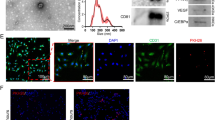
Abstract
Nanofat is a relatively recent fat grafting technique obtained involving the mechanical emulsification of adipose tissue whose preparation is produced at the patient’s bedside. Although it was initially reported to improve skin quality in intradermal applications, it is now increasingly used in regenerative medicine. However, the absence of standardized protocols and the diversity of commercial devices result in nanofat products of variable quality. This study presents the first comprehensive comparison of nanofat obtained from different commercially available preparation systems, combining both their technical performance and biological characterization. Lipoaspirates from five healthy donors were processed using eight commercially available devices for nanofat production using emulsification or micronization techniques. The technical parameters included preparation time, ease of preparation and injection, volumetric yield, and residual aqueous fraction. Biological analyses included stromal vascular fraction isolation with evaluation of cell viability, viable nucleated cell yield, immunophenotypic cell subtype characterization and clonogenic capacity. These parameters were compared using a scoring model that enabled inter-kit ranking, integrating both a technical performance score and a biological quality score. Additionally, nanofat-conditioned media were collected for extracellular vesicles (EVs) quantification and subtyping by flow cytometry, and confocal microscopy was performed to evaluate the preservation of mature adipocytes, capillary networks, and the extracellular matrix. All devices demonstrated satisfactory technical performance, with Puregraft Boost V2 and Emulsfat achieving the highest overall technical scores. Cell viability was consistently high, with median values above 85% across all devices. Adinizer provided the greatest proportion of adipose-derived stromal/stem cells and achieved the highest overall biological score. In contrast, Hy-Tissue Nanofat produced the lowest cell yields together with the highest leukocyte proportions. All nanofats contained clonogenic progenitors. Extracellular vesicles concentrations were comparable between devices, and were mainly influenced by donor variability, although Emulsfat was enriched in adipocyte-derived EVs. Microscopic analysis revealed preservation of adipocytes, vascular networks, and the extracellular matrix across devices, challenging the assumption that emulsification or micronization completely disrupts tissue architecture. Nanofat properties are strongly device dependent, with possible dissociation between technical ease and biological quality. This first comparative study highlights the need for standardized preparation methods and qualification criteria, and provides guidance for selecting devices aligned with specific clinical objectives to optimize regenerative outcomes.
Similar content being viewed by others
Data availability
All data generated during this study are included in this published article. The data that support the findings of this study are available on request from the corresponding author, upon reasonable request.
Abbreviations
- AEVs:
-
Adipocyte-derived extracellular vesicles
- ASC:
-
Adipose-derived stem/stromal cells
- ASC-EVs:
-
Adipose stromal/stem cell-derived extracellular vesicles
- AT:
-
Adipose tissue
- ATMP:
-
Advanced therapy medicinal product
- CFU-F:
-
Colony forming units-fibroblasts
- CM:
-
Conditioned media
- DAPI:
-
4',6-Diamidino-2-phenylindole (utilisé pour marquage nucléaire)
- EBM:
-
Endothelial basal medium
- ECM:
-
Extracellular matrix
- EEVs:
-
Endothelial-derived extracellular vesicles
- ErEVs:
-
Erythrocyte-derived extracellular vesicles
- EVs:
-
Extracellular vesicles
- FBS:
-
Fetal bovine serum
- FMO:
-
Fluorescence minus one
- HSA:
-
Human serum albumin
- IFATS:
-
International federation for adipose therapeutics and science
- IGF:
-
Insulin-like growth factor
- IQR:
-
Interquartile range
- ISCT:
-
International society for cellular therapy
- LEVs:
-
Leukocyte-derived extracellular vesicles
- MSC-EVs:
-
Mesenchymal stromal/stem cell-derived EVs
- ORO:
-
Oil Red O
- PBS:
-
Phosphate-buffered saline
- PDGF:
-
Platelet-derived growth factor
- PEVs:
-
Platelet-derived extracellular vesicles
- PFA:
-
Paraformaldehyde
- SVF:
-
Stromal vascular fraction
- VEGF:
-
Vascular endothelial growth factor
- VNC:
-
Viable nucleated cells
References
Coleman, S. R. Structural fat grafting: More than a permanent filler. Plast. Reconstr. Surg. 118(Suppl), 108S-120S (2006).
Meruane, M. A., Rojas, M. & Marcelain, K. The use of adipose tissue-derived stem cells within a dermal substitute improves skin regeneration by increasing neoangiogenesis and collagen synthesis. Plast. Reconstr. Surg. 130(1), 53–63 (2012).
Zuk, P. A. et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 7(2), 211–228 (2001).
Nguyen, P. S. A., Desouches, C., Gay, A. M., Hautier, A. & Magalon, G. Development of micro-injection as an innovative autologous fat graft technique: The use of adipose tissue as dermal filler. J. Plast. Reconstr. Aesthetic. Surg. JPRAS. 65(12), 1692–1699 (2012).
Ding, P. et al. Research progress on preparation, mechanism, and clinical application of nanofat. J. Burn Care Res. Off Publ. Am. Burn. Assoc. 43(5), 1140–1144 (2022).
La Padula, S. et al. Nanofat in plastic reconstructive, regenerative, and aesthetic surgery: A review of advancements in face-focused applications. J. Clin. Med. 12(13), 4351 (2023).
Tonnard, P. et al. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 132(4), 1017–1026 (2013).
Quintero Sierra, L. A. et al. Highly pluripotent adipose-derived stem cell-enriched nanofat: A novel translational system in stem cell therapy. Cell Transplant. 32, 9636897231175968 (2023).
Grünherz, L., Sanchez-Macedo, N., Frueh, F. S., McLuckie, M. & Lindenblatt, N. Nanofat applications: From clinical esthetics to regenerative research. Curr. Opin. Biomed. Eng. 10, 174–180 (2019).
Lo Furno, D. et al. Nanofat 2.0: Experimental evidence for a fat grafting rich in mesenchymal stem cells. Physiol. Res. 66(4), 663–671 (2017).
Cohen, S. R. et al. Cellular optimization of nanofat: Comparison of two nanofat processing devices in terms of cell count and viability. Aesth. Surg. J. Open Forum. 1(4), 28 (2019).
Wei, H. et al. Nanofat-derived stem cells with platelet-rich fibrin improve facial contour remodeling and skin rejuvenation after autologous structural fat transplantation. Oncotarget 8(40), 68542–68556 (2017).
Colazzo, F. et al. Shear stress and VEGF enhance endothelial differentiation of human adipose-derived stem cells. Growth Factors 32(5), 139–149 (2014).
Banyard, D. A. et al. Phenotypic analysis of stromal vascular fraction after mechanical shear reveals stress-induced progenitor populations. Plast. Reconstr. Surg. 138(2), 237e-e247 (2016).
Sultan, S. M. et al. Human fat grafting alleviates radiation skin damage in a murine model. Plast. Reconstr. Surg. 128(2), 363–72 (2011).
Klinger, M., Caviggioli, F., Vinci, V., Salval, A. & Villani, F. Treatment of chronic posttraumatic ulcers using autologous fat graft. Plast. Reconstr. Surg. 126(3), 154e-e155 (2010).
Rageh, M. A., El-Khalawany, M. & Ibrahim, S. M. A. Autologous nanofat injection in treatment of scars: A clinico-histopathological study. J. Cosmet. Dermatol. 20(10), 3198–3204 (2021).
Arcani, R. et al. Nanofat use in regenerative medicine: A systematic literature review and consensus recommendations from expert opinions. Facial Plast. Surg. Aesth. Med. 1, 1 (2025).
Lombardo, J. A., Banyard, D. A., Widgerow, A. D. & Haun, J. B. Fluidic device system for mechanical processing and filtering of human lipoaspirate enhances recovery of mesenchymal stem cells. Plast. Reconstr. Surg. 151(1), 72e–84e (2023).
Girard, P. et al. Modified nanofat grafting: Stromal vascular fraction simple and efficient mechanical isolation technique and perspectives in clinical recellularization applications. Front. Bioeng. Biotechnol. 10, 895735 (2022).
Chen, X. et al. Mechanical emulsification of lipoaspirate by different Luer-Lok connector changes the viability of adipose derived stem cells in Nanofat. J. Plast. Surg. Hand Surg. 54(6), 344–351 (2020).
Bourin, P. et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15(6), 641–648 (2013).
Bonifay, A. et al. A new strategy to count and sort neutrophil-derived extracellular vesicles: Validation in infectious disorders. J. Extracell. Vesicles 11(4), 1. https://doi.org/10.1002/jev2.12204 (2022).
Qiu, H., Jiang, Y., Chen, C., Wu, K. & Wang, H. The effect of different diameters of fat converters on adipose tissue and its cellular components: selection for preparation of nanofat. Aesth. Surg. J. 41(11), 1734–1744 (2021).
Grünherz, L., Kollarik, S., Sanchez-Macedo, N., McLuckie, M. & Lindenblatt, N. Lipidomic analysis of microfat and nanofat reveals different lipid mediator compositions. Plast. Reconstr. Surg. 154(5), 895e–905e (2024).
Sanchez-Macedo, N., McLuckie, M., Grünherz, L. & Lindenblatt, N. Protein profiling of mechanically processed lipoaspirates: Discovering wound healing and antifibrotic biomarkers in nanofat. Plast. Reconstr. Surg. 150(2), 341e-e354 (2022).
Chen, A. et al. Small extracellular vesicles from human adipose-derived mesenchymal stromal cells: A potential promoter of fat graft survival. Stem Cell Res. Ther. 12(1), 263 (2021).
Wang, Y., Li, Q., Zhou, S., & Tan, P. Contents of exosomes derived from adipose tissue and their regulation on inflammation, tumors, and diabetes. Front. Endocrinol. 15, 1374715 (2024).
François, P. et al. Inter-center comparison of good manufacturing practices-compliant stromal vascular fraction and proposal for release acceptance criteria: a review of 364 productions. Stem Cell Res Ther. 12(1), 373 (2021).
Acknowledgements
The authors declare that they have not used AI-generated work in this manuscript
Funding
This study was funded by the Société Nationale Française de Médecine Interne (SNFMI) and the Association des Hospitalo-Universitaires de Marseille (ASHUM).
Author information
Authors and Affiliations
Contributions
RA, MA, SS, VD, SR, AZ, LA, CB, SM and MV performed experiments and collected data. RA, SS, VD, SR, EJ, LA, RL, MV and JM analysed and interpreted the results. GM, AD, MV, and JM conceived the study, supervised the project, and contributed to data interpretation. GM, RL, FDG, FS, and AD provided critical input on study design, data interpretation, and manuscript revision. RA, MA, SS, SR, LA, VD, MV and JM drafted the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
GM, FS and JM are cofounders of the Remedex Network. JM received honoraria for educational support from Fidia Pharmaceuticals, Horiba, Arthrex, Horus Pharma and Macopharma. These manufacturers had no role in the development of this study or its decision for publication. The authors declare that they have no competing interests.
Ethics approval and consent to participate
All donors who participated in the study received an information and nonopposition notice and did not express any objection to the use of their adipose tissue.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Arcani, R., Abellan, M., Simoncini, S. et al. First comparison of commercial systems to prepare nanofat: technical performances and biological quality differ among obtained products. Sci Rep (2026). https://doi.org/10.1038/s41598-026-40847-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-40847-2