Abstract
Lead white is one of the most important pigments in human history, and its synthesis has promoted the development of art and cosmetics. The corrosion approach to synthesize lead white appeared in Greece during the fourth century BCE, and since then lead white has been produced on a large-scale and widely used in painting and cosmetics across Europe. However, when and how synthetic lead white appeared in east Eurasia and whether it was also involved with beauty remained unclear. Here, we investigate some white cosmetic residues from the Liangdaicun site during the eighth century BCE in northern China through FTIR, XRD, SEM-EDS, radioactive and stable carbon isotope analyses. The results show that these residues were the earliest synthesized lead white in the world to date, which was produced by the precipitation method in solution distinct from the corrosion method practiced in ancient Greece. Thus, the synthesis of lead white should have evolved independently in east and west Eurasia during the first millennium BCE. The mass production of synthetic lead white with lower cost promoted the widespread use of white makeup in China and the Mediterranean World, which triggered a cosmetic revolution and highlighted that the pursuit of beauty stimulated the development of chemistry in human history, especially the earliest wet chemistry practice in China.
Similar content being viewed by others
Introduction
Humans exploited natural mineral pigments in prehistoric sites and cave art at least 40 thousand years ago (Aubert et al., 2019; Wang et al., 2022) and then invented their chemical synthesis to satisfy the ever-growing demand in art and beauty activities. In antiquity, several raw materials have been used together to produce synthetic pigments, such as Egyptian Blue (CaCuSi4O10) (~3200 BCE), phosgenite (Pb2Cl2CO3) (~1400 BCE), China Purple (BaCuSi2O6) (eighth century BCE) and Maya blue (eighth century CE), which play a decisive role in beauty creation (Walter et al., 1999; Ma et al., 2006; Berke, 2007; Beck et al., 2018; Cartechini et al., 2021; Li et al., 2022) and are important for the development of art, cosmetics and chemistry (Pulsifer, 1888; Schafer, 1956; Needham et al., 1976; Becker, 2022).
The lead-based white pigment, lead white, is usually referred to as a mixture of two main lead carbonates, hydrocerussite ((PbCO3)2·Pb(OH)2) and cerussite (PbCO3). Lead white has been a famous material for make-up use since antiquity (Gliozzo and Ionescu, 2021) and one of the most important, employed and valued white pigments in paintings and murals, with early reports in Egyptian cartonnage from the Graeco-Roman Period (Scott et al., 2003), and used in painting workshops on Fayum portraits as early as the second century CE (Salvant et al., 2018). Lead white then became ubiquitously used in European oil paintings since the late Middle Ages (Stols-Witlox, 2014; Gonzalez et al., 2017; De Meyer et al., 2019). In ancient China, lead white was found on the pigment layers of Terra-Cotta Warriors (the late third century BCE), which is currently the earliest application in east Asia (Li, 1983).
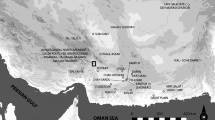
The earliest method to obtain lead white was simply mining natural cerussite during the 5th–2nd millennium BCE in southern Europe (~4000 BCE) (Kramberger et al., 2021), Egypt (~3000 BCE) (Beck et al., 2018), Mesopotamia (~2500 BCE) (Hauptmann et al., 2016), Iran (~3000 BCE) (Vidale et al., 2012), and Indus Valley (~2500 BCE) (Vidale et al., 2016) (Fig. 1). Then, the synthetic production method was adopted in the mid-first millennium BCE. In Europe, the corrosion method to synthesize lead white first appeared during the fourth century BCE in ancient Greece (Fig. 1), followed by Theophrastus, Vitruvius, Pliny the Elder and Dioscorides recording the recipes (Pulsifer, 1888; Katsaros et al., 2010). In the corrosion process, vinegar vapor, heat, and carbon dioxide played key roles in transforming lead into lead acetate and then carbonated into lead white (equations 1–4 in the Supplementary Material), but their functions were usually ignorant and poorly described in ancient recipes (Stevenson, 1955; Gonzalez et al., 2019; Photos-Jones et al., 2020). The corrosion process is predominantly used in antiquity (Pulsifer, 1888), and the synthetic nature of early Greek lead white has been confirmed by AMS radiocarbon dating (Beck et al., 2018).
The symbol shapes represent different regions, and their colors represent different natures of cerussite. This worldwide scenario of lead white use before the Common Era was drawn according to the references (Vidale et al., 2012; Hauptmann et al., 2016; Vidale et al., 2016; Beck et al., 2018; Kramberger et al., 2021).
However, there is no historical record or scientific analysis that clearly described lead white synthesis in the first millennium BCE in east Eurasia (Fig. 1). Thus, different hypotheses were proposed about the origin of the lead white synthesis in east Eurasia. Joseph Needham thought that lead white synthesis should have been invented in the fifth century BCE in China through interpreting an ancient Chinese recipe (No. 1 in Table S1) and then spread to Europe (Needham et al., 1976). On the other hand, it is argued that the technology of lead white synthesis was possibly introduced into China from Europe (Wai and Liu, 1991) or ancient Egypt (Huang and Qin, 2010).
Currently, an investigation of archeological findings about lead white in east Eurasia is lacking to clarify the nature and synthesis method of lead white during the first millennium BCE, e.g., the absence of direct 14C dating for lead carbonate. Thus, the worldwide scenario on early lead white synthesis is incomplete and in debate. In this study, some archeologically excavated white residue samples within miniature cosmetic containers at the Liangdaicun site (Figs. 2 and 3) were collected and analyzed by microscopy observation, Fourier transform infrared spectroscopy (FTIR), X-ray powder diffraction (XRD), stable isotope 13C analysis and radioactive 14C dating. The chemical analysis allows, for the first time, to reveal the earliest known case of man-made lead white in the world.
a The location of the Liangdaicun site in northern China and b the location of tombs M26, M27 and M300 within the Liangdaicun site. In b, the yellow symbols (mostly with tomb passage) represent tombs of the aristocratic class, and the gray symbols represent tombs of civilians. Most of the tombs above the dashed line are not shown in the map except M300, M33, M502, M560 and M586. c is the plane graph of tomb M27 (occupier Ruigong, a king of Rui state) and d is the plane graph of tomb M26 (occupier Zhongjiang, a queen of Rui state). The graphs are in reference to the archeological report of the Liangdaicun site. M26 is a “甲”-shaped tomb with one tomb passage, while M27 is a “中”-shaped tomb with two tomb passages (“甲” and “中” are typical tomb shapes for nobilities and elite burials during that period).
a Miniature bronze items M26:138, M26:137, M26:261, M26:139, M26:135, and M26:136 as a set of funerary items; b vertical view of tomb M26. In b, the blue box indicates the location of the items in the outer coffin sitting northeast of the head of the occupier in tomb M26. Decorative patterns of these miniature vessels are illustrated in Fig. S1, Supplementary Material.
Materials and Methods
Archeological background
The Liangdaicun site is located in Hancheng city, Shaanxi Province, northern China (Fig. 2). It was the nobility cemetery of Rui state in the early Spring and Autumn Period (~8th century BCE) (Sun et al., 2007; Sun et al., 2008; Han et al., 2022). Many valuable artifacts have been unearthed from nobilities’ tombs, including tomb M26 (occupier Zhongjiang, a queen of Rui state), tomb M27 (occupier Ruigong, a king of Rui state) and M300 (a female occupier from aristocratic class). In these tombs, several small and ornate miniature bronze artifacts were unearthed (Figs. 2–4, Figs. S1–S6, Supplementary Material), notably, a set of ornate miniature bronze containers in the queen’s tomb M26 (Fig. 3, Fig. S1, Supplementary Material). These miniature containers, discovered in the tombs of the contemporary aristocratic class, were deduced as cosmetic containers (Li, 2009). White residue aggregates were found inside six miniature bronze containers (Figs. 3 and 4; Figs. S3–S6). Some samples were taken from these containers and coded as LW-1 to LW-6, whose detailed information is listed in Table 1.
a The miniature bronze container of Fu (code M26:138); b the white residue (LW-1a) on its lid; c the SEM observation of sample LW-1a with multiple stacks layout; d numerous pure lead white residue particles scattered within the soil off the bottom of M26:138; e some elongated needles and prismatic lead white (LW-1c) gently separated from the soil of d; f the SEM observation of sample LW-1c; g the miniature bronze container of round-foot jar (code M26:137); h the white residue (LW-2a) on its lid; i the SEM observation of sample LW-2a with prismatic shape.
Microscopic observation
Microscopic observation was conducted using a Keyence VHX600 digital microscope. Scanning electron microscopy (SEM) was conducted on a Phenom XL coupled with an energy dispersive X-ray spectrometer (EDS) system. For SEM analysis, the specimens were sprinkled on carbon adhesive tape. SEM observation was conducted at 15 kV with a spot size of 1.2 nA, and EDS analysis was conducted at 15 kV with a spot size of 8 nA.
FTIR analysis
FTIR analysis was conducted using a Nicolet 6700 (Thermo Scientific) FTIR spectrometer with an attenuated total reflectance (ATR) accessory. A tiny portion of the residue was placed on the ATR testing platform using a cleaned dissecting needle. Spectra were acquired over the range of 4000–500 cm−1 using a resolution of 4 cm−1, with 32 scans per spectrum.
XRD analysis
XRD analysis was applied to the residue samples by a Rigaku MiniFlex II Desktop X-ray diffractometer using Cu Kα (λ = 1.5406 Å) radiation. A small amount of the residue was ground into a fine powder and then dispersed evenly on a square silicon tablet (1.5 × 1.5 cm) using ethanol solution. The sample was then left to dry. The analysis was conducted with the following parameters: voltage of 30 kV, current of 15 mA, divergence slit of 1.25°, anti-scattering slit of 1.25°, and receiving slit width of 0.3 mm. Samples were scanned over an angular 2θ range from 10 to 75° with a scanning speed of 3°/min. The approximate contents of PbCO3 and Pb2Cl2CO3 were calculated using their intensive peak areas.
Carbon and oxygen isotope analysis
Subsequent to physical examination, sample material was bathed in less than 0.1 N hydrochloric acid (HCl) at room temperature to effectively remove all surface carbonate (potentially containing carbon from secondary sources). Sonication was applied to shake loose debris. After rinsing to neutral, the sample was dried at 110 °C for 12–14 h and then examined under a microscope. After the pretreatment of acid etching, the samples are crushed and placed in the reaction vessel, pulled to vacuum, purged two times with CO2 free air and pulled to vacuum. Since the samples are pure lead carbonates (without mixing other carbonates, as in painting artworks (Beck et al., 2019; Hendriks et al., 2020a)), the acid hydrolysis method was applied to generate CO2. A 10% reagent phosphoric acid/deionized water solution was then slowly added to the reaction vessel containing the sample. More acid was added slowly until the reaction appeared to have stopped. The vessel is placed into a beaker with water in it and then onto a hotplate and is heated at 80 °C for 2 h to facilitate full completion of the reaction. The CO2 is then collected from the vessel. The radiocarbon ages and carbon and oxygen isotopes were measured by accelerator mass spectrometry (AMS) and isotope ratio mass spectrometry (IRMS) at the Beta Analytic Radiocarbon Dating Laboratory (Miami, Florida, USA). Calibration of the radiocarbon dates was against the IntCal20 atmospheric calibration curve (Reimer et al., 2009; Ramsey, 2017).
Results
The identification of lead carbonate in white cosmetic residues
The FTIR analysis of all the samples (Fig. 5, Figs. S2–S6, Supplementary Material) shows the characteristic peaks of cerussite (PbCO3), including 678 cm−1 (a ν4-associated band produced by the in-plane bending vibration of the CO32− group), 838 cm−1 (a ν2-associated band produced by the out-of-plane bending vibration of the CO32− group) and 1050 cm−1 (a ν1-associated band produced by the symmetric stretching vibration of the CO32- group) (Catalli et al., 2005; Jones, Jackson, 2012). Almost all the samples are composed of pure PbCO3, except for samples LW-1a and LW-5, whose peaks also include 758, 648, and 638 cm−1, which imply the presence of phosgenite (Pb2Cl2CO3) (Fig. 5) (Stanley et al., 1991; Jones and Jackson, 2012). The residue compositions were further confirmed by XRD analysis, which showed that the main component was PbCO3 (Figs. S3–S6). Pb2Cl2CO3 was detected as a minor component (~5%) in sample LW-1a and a major component (~60%) in sample LW-5 (Fig. 5, Fig. S5, and Table 1).
The morphology of the lead white crystal
The cerussite crystals in the Liangdaicun cosmetic residue present elongated pseudohexagonal, acicular and prismatic morphologies (Fig. 4 and Figs. S7–S9), which reveal its formation process. The corrosion synthetic process generally produced grainy and short prismatic crystals and was usually a mixture of hydrocerussite and cerussite (Welcomme et al., 2006; Sánchez-Navas et al., 2013; Gonzalez et al., 2019), while the cerussite crystals precipitated in solution would contain pseudohexagonal needle and rod crystal shapes in both natural and artificial environments (Mellor, 1923; Sánchez-Navas et al., 2013; Gonzalez, 2016). Thus, the cerussite crystal in this study should be formed through precipitation in solution.
The carbon sources of lead carbonate
The δ13C values can serve as an evaluation of carbon sources in lead carbonate crystal formation. A previous study showed that cerussite, which has light δ13C values (e.g., −13.5‰ to −19.1‰), was consistent with crystal formation from a carbon source with a significant organic carbon contribution (e.g., C3 plants) (Melchiorre et al., 2001; Gilg et al., 2008). Differences in the δ13C values of organic-derived carbonates and atmosphere-derived carbonate were also observed in other minerals (Oskierski et al., 2013). The δ13C values in the Liangdaicun cosmetic lead white residues were light (−15.6‰ and −18.8‰, Table S2, Supplementary Material), which indicates its origin with a noteworthy organic carbon contribution, especially C3 plants, rather than only the in-take of atmospheric CO2 in crystal formation (Gilg et al., 2008).
Radioactive 14C dating serves as another proxy to determine the age of carbon sources and can also distinguish synthetic lead carbonate from natural cerussite. By principle, natural cerussite that carried 14C signals during their mineral formation would be significantly earlier (e.g., ~5000 years, as reported in Beck et al., 2018) than the tomb age. In lead carbonate synthesis, atmospheric carbon dioxide through different routes was incorporated at the time of synthesis. Accordingly, the radioactive isotope 14C was fixed in the crystal in the form of carbonate and decayed over time after its incorporation, thus making it possible for radiocarbon dating. The method was applied on the precondition that the 14C date of synthetic PbCO3 carried the 14C signal of the coeval atmosphere and would theoretically match the burial date of the corresponding tomb. This method was tested in the analysis of ancient Greece cerussite (e.g., sample AGER-CA508 in Fig. 6e), which was proven to be a synthetic product using atmospheric CO2 in the corrosion process (Beck et al., 2018).
a and b are the calibrated dates for the lead white sample LW-1a and sample LW-2a from tomb M26 in the Liangdaicun site, respectively; c and d are the calibrated dates for tomb M26 and M27, respectively (Chen et al., 2009); e the calibrated dates for the lead white sample AGER-CA508 in ancient Greece (Beck et al., 2018). The bars underneath the probability distributions are the 95.4% confidence intervals. The red column represents the supposed burial dates of tombs M26 and M27 according to the archeological context. The blue column represents the burial date of sample AGER-CA508 (Beck et al., 2018).
Samples LW-1a (cerussite & phosgenite) and LW-2a (cerussite) from tomb M26 have been submitted to AMS radiocarbon dating analysis because both samples are abundant in quantity. The 14C analysis results are presented in Fig. 6. The measured radiocarbon concentrations for both samples are 69.78 ± 0.26 pMC and 70.22 ± 0.26 pMC (Table S2, Supplementary Material). Their calibrated calendar dates present a short offset that was earlier than the burial date of tomb M26 dated by 14C measurement of the lacquer on the coffin (Fig. 6c) (Chen et al., 2009). Given the depleted 13C isotope values, the organic plant carbon involved in lead carbonate crystal formation may account for the short offset in 14C dating.
Discussion
The synthetic nature of lead carbonate
The FTIR and XRD analysis showed that the cosmetic residues were pure cerussite or a mixture with phosgenite in two samples. The morphological features indicate that the cerussite crystals were precipitated in a solution. The carbon δ13C isotope analysis demonstrates that the carbon source was mainly from C3 plants. Although the age of the lead carbonates does not fall exactly within the burial date of the tomb (Fig. 6), it still reveals the synthetic origin of ancient samples because natural cerussite was observed to have a significantly larger offset (more than 5000 years) (Beck et al., 2018). In fact, different carbon sources have a great influence on the radiocarbon dating results of synthetic lead carbonate (Hendriks et al., 2019; Hendriks et al., 2020b). For instance, when CO2 released from coal burning (fossil carbon source, much depleted in 14C concentration) was involved in the synthesis reaction, it produced an offset of more than 10,000 years (Hendriks et al., 2019); when wood-derived carbon (either CO2 released from burning or carbonate obtained from plant ash) was involved, it produced short offset ahead of the burial time (as the observation from Liangdaicun samples), while when CO2 in the atmosphere or released from dung fermentation was involved, it falls in the burial time range due to the short living period of herbs (as the fodder of the livestock) (Beck et al., 2018; Hendriks et al., 2019; Quarta et al., 2020; Hendriks et al., 2020b). Nevertheless, determining the precise source of carbon involved in the chemical reaction can be complex and is still an open question (Beck et al., 2018).
The carbon from the C3 plants involved in the lead white synthesis could account for the 14C dating offset observed in the Liangdaicun samples. The wood carbon sources would have slightly depleted values in 14C concentration compared to the 14C signal of the coeval atmosphere of the tomb burial date, thus resulting in a short offset ahead of the tomb burial time. Moreover, as an explanation by Lucile Beck et al., a mixture of a small portion of the old aged natural cerussite or synthesized cerussite of early date in the residue might also cause this offset (Beck et al., 2018).
Apart from the carbon sources, other evidence also indicates the synthetic nature of the cosmetic residues. The detection of phosgenite (Pb2Cl2CO3) in the cosmetic samples (LW-1a and LW-5) indicates their synthetic origin, given its rare natural occurrence (Walter et al., 1999; Beck et al., 2018). The large number of pure PbCO3 particles with acicular and prismatic shapes scattered in the soil off the bronze wall (showing no signs of corrosion) of M26:138 (Fig. 4d–f) indicated that lead white was purposefully placed inside. The inner surface where the lead white attaches to has no sign of corrosion after removing the white PbCO3 during the sampling process. These large aggregations of white residues are only found in the miniature bronze containers and have not been observed in other bronze wares of the same tomb. Additionally, bronze corrosion products of lead compounds are always mixed with different copper compounds, which are difficult to separate, while the white samples in this study mainly contain pure PbCO3, and they were only found inside the containers. Trace pyromorphite (Pb5(PO4)3Cl), usually as an impurity in natural cerussite in China (Wang et al., 2008), has not been observed in FTIR spectra, and phosphorus is not detected in EDS analysis of all samples (Fig. 5, Fig. S8, Supplementary Material). These facts further confirm the synthetic nature of the lead carbonates from miniature cosmetic containers (Table 1).
The synthetic route by the precipitation method
The ancient recipe of phosgenite synthesis shows a mixture of PbO and salt (Cl−) in water (Walter et al., 1999; Beck et al., 2018), so the presence of phosgenite in two Liangdaicun samples further indicates that the formation of lead carbonate crystals was in solution, whose synthesis should start with PbO to firstly generate soluble lead salts: one of the most important ingredients in the precipitation method. Two water-soluble lead salts (lead acetate and lead nitrate) are usually used to react with CO32− in the solution for precipitation (equations 5–7 in the Supplementary Material). Lead acetate can be easily produced through the reaction of PbO and acetic acid (vinegar), of which the ingredients are readily available in antiquity. Meanwhile, lead nitrate was rarely used in ancient times to precipitate lead carbonate until modern times, when it was industrially available (Pérez-Villares and Bailon-Moreno, 2017).
Thus, the synthetic route of the precipitation method can be divided into sequential steps. The acquisition of water-soluble lead acetate was the first step in the synthesis. This can be easily achieved by dissolving PbO in vinegar to produce lead acetate solution. The carbonation of lead acetate into lead carbonate is the second yet key step in lead carbonate formation. In this step, there are two general ways to provide a carbon source: one is to increase the dissolving of CO2 by slowly and constantly bubbling CO2 into the lead acetate solution to form CO32− or increasing the CO2 concentration above the solution, e.g., the smoke of burning plants. Putting the lead acetate solution in a room filled with an elevated concentration of CO2 released from wood burning could favor the process; nevertheless, it could take quite a long time for the carbonation process. It would be more efficient to bubble CO2 into lead acetate solution (Eckert and Meldrum, 1972), while there is currently no evidence that such a bubbling device has existed in the first millennium BCE in China.
The other way that can provide CO32− was using water-soluble carbonate in plant ash. In ancient times, potassium carbonate (K2CO3) was enriched in leaching solution from wood ash (Gunneweg et al., 2010). By soaking, filtering and concentrating, a solution containing potassium carbonate can be extracted from plant ash, and when it is added into the lead acetate solution, CO32− can react with Pb2+ to precipitate lead carbonate. Moreover, the plant ash leaching solution or vinegar may include other ions, such as Cl−, which would promote the formation of phosgenite. In the presence of an elevated concentration of Cl−, Pb2+ can react with Cl− to precipitate water-insoluble PbCl2. Then, the dechlorination of PbCl2 can occur by adding basic carbonate solutions and converting PbCl2 into phosgenite (pH 8–9) or pure cerussite (pH 10), decisively controlled by the solution pH, which is observed in the basic ammonium bicarbonate solution (Chen et al., 2017). Similarly, for samples LW-1a and LW-5, the presence of Pb2+ and Cl− would precipitate PbCl2, and then adding a weak alkaline solution of K2CO3 from wood ash could convert it into a phosgenite/cerussite mixture.
The debut of synthetic lead white in east Eurasia during the first millennium BCE
The precipitation synthetic approach, as revealed by Liangdaicun lead carbonate cosmetic residue, sheds light on the re-explanation of an ancient recipe, which is speculated to have appeared during the first millennium BCE: 黑铅之错化为黄丹,丹之再化之成水粉, literally, “transform black lead into yellow litharge (PbO, 黄丹), and litharge can transform into lead white powder”. However, the recipe is written quite briefly and does not describe some important factors, such as heat in the oxidation reaction of lead (Pb→PbO) and heat & vinegar & carbon source in releasing Pb2+ in the solution and successive carbonation processes (PbO→Pb(CH3COO)2 → PbCO3/(PbCO3)2·Pb(OH)2), so it is difficult to interpret this recipe (Schafer, 1956; Needham et al., 1976; Zhu, 1983; Wai and Liu, 1991). With acquired information about the synthetic lead carbonate in Liangdaicun cosmetics, this recipe could be explained as recording a precipitation method for lead white production and had been used in the first millennium BCE (Schafer, 1956; Zhu, 1983), much earlier than Galen’s recipe in the second century CE and reintroduced in France in 1803 (Homburg, De Vlieger, 1996). However, ancient people may not recognize that when Cl− is present in solution, phosgenite can be simultaneously produced with cerussite.
The production of vinegar should have a great influence on the debut of synthetic lead white in ancient China during the first millennium BCE. The Zhou Dynasty in China (1046–256 BCE) witnessed the large-scale production of vinegar, and the central government set up a specialized official Xiren (醯人) in charge of vinegar production in noble or royal households (Mazza and Murooka, 2009). The discovery of Liangdaicun precipitated lead white provided evidence for the recognition of the power of vinegar (acetic acid) in the reaction to dissolve and solubilize metal oxide for matter transformation in the first millennium BCE. As a cheap, simple and nearly ubiquitous condiment, vinegar was later widely used in pigment production and as a key reagent in aqueous reactions that the alchemists experimented extensively in their prescientific investigations (Ping-Yü and Needham, 1959; T’Ien-Ch’in, et al., 1959; Pregadio, 2002; Bourgeois and Barja, 2009; Mastrotheodoros et al., 2010; Ferrari et al., 2021). As this study demonstrates, the use of vinegar in wet chemistry is almost 1000 years earlier than the earliest literature recording wet chemistry practice in ancient China, in which vinegar was added as a reactive reagent with explicit directions (36 Shui Jing, Thirty-Six Methods for the Bringing of Solids into Aqueous Solutions, reference T’Ien-Ch’in et al., 1959). Although the evolutionary trajectory of vinegar production and usage through the eighth century BCE to the third century CE remain largely unknown, our discovery forecasts the use of vinegar as a key reagent in aqueous alchemy practice.
In addition, exquisite bronze extraordinarily arose in large quantities during the late Shang and Zhou Dynasties (thirteenth–third century BCE), and ancient craftsmen would have experimented with metallic lead for chemical reactions, as it was a key material in bronze production or pure lead funeral objects (Chen et al., 2021). This should have contributed to the use of lead/litharge and vinegar to make lead white. Interestingly, residue analysis inside miniaturized bronze vessels also provides a new perspective on the diversified function of bronze vessels, such as cauldrons (miniaturized), which are widely distributed across Eurasia (Wang et al., 2019; Bârcă, 2020; Liu et al., 2022).
The favorite of white color as an ideal physical esthetic complexion has historically embedded in traditional culture in China and has been associated with high social and economic status. For instance, in the Classic of Poetry (the oldest existing collection of Chinese poetry in the eleventh to seventh century BCE), attractive figures were described by likening the complexion to “jade” (有女如玉), “creamy” (肤如凝脂), and “white moonlight” (月出皓兮), and the analogies directly showed the white color with the connotation of pulchritude, pureness and nobleness. The eagerness to white created a huge demand for white makeup, especially among the noble class possessing a large number of social resources. This cultural and social background urged the debut of synthetic lead white in China during the first millennium BCE.
The debut of synthetic lead white in east Eurasia during the first millennium BCE marked renewed interest in the development of chemical practices in China. The precipitation method of the Liangdaicun lead white was distinct from the lead corrosion synthetic approach that was predominantly used in ancient Europe. Since east and west Eurasia have different synthetic approaches, the synthesis of lead white should be invented and practiced independently in east and west Eurasia during the first millennium BCE. This can also be witnessed by scientific analysis of archeological lead white in both regions. The early production of lead white in ancient Greek (psimythion) and Roman (cerussa) used the corrosion synthetic approach mostly using fermentable materials as heat and carbon sources (Pulsifer, 1888; Beck et al., 2018; Gonzalez et al., 2019), and the products were mainly a mixture of cerussite and hydrocerussite. However, at the Liangdaicun site in China, lead white production used litharge and vinegar to produce lead acetate, which was then added carbonate salt solution or CO2 for the carbonation of Pb2+ ion. The main products include pure cerussite or a mixture with phosgenite, distinct from European practice.
As this study demonstrates, the earliest wet chemical practice in ancient China was observed for making lead white during the eighth century BCE, which shed light on the earliest beginning of inorganic reactions in aqueous medium insofar, much earlier than the previous knowledge that wet chemistry in China commenced at least with alchemist Ge Hong (葛洪, 283–363 CE) (T’Ien-Ch’in et al., 1959). Notably, lead white and lead acetate later became important elements explored by alchemists in ancient Chinese proto-chemistry activities (Schafer, 1956). Nevertheless, the evolutionary trajectory of synthetic lead white by the precipitation method after the first millennium BCE remains largely unknown. Knowledge of matter transformation in ancient times was an empirically based practice, and the role of science has been used to explain and provide better control rather than to open up new areas and disciplines (Smith, 1965). Moreover, the illiteracy of ancient empiricists/technicians/alchemists, or more likely his/her desire to maintain secrecy, makes it difficult to conduct a full comparative study on the development of ancient chemistry (Levey, 1956; Leicester, 1961). Nevertheless, chemical knowledge was transmitted and carried forward continuously by the oral traditions of father to son through the organization of craftsmen, as well as commercial intercourse and industrial trades (Levey, 1960). For instance, the appearance of the precipitation recipe for lead white in the west and the corrosion recipe for lead white in the east (Table S1) implies cultural and technical exchanges across the Eurasian continent.
Worldwide cosmetic revolution triggered by synthetic lead white
Technical innovations are topics of enduring interest among broader social sciences (Erb-Satullo Nathaniel, 2020), among which archaeomaterial studies have shown enormous potential in outlining cosmetic development in ancient China (Mai et al., 2016; Yu et al., 2017; Han et al., 2021). Previously, when discussing the worldwide scenario of lead white use before the Common Era, the Asian region was usually ignored. As shown in Fig. 1, although archeological evidence of cerussite use was reported in southern Europe (Kramberger et al., 2021), Egypt (Beck et al., 2018), Mesopotamia (Hauptmann et al., 2016), Iran (Vidale et al., 2012; Holakooei et al., 2022) and Indus Valley (Vidale et al., 2016), to date, no discovery has been reported in the corresponding period (5th–2nd millennium BCE) in the East Asian region. Clear historical descriptions also helped recover the lead white synthesis in west Eurasia that the corrosion synthetic approach first appeared in the fourth century BCE, which was predominantly used until modern times (Pulsifer, 1888). In east Eurasia, however, there were no clear descriptions before the Common Era.
The discovery of synthetic lead white at the Liangdaicun site highlights its earliest cosmetic usage in China, which further validates the rise of the cosmetic industry in the early phase of the Spring and Autumn period witnessed by the emergence of miniaturized cosmetic containers (Fig. S10, Li, 2009; Han et al., 2021). The innovative approach of lead white making constitutes a highly influential contribution to the cosmetic industry development during the first millennium BCE, which can be witnessed both literarily and archeologically. Lead white was used not only by the king/queen but also by the aristocratic woman (e.g., M300), indicating its wide social use. In addition to women, noble men of Rui state, the king from tomb M27 and the noble man from tomb M49 at the Liujiawa site (Han et al., 2021), also used white cosmetics. Male use of white make-up exemplified the earliest men’s fashion of facial luminance in Chinese history. Cosmetics then became more popular in the subsequent Warring States Period (475–221 BCE) as an emerging era for white makeup advocating facial attractiveness with white luminance, which is reflected in contemporary historical records such as Han Feizi, Deng Tuzi Fond of Beauty and Chu Ci, e.g., white face and black eyebrow (粉白黛黑) is inferred as the use of lead white (Zhu, 1983). Apart from cosmetic use, lead white was later continuously used in wall paintings, murals or whitewash on the walls of buildings since the Han Dynasty (second century BCE) (Schafer, 1956). Lead white also finds its way into traditional Chinese medicine and alchemy elixir.
In western Eurasia, lead white has been made in notable quantities since the fifth century BCE by Grecian and Roman craftsmen. Its wide cosmetic use by females was archeologically witnessed in the Greco-Roman world, while males were also found to have started to use lead white cosmetic powders since the Classical/Hellenistic period of Greece (Welcomme et al., 2006; Katsaros et al., 2010; Beck et al., 2018; Photos-Jones et al., 2020).
Lead white has been widely used since the invention of its synthesis approaches because its production is much simpler with lower cost in comparison to phosgenite synthesis, which involves repetitive operations (Walter et al., 1999; Beck et al., 2018) and is suitable for large-scale production, as witnessed from the late successful mass production of lead white pigment via the Stack/Dutch process (D’Imporzano et al., 2021). The invention of synthetic lead white triggered a cosmetic revolution in human history since the mid-first millennium BCE, promoting its prominent and widespread use as a readily available pigment in cosmetics and painting in antiquity.
Inorganic pigments are usually mixed with lipid substances in cosmetic applications with nuanced differences in different backgrounds (Evershed et al., 2004; Pérez-Arantegui et al., 2009; Giachi et al., 2013; Mariotti and Giachi, 2021; Pérez-Arantegui, 2021). Lead white is also mixed with oil in painting and cosmetic use (Walter et al., 1999; Polkownik, 2018; Polkownik and Emmett, 2020). In ancient China, an inorganic–organic mixture cosmetic recipe was also found (monohydrocalcite + ruminant adipose fat) (Han et al., 2021), while lipid substances were not identified in the Liangdaicun residues of this study. As a plausible explanation, the six miniature vessels unearthed as a set in the queen’s tomb may indicate their function as storage or making utensils, while the lead white will be processed and mixed with oil upon application to the face or body.
Conclusion
This study reports the earliest known case of synthetic lead white produced by the precipitation method in the eighth century BCE. This chemical technique was practiced ~1000 years earlier than the recorded inorganic reactions in aqueous medium in the third–fourth century CE in China, which promoted the study of the history of chemistry practice in the Asian region. The origin of lead white synthesis is of great interest for research on science and technology development in ancient China, and knowledge of lead white use in Asia allows us to compare its worldwide use across the world. The synthetic lead white found at the Liangdaicun site was several hundred years earlier than that of ancient Greece, and both regions have different synthetic approaches, indicating the independent origins and development of lead white synthesis between east and west Eurasia during the first millennium BCE. The large-scale production of lead white triggered revolutionary worldwide use of white makeup and art pigment, helping establish beauty roles cohering to different populations with sociocultural identities and symbolisms. Lead white also finds its way into one of the most valued white pigments in western oil paintings and Chinese murals that profoundly influenced art development. Whether in east or west Eurasia, synthetic lead carbonates were always first used for beauty purposes, which highlights that beauty played a critical role in the development of chemistry practice.
As a further study of the topic, the review of the subsequent development of lead white production, as well as the exploration of the divergent patterns between the east and west Eurasia, is an interesting topic that would need comprehensive research from broader humanities and natural and social sciences. Further work as well as attention and perspective from a wider range of disciplines would elaborate more details of the topic.
Data availability
The data used in the study are available from the corresponding author upon reasonable request. Analysis information is also provided in the Supplementary Information.
References
Aubert M, Lebe R, Oktaviana A et al. (2019) Earliest hunting scene in prehistoric art. Nature 576(7787):442–445
Bârcă V (2020) The bronze cauldron of Piatra Șoimului (Calu)(Neamț County, Romania). Notes on the distribution, dating and use of such type cauldrons in the Sarmatian environment. J Anc Hist Archaeol 7(1):79–105
Becker H (2022) Pigment nomenclature in the ancient Near East, Greece, and Rome. Archaeol Anthropol Sci 14(1):1–25
Beck L, Caffy I, Delqué-Količ E et al. (2018) Absolute dating of lead carbonates in ancient cosmetics by radiocarbon. Comm Chem 1(1):1–7
Beck L, Messager C, Coelho S et al. (2019) Thermal decomposition of lead white for radiocarbon dating of paintings. Radiocarbon 61(5):1345–1356
Berke H (2007) The invention of blue and purple pigments in ancient times. Chem Soc Rev 36(1):15–30
Bourgeois J, Barja F (2009) The history of vinegar. Arch Sci 62:147–160
Cartechini L, Miliani C, Nodari L et al. (2021) The chemistry of making color in art. J Cult Herit 50:188–210
Chen D, Qiao B, Luo W (2021) Archaeometallurgical research on the manufacture of lead mingqi from Nanyang in Chu state during the Warring States period. Archaeometry 63(4):810–825
Catalli K, Santillan J, Williams Q (2005) A high pressure infrared spectroscopic study of PbCO 3-cerussite: constraints on the structure of the post-aragonite phase. Phys Chem Miner 32(5-6):412–417
Chen J, Yang J, Sun B et al. (2009) Manufacture technique of bronze-iron bimetallic objects found in M27 of Liangdaicun Site, Hancheng, Shaanxi. Sci China Ser E-Technol Sci 52(10):3038–3045
Chen Y, Ye L, Xue H et al. (2017) Conversion of lead chloride into lead carbonate in ammonium bicarbonate solution. Hydrometallurgy 173:43–49
D’Imporzano P, Keune K, Koornneef J, Hermens E et al. (2021) Time-dependent variation of lead isotopes of lead white in 17th century Dutch paintings. Sci Adv 7(49):eabi5905
De Meyer S, Vanmeert F, Vertongen R et al. (2019) Macroscopic X-ray powder diffraction imaging reveals Vermeer’s discriminating use of lead white pigments in Girl with a Pearl Earring. Sci Adv 5(8):eaax1975
Eckert F, Meldrum D (1972) Method for producing pearlescent basic lead carbonate, Google Patents
Erb-Satullo Nathaniel L (2020) Archaeomaterials, innovation, and technological change. Adv Archaeomater 1(1):36–50
Evershed R, Berstan R, Grew F et al. (2004) Formulation of a Roman cosmetic. Nature 432(7013):35–36
Ferrari E, Mercier F, Foy E, Téreygeol F (2021) New insights on the interpretation of alum-based fake silver recipes from 3rd century CE by an experimental archaeology approach in the laboratory. J Archaeol Sci Rep 36:102742
Giachi G, Pallecchi P, Romualdi A et al. (2013) Ingredients of a 2,000-y-old medicine revealed by chemical, mineralogical, and botanical investigations. Proc Natl Acad Sci USA 110(4):1193–1196
Gilg A, Boni M, Hochleitner R et al. (2008) Stable isotope geochemistry of carbonate minerals in supergene oxidation zones of Zn–Pb deposits. Ore Geol Rev 33(2):117–133
Gliozzo E, Ionescu C (2021) Pigments—Lead-based whites, reds, yellows and oranges and their alteration phases. Archaeol Anthropol Sci 14(1):17
Gonzalez V (2016) Caractérisation micro-structurale et luminescence des carbonates de plomb: apport à la discrimination des pigments blancs de plomb des œuvres peintes, UPMC-Université Paris 6 Pierre et Marie Curie
Gonzalez V, Wallez G, Calligaro T et al. (2017) Synchrotron-based high angle resolution and high lateral resolution X-ray diffraction: revealing lead white pigment qualities in old masters paintings. Anal Chem 89(24):13203–13211
Gonzalez V, Wallez G, Calligaro T et al. (2019) Synthesizing lead white pigments by lead corrosion: New insights into the ancient manufacturing processes. Corrosion Sci 146:10–17
Gunneweg J, Adriaens A, Dik J (2010) Holistic Qumran: trans-disciplinary research of Qumran and the Dead Sea Scrolls, Brill
Han B, Chong J, Sun Z et al. (2021) The rise of the cosmetic industry in ancient China: Insights from a 2700-year-old face cream. Archaeometry 63(5):1042–1058
Han B, Sun Z, Chong J et al. (2022) Lipid residue analysis of ceramic vessels from the Liujiawa site of the Rui State (early Iron Age, north China). J Quat Sci 37(1):114–122
Hauptmann A, Klein S, Zettler R et al. (2016) On the making and provenancing of pigments from the Early Dynastic Royal Tombs of Ur. Mesopotamia Metalla 22(1):41–74
Hendriks L, Caseri W, Ferreira S et al. (2020a) The ins and outs of 14C dating lead white paint for artworks application. Anal Chem 92(11):7674–7682
Hendriks L, Hajdas I, Ferreira S et al. (2019) Selective dating of paint components: radiocarbon dating of lead white pigment. Radiocarbon 61(2):473–493
Hendriks L, Kradolfer S, Lombardo T et al. (2020b) Dual isotope system analysis of lead white in artworks. Analyst 145(4):1310–1318
Holakooei P, Karimy A, Piovesan R, Hosseinzadeh J et al. (2022) Make up in the grave: scientific analysis of contents of the so-called kohl pots at the archaeological site of Estark–Joshaqan, central Iranian plateau. Archaeol Anthropol Sci 14(4):1–15
Homburg E, De Vlieger J (1996) A victory of practice over science: the unsuccessful modernisation of the Dutch white lead industry (1780–1865). Hist Technol 13(1):33–52
Huang H, Qin Y (2010) A new perspective on ancient technique communication of cosmetics between the East and the West, based on the analysis of Chinese cosmetics-“Hu” powder. Almagest 1(2):106–115
Jones G, Jackson B (2012) Infrared transmission spectra of carbonate minerals, Springer Science & Business Media
Katsaros T, Liritzis I, Laskaris N (2010) Identification of Theophrastus’ pigments egyptios yanos and psimythion from archaeological excavations. A case study. ArcheoSciences. Revue d’archéométrie 34:69–80
Kramberger B, Berthold C, Spiteri C (2021) Fifth millennium BC miniature ceramic bottles from the south-eastern Prealps and Central Balkans: a multidisciplinary approach to study their content and function. J Archaeol Sci Rep 38:102993
Leicester H (1961) Chemistry, chemical technology, and scientific progress. Technol Cult 2(4):352–356
Levey M (1956) Babylonian chemistry: a study of Arabic and second millenium BC perfumery. Osiris 12:376–389
Levey M (1960) Early Muslim chemistry: its debt to ancient Babylonia. Chymia 6:20–26
Li L, Zhuang G, Li M et al. (2022) Influence of indigo-hydroxyl interactions on the properties of sepiolite-based Maya blue pigment. Dyes Pigm 2022:200–110138
Li L (2009) A discussion of the Du: women’s items in early China-boxes for headgear,makeup and perfumes. Palace Museum J 03:69–86+160–161
Li Y (1983) Research on the painted pigments of the terracotta warriors and the history of the pigments in the Qin Dynasty. Cult Relics Archaeol 3:62–66
Liu C, Liu R, Zhu S, et al. (2022) New scientific analyses reveal mixing of copper sources in the early Iron Age metal production at Ili, western China. Archaeometry
Ma Q, Portmann A, Wild F et al. (2006) Raman and SEM studies of man-made barium copper silicate pigments in ancient Chinese artifacts. Stud Conserv 51(2):81–98
Mai H, Yang Y, Abuduresule I et al. (2016) Characterization of cosmetic sticks at Xiaohe Cemetery in early Bronze age Xinjiang, China. Sci Rep 6(1):1–9
Mariotti L, Giachi G (2021) Pollen content in a II century BC remedy from the Pozzino shipwreck (Tuscany, Italy): its sources and association with the ingredients. Palynology 45(1):87–93
Mastrotheodoros G, Beltsios K, Zacharias N (2010) Assessment of the production of antiquity pigments through experimental treatment of ochres and other iron based precursors. Mediterr Archaeol Archaeom 10(1):37–59
Mazza S, Murooka Y (2009) Vinegars through the ages. In: Solieri L, Giudici P (eds). Vinegars of the world, Springer. pp. 17–39
Melchiorre B, Williams A, Bevins E (2001) A low temperature oxygen isotope thermometer for cerussite, with applications at Broken Hill, New South Wales, Australia. Geochim Cosmochim Acta 65(15):2527–2533
Mellor W (1923) A comprehensive treatise on inorganic and theoretical chemistry. Longmans, Green and Company
Needham J, Bray F, Harbsmeier, C (1976) Science and civilisation in China: chemistry and chemical technology: pt. 3. Spagyrical discovery and invention: historical survey, from cinnabar elixirs to synthetic insulin. Cambridge University Press
Oskierski C, Dlugogorski Z, Jacobsen G (2013) Sequestration of atmospheric CO2 in chrysotile mine tailings of the Woodsreef Asbestos Mine, Australia: Quantitative mineralogy, isotopic fingerprinting and carbonation rates. Chem Geol 358:156–169
Pérez-Arantegui J (2021) Not only wall paintings—pigments for cosmetics. Archaeol Anthropol Sci 13(11):1–10
Pérez-Arantegui J, Cepriá G, Ribechini E et al. (2009) Colorants and oils in Roman make-ups–an eye witness account. Trac-Trends Anal Chem 28(8):1019–1028
Pérez-Villares N, Bailon-Moreno R (2017) New simple procedure to produce white lead for special use in the plastic arts and in restoration. J Cult Herit 27:164–169
Photos-Jones E, Bots P, Oikonomou E et al. (2020) On metal and ‘spoiled’wine: analysing psimythion (synthetic cerussite) pellets (5th–3rd centuries BCE) and hypothesising gas-metal reactions over a fermenting liquid within a Greek pot. Archaeol. Archaeol Anthropol Sci 12(10):1–14
Ping-Yü H, Needham J (1959) Theories of categories in early mediaeval Chinese alchemy. J Warbg Court Inst 22(3-4):173–210
Polkownik C, Emmett T (2020) A technical examination of the nature and significance of prismatic lead white. J Inst Conserv 43(2):174–186
Polkownik C (2018) Lead white: how variations in the crystalline ratios influence the optical and handling properties of oil paint. Hamilton Kerr Inst Bull 7:59–68
Pregadio F (2002) The early history of the Zhouyi cantong qi. J Chin Relig 30(1):149–176
Pulsifer WH (1888) Notes for a history of lead: and an inquiry into the development of the manufacture of white lead and lead oxides, D. Van Nostrand
Quarta G, D’Elia M, Paparella S et al. (2020) Characterisation of lead carbonate white pigments submitted to AMS radiocarbon dating. J Cult Herit 46:102–107
Ramsey C (2017) Methods for summarizing radiocarbon datasets. Radiocarbon 59(6):1809–1833
Reimer J, Baillie M, Bard E et al. (2009) IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon 55(4):1869–1887
Salvant J, Williams J, Ganio M et al. (2018) A Roman Egyptian painting workshop: Technical investigation of the portraits from Tebtunis, Egypt. Archaeometry 60(4):815–833
Sánchez-Navas A, López-Cruz O, Velilla N et al. (2013) Crystal growth of lead carbonates: Influence of the medium and relationship between structure and habit. J Cryst Growth 376:1–10
Schafer H (1956) The early history of lead pigments and cosmetics in China. T’oung Pao 44(Livr. 4/5):413–438
Scott A, Dennis M, Khandekar N et al. (2003) An Egyptian cartonnage of the Graeco-Roman period. Stud Conserv 48(1):41–56
Smith C (1965) Materials and the development of civilization and science: empiricism and esthetic selection led to discovery of many properties on which material science is based. Science 148(3672):908–917
Stanley C, Jones G, Hart A et al. (1991) Barstowite, 3PbCl2. PbCO3. H2O, a new mineral from Bounds Cliff, St Endellion, Cornwall. Mineral Mag 55(378):121–125
Stevenson L (1955) On the meaning of the words cerussa and psimithium (psimythion). J Hist Med Allied Sci 10(1):109
Stols-Witlox M (2014) Historical recipes for preparatory layers for oil paintings in manuals, manuscripts and handbooks in North West Europe, 1550–1900: analysis and reconstructions. Ph.D thesis, Faculty of Humanities (FGw), Amsterdam School of Historical Studies (ASH)
Sun B, Li G, Cheng R, et al. (2007) A brief excavation report on the Tomb M27 located in Liangdai Village, Hancheng City, Shaanxi. Archaeol Cult Relic 2007:3–22
Sun B, Cheng R, Zhang W, et al. (2008) A brief excavation report on the Tomb M26 located in Liangdai Village, Hancheng City, Shaanxi. Cult Relic 2008: 4–21
T'Ien-Ch'In A, Ping-Yü H, Needham J (1959) An early mediaeval Chinese alchemical text on aqueous solutions. Ambix 7(3):121–155
Vidale M, Craig O, Desset F et al. (2012) A chlorite container found on the surface of shahdad (Kerman, Iran) and its cosmetic content. Iran 50(1):27–44
Vidale M, Salviulo G, Zorzi F et al. (2016) Cosmetics and cosmetology at Shahr-i Sokhta. Iran 54(2):1–24
Wai C, Liu K (1991) The origin of white lead-from the east or the west. J. Chem Educ 68(1):25
Walter P, Martinetto P, Tsoucaris G et al. (1999) Making make-up in ancient Egypt. Nature 397(6719):483
Wang F, Yang S, Ge J et al. (2022) Innovative ochre processing and tool use in China 40,000 years ago. Nature 603(7900):284–289
Wang L, Chen F, Wang Y et al. (2019) Copper metallurgy in prehistoric upper Ili Valley, Xinjiang, China. Archaeol. Anthropol Sci 11(6):2407–2417
Wang Z, Mao Z, Zhong H, et al. (2008) Primary study on cosmetics excavated from Yuan Dynasty tomb in Huashizui. Rock Miner Anal 27: 255–258
Welcomme E, Walter P, Van Elslande E et al. (2006) Investigation of white pigments used as make-up during the Greco-Roman period. Appl Phys A 83(4):551–556
Yu Z, Wang X, Su B et al. (2017) First evidence of the use of freshwater pearls as a cosmetic in ancient China: analysis of white makeup powder from a Northern Song dynasty Lv tomb (Lantian, Shaanxi province, China). Archaeometry 59(4):762–774
Zhu C (1983) The chemical knowledge of lead in ancient China. Chemistry 4:52–55
Acknowledgements
This work was supported by the China Postdoctoral Science Foundation, the Fundamental Research Funds for the Central Universities, the National Young Top-Notch Talent Support Program, and the National Social Science Fund of China (20&ZD253). The authors would like to thank Mr. Huaiyu Liang, Ms. Yanli Xue, and Mr. Qi Fang of Ruiguo Site Museum, Mr. Sizhe Liu of Shaanxi Academy of Archeology for sampling and providing sample background information, and to thank Prof. Changsui Wang and Prof. Hongen Jiang of University of Chinese Academy of Sciences, Dr. Patrick Roberts of Max Planck Institute for the Science of Human History, for their constructive suggestions.
Author information
Authors and Affiliations
Contributions
Conceptualization: YY, SZ, CJ; methodology: YY, CJ, SZ, HB; investigation: HB, ZB, CJ, SZ, YY; funding acquisition: YY, CJ, HB; project administration: YY, CJ; supervision: YY; writing—original draft: BH, YY; writing—review & editing: HB, ZB, CJ, SZ, YY.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This research did not require any ethical approval.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, B., Zhang, B., Chong, J. et al. Beauty and chemistry: the independent origins of synthetic lead white in east and west Eurasia. Humanit Soc Sci Commun 9, 281 (2022). https://doi.org/10.1057/s41599-022-01290-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1057/s41599-022-01290-6
This article is cited by
-
A Bronze Age lip-paint from southeastern Iran
Scientific Reports (2024)
-
Multi-analysis technique researches the painting materials and technics of polychrome arhat statue in Lingyan Temple, Shandong Province, China
Archaeological and Anthropological Sciences (2024)
-
A study of diverse cosmetics from the Tang dynasty
Archaeological and Anthropological Sciences (2024)