Abstract
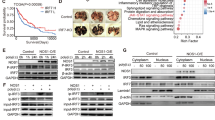
Nitric oxide (NO) is a key signaling molecule that plays a vital role in maintaining homeostasis of physiological processes such as immune responses and neurotransmission. However, excessive NO production during inflammatory responses to infection can lead to cytotoxicity and tissue damage. The nasal epithelial barrier is a crucial first line of immunological defense against viral infections, and it is likely exposed to excessive NO levels during chronic inflammation. Therefore, clarifying the effects of NO on this barrier is thus critical. In this study, we investigated the biological effects of sustained NO exposure on RPMI2650 human nasal epithelial cells. Post-NO exposure transcriptomic analyses revealed significant upregulation of genes involved in the p53 signaling pathway. RT-qPCR analyses confirmed the temporal upregulation of p53 target genes associated with apoptosis and cell cycle regulation. These gene expression changes downregulated cell proliferation and induced cell death. Our findings suggest that excessive NO exposure induces nasal epithelial cell death via the p53 pathway, which over the long term can result in tissue damage and dysfunction under inflammatory conditions. These results provide new insights into how prolonged NO exposure affects the nasal epithelial cells and may contribute to the progression of chronic infectious diseases.
Similar content being viewed by others
Data availability
RNA sequencing data were deposited in the DDBJ (https://ddbj.nig.ac.jp/search/en) under accession number PRJDB35845.
References
Uehara, T. et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441, 513–517. https://doi.org/10.1038/nature04782 (2006).
Nakato, R. et al. Regulation of the unfolded protein response via S-nitrosylation of sensors of endoplasmic reticulum stress. Sci. Rep. 5, 14812. https://doi.org/10.1038/srep14812 (2015).
Numajiri, N. et al. On–off system for PI3-kinase–Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN). Proc. Natl. Acad. Sci. U. S. A. 108, 10349–10354. https://doi.org/10.1073/pnas.1103503108 (2011).
Okuda, K. et al. Pivotal role for S-nitrosylation of DNA methyltransferase 3B in epigenetic regulation of tumorigenesis. Nat. Commun. 14, 621. https://doi.org/10.1038/s41467-023-36232-6 (2023).
Sharma, J. N., Al-Omran, A. & Parvathy, S. S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 15, 252–259. https://doi.org/10.1007/s10787-007-0013-x (2007).
Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2, 907–916. https://doi.org/10.1038/ni1001-907 (2001).
Cinelli, M. A., Do, H. T., Miley, G. P. & Silverman, R. B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 40, 158–189. https://doi.org/10.1002/med.21599 (2020).
Daniela, B.-G., Thomas, R. & Kolb-Bachofen, V. Nitric oxide in human skin: Current status and future prospects. J. Invest. Dermatol. 110, 1–7. https://doi.org/10.1046/j.1523-1747.1998.00084.x (1998).
Ikeyama, K., Fuziwara, S. & Denda, M. Topical application of neuronal nitric oxide synthase inhibitor accelerates cutaneous barrier recovery and prevents epidermal hyperplasia induced by barrier disruption. J. Invest. Dermatol. 127, 1713–1719. https://doi.org/10.1038/sj.jid.5700742 (2007).
Tonelli, L. H. & Postolache, T. T. Airborne inflammatory factors: “From the nose to the brain”. Front. Biosci. (Schol. Ed.) 2, 135–152 (2010).
Lafever, B. J. & Imamura, F. Effects of nasal inflammation on the olfactory bulb. J. Neuroinflammation 19, 294. https://doi.org/10.1186/s12974-022-02657-x (2022).
Butowt, R., Bilinska, K. & Von Bartheld, C. S. Olfactory dysfunction in COVID-19: New insights into the underlying mechanisms. Trends Neurosci. 46, 75–90. https://doi.org/10.1016/j.tins.2022.11.003 (2023).
Chen, S. & Wang, S. The immune mechanism of the nasal epithelium in COVID-19–related olfactory dysfunction. Front. Immunol. 14, 1045009. https://doi.org/10.3389/fimmu.2023.1045009 (2023).
Gamage, A. M. et al. Human nasal epithelial cells sustain persistent SARS-CoV-2 infection in vitro, despite eliciting a prolonged antiviral response. mBio 13, e0343621. https://doi.org/10.1128/mbio.03436-21 (2022).
Gelzo, M. et al. Inducible nitric oxide synthase (iNOS): Why a different production in COVID-19 patients of the two waves?. Viruses 14, 534. https://doi.org/10.3390/v14030534 (2022).
Zhang, Y. et al. S-nitrosylation of the Peroxiredoxin-2 promotes S-nitrosoglutathione-mediated lung cancer cells apoptosis via AMPK-SIRT1 pathway. Cell Death Dis. 10, 329. https://doi.org/10.1038/s41419-019-1561-x (2019).
Joo, T. et al. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi J. Biol. Sci. 21, 427–435. https://doi.org/10.1016/j.sjbs.2014.04.003 (2014).
Polyak, K., Xia, Y., Zweier, J. L., Kinzler, K. W. & Vogelstein, B. A model for p53-induced apoptosis. Nature 389, 300–305. https://doi.org/10.1038/38525 (1997).
Aubrey, B. J., Kelly, G. L., Janic, A., Herold, M. J. & Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression?. Cell Death Differ. 25, 104–113. https://doi.org/10.1038/cdd.2017.169 (2018).
Lavin, M. F. & Gueven, N. The complexity of p53 stabilization and activation. Cell. Death. Differ 13, 941–950. https://doi.org/10.1038/sj.cdd.4401925 (2006).
Kubbutat, M. H. G., Jones, S. N. & Vousden, K. H. Regulation of p53 stability by Mdm2. Nature 387, 299–303. https://doi.org/10.1038/387299a0 (1997).
Zhu, J., Singh, M., Selivanova, G. & Peuget, S. Pifithrin-α alters p53 post-translational modifications pattern and differentially inhibits p53 target genes. Sci. Rep. 10, 1049. https://doi.org/10.1038/s41598-020-58051-1 (2020).
Ding, M. et al. Inducible nitric-oxide synthase and nitric oxide production in human fetal astrocytes and microglia. J. Biol. Chem. 272, 11327–11335. https://doi.org/10.1074/jbc.272.17.11327 (1997).
Meßmer, U. K., Ankarcrona, M., Nicotera, P. & Brüne, B. p53 expression in nitric oxide‐induced apoptosis. FEBS Lett. 355, 23–26. https://doi.org/10.1016/0014-5793(94)01161-3 (1994).
Brüne, B. The intimate relation between nitric oxide and superoxide in apoptosis and cell survival. Antioxid. Redox Signal. 7, 497–507. https://doi.org/10.1089/ars.2005.7.497 (2005).
Sagiv, A. et al. P53 in bronchial club cells facilitates chronic lung inflammation by promoting senescence. Cell. Rep. 22, 3468–3479. https://doi.org/10.1016/j.celrep.2018.03.009 (2018).
He, W., Tang, M., Wu, X., Mu, X. & Nie, X. The role of p53 in regulating chronic inflammation and PANoptosis in diabetic wounds. Aging Dis. 16, 373–393. https://doi.org/10.14336/ad.2024.0212 (2024).
Schonhoff, C. M., Daou, M.-C., Jones, S. N., Schiffer, C. A. & Ross, A. H. Nitric oxide-mediated inhibition of Hdm2-p53 binding. Biochemistry 41, 13570–13574. https://doi.org/10.1021/bi026262q (2002).
Baldelli, S. & Ciriolo, M. R. Altered S-nitrosylation of p53 is responsible for impaired antioxidant response in skeletal muscle during aging. Aging 8, 3450–3467. https://doi.org/10.18632/aging.101139 (2016).
Loxham, M., Davies, D. E. & Blume, C. Epithelial function and dysfunction in asthma. Clin. Exp. Allergy 44, 1299–1313. https://doi.org/10.1111/cea.12309 (2014).
Ye, Q., Wang, B. & Mao, J. The pathogenesis and treatment of the `cytokine storm’ in COVID-19. J. Infect. 80, 607–613. https://doi.org/10.1016/j.jinf.2020.03.037 (2020).
Milani, D. et al. P53/NF-kB balance in SARS-CoV-2 infection: From OMICs, genomics and pharmacogenomics insights to tailored therapeutic perspectives (COVIDomics). Front. Pharmacol. 13, 871583. https://doi.org/10.3389/fphar.2022.871583 (2022).
Salib, R. J., Lau, L. C. & Howarth, P. H. The novel use of the human nasal epithelial cell line RPMI 2650 as an in vitro model to study the influence of allergens and cytokines on transforming growth factor-β gene expression and protein release. Clin. Exp. Allergy 35, 811–819. https://doi.org/10.1111/j.1365-2222.2005.02258.x (2005).
Wang, H. et al. Three-dimensional cultured human nasal epithelial cell model for testing respiratory toxicity and neurotoxicity of air pollutants. Environ. Sci. Technol. 59, 6452–6463. https://doi.org/10.1021/acs.est.4c13205 (2025).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 16, 284–287. https://doi.org/10.1089/omi.2011.0118 (2012).
Kubota, S. et al. Chromatin modifier Hmga2 promotes adult hematopoietic stem cell function and blood regeneration in stress conditions. EMBO J. 43, 2661–2684. https://doi.org/10.1038/s44318-024-00122-4 (2024).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D677. https://doi.org/10.1093/nar/gkae909 (2025).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Tsuchida, T. et al. Epigenetic regulation of CXC chemokine expression by environmental electrophiles through DNA methyltransferase inhibition. Int. J. Mol. Sci. 25, 11592. https://doi.org/10.3390/ijms252111592 (2024).
Acknowledgements
We thank Kotoe Sueyoshi and Eriko Nishimura for technical assistance.
Funding
This research was funded by grants-in-aid for Challenging Exploratory Research (22K19380 to T.U.) and Scientific Research (A) (24H00678 to T.U.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. Funding was also provided by the Smoking Research Foundation (to T.U.) and the JSPS Program for Forming Japan’s Peak Research Universities (J-PEAKS) (JPJS00420230010).
Author information
Authors and Affiliations
Contributions
T.U. conceived and designed the study. S.K. (Shizuki Kamiuezono) acquired and initially analyzed the data. S.K. (Shizuki Kamiuezono), S.K. (Sho Kubota), and T.T. conducted further analyses. S.K. (Shizuki Kamiuezono) drafted the manuscript. S.K. (Sho Kubota) and N.T. substantively revised the manuscript. All authors approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kamiuezono, S., Kubota, S., Tsuchida, T. et al. Nitric oxide induces p53-mediated cell death in human nasal epithelial cells. Sci Rep (2026). https://doi.org/10.1038/s41598-026-40908-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-40908-6