10 and 15 were reached. The corresponding wattages were then used as exercise intensities during the experimental phases.
Measurement of oesophageal pressure swing
A nasogastric single-balloon PesoCath© oesophageal catheter (Loewenstein medical, Bad Ems Germany) was used. Insertion was facilitated by local anaesthesia of the nose and pharynx using lidocaine spray and catheter preparation with lidocaine gel. The probe was inserted via a nostril aiming at initial infra-diaphragmatic placement. The oesophageal balloon was then inflated with 6 ml of air, followed by deflation of 2 ml to achieve a final volume of 4 ml. Infra- diaphragmatic placement was confirmed by positive inspiratory pressure swings. The probe was then retracted until negative pressure swings during inspiration and maximal cardiac oscillations confirmed correct placement. ΔPoes was calculated as the difference between the end-expiratory and end-inspiratory Poes.
Ultrasonographic measurements
As respiratory muscle ultrasound is not routinely performed at our facility, the sonographer completed standardized training on the study protocol prior to data acquisition, and all ultrasound measurements were obtained by a single, trained investigator. Initial examinations were performed under supervision. Ultrasound data were acquired using a GE Vivid E9 ultrasound system (GE Healthcare, Chicago, IL, USA) equipped with a 9 L-D linear array transducer (frequency range 2.4–10.0 MHz). For diaphragm ultrasound, the transducer was placed in the right mid-axillary line to visualize the zone of apposition, where the diaphragm inserts into the rib cage18. In each phase, B-mode images were acquired to measure diaphragm thickness at end-inspiration (DTinsp) and end-expiration (DTexsp). The diaphragm thickening fraction (DTF) was then calculated as a percentage according to Wait, Nahormek19using the following formula:
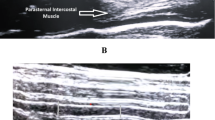
For the intercostal ultrasound, the probe was placed vertically in the parasternal region between the second and third ribs, approximately 2–4 cm lateral to the sternum. The parasternal intercostal muscle appears as a three-layered biconcave structure. Two hyperechoic membranes outline the central muscle portion. Intercostal muscle thickness was measured at end-inspiration (ITinsp) and end-expiration (ITexsp) in B-mode between the inner and outer hyperechoic fascial borders. ITF was calculated as11:
Exemplary images of DTF and ITF measurements are provided as eFigure 1 and supplemental electronic material.
Study phases
The study phases were randomized, ensuring that each participant underwent the same sequence within each set. The study consisted of three sets: seated at rest, ergometry with a value of 10 and 15 on the Borg RPE scale, respectively. Each set was further divided into five study phases, with the order of the phases randomised before the start of the experiment. During phase 0, participants were not connected to a ventilator. The ventilation settings during the different phases are summarised in Table 1. A Carescape™ R860 (GE HealthCare®, Chicago, USA) ventilator was used for ventilation and oesophageal pressure measurements. The order of the study phases within each set was randomised using a concealed allocation procedure with sealed, unmarked envelopes. The sonographer was blinded to NIV settings and exercise levels.
Measurements were taken after a uniform breathing pattern had been established, with a minimum waiting time of two minutes. This included DTF, ITF, ΔPoes, airway pressures, minute ventilation, blood pressure, heart rate, and peripheral oxygen saturation. The measurement and calculation of DTF, ITF, and ΔPoes were based on two consecutive breaths.
Statistical analysis
Repeated measures correlations between index tests were calculated using R software version 4.3.1 with the rmcorr-package20 version 0.5.4. All other statistical analyses were performed using IBM® SPSS® Statistics Version 29.0.1.0 (IBM, Armonk, US-NY). Due to the limited sample size, non-normally distributed data were assumed. Differences in measurements between exercise loads were compared using related-samples Friedman’s two-way analysis of variance by ranks (Friedman test). Summaries of categorical variables are given in terms of absolute numbers and group-related percentages. Summaries of metric variables are given as medians and interquartile ranges (IQR).
Results
Participants
43 subjects agreed to participate. In two subjects the nasogastric probe could not be placed. In three subjects, the study was prematurely terminated due to intolerance of the nasogastric probe. A total of 38 participants completed all measurements. A study flow chart is provided as eFigure 2.
Median age was 24.5 (IQR 22.0–28.0) years, median body mass index 25.3 (IQR 22.3–27.3) kg/m2 and 18 (52%) were female. At rest and during spontaneous breathing without connection to the ventilator the heart rate was 74 (IQR 71–86) bpm, DTF was 44.6 (IQR 15.6–78.2) % and ITF was 0.54 (IQR − 3.24–3.17) %. ΔPoes was 7 (IQR 5–9) cmH2O. The baseline criteria of the 38 participants are shown in Table 2.
Correlation between the parameters DTF, ITF and ΔPoes
Each participant completed three study sequences, each consisting of five randomised ventilation settings. One set of measurements was obtained for each combination of load and ventilatory support. Repeated measures correlation analysis revealed a moderate correlation between DTF and ΔPoes (ρ = 0.419, p < 0.001). In contrast, no significant correlation was found between DTF and ITF (ρ = − 0.025, p = 0.572) or between ITF and ΔPoes (ρ = − 0.040, p = 0.361). Repeated measures correlation plots are provided as eFigures 3–5.
Discrimination between exercise loads
In all ventilatory setups, both DTF and ΔPoes increased with exercise load. While DTF permitted statistical discrimination between different exercise phases, ΔPoes showed a clearer differentiation across all phases, as confirmed by the Friedman test and illustrated in Figs. 1 and 2. In contrast, ITF did not discriminate between the exercise loads (see Fig. 3). Table 3 summarises the median values of all respiratory parameters across the different study phases, together with the mean ranks used for the Friedman analysis.
Discrimination of Poes between exercise load according to respiratory phase. I = No ventilator; p < 0.001. II = EPAP 0 cmH2O/IPAP 0 cmH2O; p < 0.001. III = EPAP 5 cmH2O/IPAP 5 cmH2O; p < 0.001. IV = EPAP 5 cmH2O/IPAP 10 cmH2O; p < 0.001. V = EPAP 5 cmH2O/IPAP 15 cmH2O; p < 0.001. Circles indicate data outliers (third quartile + 1.5 interquartile range or first quartile – 1.5 interquartile range). Triangles indicate extreme outliers (third quartile + 3 interquartile range or first quartile – 3 interquartile range).
Discrimination of DTF between exercise load according to respiratory phase. I = No ventilator; p < 0.001. II = EPAP 0 cmH2O/IPAP 0 cmH2O; p < 0.001. III = EPAP 5 cmH2O/IPAP 5 cmH2O; p < 0.001. IV = EPAP 5 cmH2O/IPAP 10 cmH2O; p < 0.001. V = EPAP 5 cmH2O/IPAP 15 cmH2O; p < 0.002. Circles indicate data outliers (third quartile + 1.5 interquartile range or first quartile – 1.5 interquartile range). Triangles indicate extreme outliers (third quartile + 3 interquartile range or first quartile – 3 interquartile range).
Discrimination of ITF between exercise load according to respiratory phase. I = No ventilator; p = 0.054. II = EPAP 0 cmH2O/IPAP 0 cmH2O; p = 0.855. III = EPAP 5 cmH2O/IPAP 5 cmH2O; p = 0.382. IV = EPAP 5 cmH2O/IPAP 10 cmH2O; p = 0.963. V = EPAP 5 cmH2O/IPAP 15 cmH2O; p = 0.913. Circles indicate data outliers (third quartile + 1.5 interquartile range or first quartile – 1.5 interquartile range). Triangles indicate extreme outliers (third quartile + 3 interquartile range or first quartile – 3 interquartile range).
Discussion
In this study, we demonstrated a moderate correlation between DTF and ΔPoes at different ventilatory settings. These findings are consistent with the results of a previous study of our group, where both ΔPoes and DTF showed similar behaviour9. Although associations between DTF and indices of respiratory effort have also been reported in critically ill patients, their strength varies across studies and clinical settings. Vivier et al. have described moderate to strong correlations under NIV after extubation21, whereas Menis et al. have not observed a clear relationship22. This could be attributable to a higher incidence of diaphragm dysfunction in invasively ventilated individuals, which may develop within a few days of mechanical ventilation23,24. In contrast, healthy individuals in our study showed no signs of diaphragm weakness. Evidence regarding diaphragm dysfunction in patients with de novo respiratory failure is limited, but preserved diaphragm function is likely more common in early disease stages, allowing sonographic evaluation of respiratory effort during NIV25.
No correlation was observed between ΔPoes and ITF or between ITF and DTF. Furthermore, ITF did not discriminate between different levels of respiratory effort in our study, suggesting that ITF was not a reliable marker under the given conditions. These findings are in line with previous observations in spontaneously breathing healthy individuals where no significant change in intercostal muscle thickness during different respiratory manoeuvres was reported15. This could be due to the predominantly isometric contraction of the intercostal muscles, which stabilise the thoracic cage and prevent paradoxical movements without significant muscle shortening or thickening15despite the presence of inspiratory electrical activity in the parasternal intercostal muscles26. Additionally, the limited usefulness of ITF in our study may in part reflect the fact that parasternal intercostals do not necessarily represent the workload of the entire group of accessory inspiratory muscles in healthy subjects27. While this may be true for resting or moderate breathing, Yoshida et al. demonstrated that the thickness of the parasternal intercostal muscles can reflect maximal breathing efforts compared to normal breathing in healthy men14. As differentiation between these states can usually be attained by clinical inspection alone, such extreme respiratory efforts were not intended and not performed by any of the participants in the present study.
ITF could be more informative in patients with diaphragmatic dysfunction12,13. Dres et al. suggested that parasternal intercostal ultrasound may help to assess the imbalance between respiratory effort and respiratory capacity in mechanically ventilated patients12. Umbrello et al. found that combining diaphragm and intercostal thickening measurements in mechanically ventilated patients could help to differentiate between true low respiratory effort and high effort in the presence of diaphragmatic dysfunction13. As those individuals should rely on the accessory respiratory muscles more than others28, a more significant recruitment of the intercostal muscles could be observable.
Taken together, these findings suggest that in healthy individuals, increased respiratory demands can still be compensated for by the diaphragm alone, whereas in the presence of diaphragm dysfunction, the intercostal muscles may play a greater role.
Limitations of this study
This study was conducted in healthy young volunteers. The sample size was relatively small, which may have limited the statistical power to detect smaller effects. Finally, the measurements were made under standardised conditions and without long-term follow-up, so the results may not reflect the variability and complexity of clinical scenarios over time. No direct conclusions for clinical practice or patient management should be drawn from these experimental findings.
Conclusions
While DTF showed a moderate correlation with ΔPoes and allowed reasonable discrimination between exercise loads in noninvasively ventilated healthy subjects, ITF did not correlate with ΔPoes or DTF and did not discriminate between exercise levels. DTF may reflect relative changes in respiratory effort and may support individual titration of noninvasive respiratory support. In healthy individuals, ITF was not informative. Further studies are needed to clarify the clinical utility of ITF in diseased noninvasively ventilated patients.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ΔPinsp :
-
Inspiratory pressure support
- ΔPoes :
-
Tidal oesophageal pressure swings
- DTF:
-
Diaphragm thickening fraction
- ITF:
-
Intercostal thickening fraction
References
Brochard, L., Mancebo, J. & Elliott, M. W. Noninvasive ventilation for acute respiratory failure. Eur. Respir J. 19 (4), 712–721 (2002).
Tonelli, R. et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in de Novo respiratory Failure. A pilot study. Am. J. Respir Crit. Care Med. 202 (4), 558–567 (2020).
MacIntyre, N. R. Physiologic effects of noninvasive ventilation. Respir Care. 64 (6), 617–628 (2019).
Vignaux, L. et al. Patient-ventilator asynchrony during non-invasive ventilation for acute respiratory failure: a multicenter study. Intensive Care Med. 35 (5), 840–846 (2009).
Goligher, E. C. et al. Lung- and Diaphragm-Protective ventilation. Am. J. Respir Crit. Care Med. 202 (7), 950–961 (2020).
Bellani, G. & Pesenti, A. Assessing effort and work of breathing. Curr. Opin. Crit. Care. 20 (3), 352–358 (2014).
Kaur, A. et al. Sonographic assessment of diaphragmatic thickening and excursion as predictors of weaning success in the intensive care unit: A prospective observational study. Indian J. Anaesth. 66 (11), 776–782 (2022).
Umbrello, M. et al. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit. Care. 19 (1), 161 (2015).
Lindner, S. et al. Correlation of diaphragm thickening fraction and oesophageal pressure swing in non-invasive ventilation of healthy subjects. BMC Pulm Med. 24 (1), 289 (2024).
Schepens, T., Fard, S. & Goligher, E. C. Assessing diaphragmatic function. Respir Care. 65 (6), 807–819 (2020).
Formenti, P., Umbrello, M., Dres, M. & Chiumello, D. Ultrasonographic assessment of parasternal intercostal muscles during mechanical ventilation. Ann. Intensive Care. 10 (1), 120 (2020).
Dres, M. et al. Usefulness of parasternal intercostal muscle ultrasound during weaning from mechanical ventilation. Anesthesiology 132 (5), 1114–1125 (2020).
Umbrello, M. et al. Oesophageal pressure and respiratory muscle ultrasonographic measurements indicate inspiratory effort during pressure support ventilation. Br. J. Anaesth. 125 (1), e148–e57 (2020).
Yoshida, R. et al. Measurement of intercostal muscle thickness with ultrasound imaging during maximal breathing. J. Phys. Ther. Sci. 31 (4), 340–343 (2019).
Cala, S. J. et al. Respiratory ultrasonography of human parasternal intercostal muscle in vivo. Ultrasound Med. Biol. 24 (3), 313–326 (1998).
Goligher, E. C. et al. Evolution of diaphragm thickness during mechanical Ventilation. Impact of inspiratory effort. Am. J. Respir Crit. Care Med. 192 (9), 1080–1088 (2015).
Borg, G. A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14 (5), 377–381 (1982).
Matamis, D. et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 39 (5), 801–810 (2013).
Wait, J. L., Nahormek, P. A., Yost, W. T. & Rochester, D. P. Diaphragmatic thickness-lung volume relationship in vivo. J. Appl. Physiol. (1985). 67 (4), 1560–1568 (1989).
Bakdash, J. Z. & Marusich, L. R. Repeated measures correlation. Front. Psychol. 8, 456 (2017).
Vivier, E. et al. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med. 38 (5), 796–803 (2012).
Menis, A. A. et al. Diaphragmatic ultrasound and its relationship to breathing effort and load: a prospective observational study. Crit. Care. 29 (1), 190 (2025).
Powers, S. K. Ventilator-induced diaphragm dysfunction: phenomenology and mechanism(s) of pathogenesis. J. Physiol. 602 (19), 4729–4752 (2024).
Wu, H. & Chasteen, B. Rapid review of ventilator-induced diaphragm dysfunction. Respir Med. 223, 107541 (2024).
Supinski, G. S., Morris, P. E., Dhar, S. & Callahan, L. A. Diaphragm dysfunction in critical illness. Chest 153 (4), 1040–1051 (2018).
De Troyer, A., Kirkwood, P. A. & Wilson, T. A. Respiratory action of the intercostal muscles. Physiol. Rev. 85 (2), 717–756 (2005).
Sampson, M. G. & De Troyer, A. Role of intercostal muscles in the rib cage distortions produced by inspiratory loads. J. Appl. Physiol. Respir Environ. Exerc. Physiol. 52 (3), 517 (1982).
De Troyer, A. & Estenne, M. Limitations of measurement of transdiaphragmatic pressure in detecting diaphragmatic weakness. Thorax 36 (3), 169–174 (1981).
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was supported by the non-profit E&H Knorr Foundation. Daniel Duerschmied is supported by the German Research Foundation (DFG CRC1366 B08, Project #394046768) and the German Centre for Cardiovascular Research (MaBo-05).
Author information
Authors and Affiliations
Contributions
Conceptualization: CH, BN, JDMZ, SB, SL. Methodology and investigation: BL, LSD, LD, SL. Formal analysis: MB, SL. Resources and supervision: DD, FJFH. Writing - Original Draft: CH, SL. Writing - Review & Editing BL, LSD, LD, BN, FJFH, MB, DD, SB. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the principles of the 1964 Helsinki declaration and its later amendments. The ethics committee II of the university Heidelberg, medical faculty Mannheim approved this study, approval number 2024−564. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Material 2
Supplementary Material 3
Supplementary Material 4
Supplementary Material 5
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoermann, C., Drotleff, L.S., Link, B. et al. Intercostal thickening fraction adds no value to diaphragm thickening fraction in healthy subjects undergoing noninvasive ventilation. Sci Rep 16, 7165 (2026). https://doi.org/10.1038/s41598-026-40192-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-026-40192-4