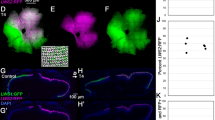
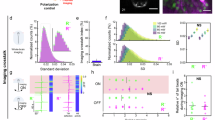
Abstract
Zebrafish are widely used to investigate visual function, owing to their well-defined retinal organization and optical transparency during early developmental stages. Here, we evaluate the effectiveness of a color vision optomotor response (CV-OMR) assay for assessing zebrafish with red cone ablation. Tg (thrb:gal4;UAS:epNTR-p2a-mCherry) transgenic zebrafish were used to ablate red cones via the addition of metronidazole (MTZ). Transgenic zebrafish larvae were treated with MTZ for 0, 12 and 24 h at 5 days post-fertilization, resulting in Tg(+)MTZ(−), Tg(+)MTZ(+)12 h and Tg(+)MTZ(+)24 h groups, respectively. The areas of mCherry-expressing cells, representing red cones in tissue sections, were compared. The mean mCherry expression area was smallest in the Tg(+)MTZ(+)24 h group (16.5 ± 7.6 μm2), followed by the Tg(+)MTZ(+)12 h (404.1 ± 200.9 μm2) and Tg(+)MTZ(−) groups (1,066.6 ± 252.2 μm2; P < 0.001). At 6 days post-fertilization, zebrafish larvae were evaluated using the CV-OMR assay comprising two colors. Results were reported as the area under the curve of the ratio of larvae at the starting point curve. The ratio of larvae at the starting point decreased most rapidly in the Tg(+)MTZ(−) group and most slowly in the Tg(+)MTZ(+)24 h group. There were significant differences in the area under the curve of the ratio of larvae at the starting point curve among the three groups. In conclusion, the CV-OMR assay demonstrated the ability to differentiate color vision impairment based on the extent of red cone ablation. The CV-OMR assay used in this study may prove valuable for evaluating color vision in zebrafish with various eye diseases.
This is a preview of subscription content, access via your institution
Access options






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Thoreson, W. B. & Dacey, D. M. Diverse cell types, circuits, and mechanisms for color vision in the vertebrate retina. Physiol. Rev. 99, 1527–1573 (2019).
Deveau, C. et al. Thyroid hormone receptor beta mutations alter photoreceptor development and function in Danio rerio (zebrafish). PLoS Genet. 16, e1008869 (2020).
Lucas, P. W. et al. Evolution and function of routine trichromatic vision in primates. Evolution 57, 2636–2643 (2003).
Surridge, A. K., Osorio, D. & Mundy, N. I. Evolution and selection of trichromatic vision in primates. Trends Ecol. Evol. 18, 198–205 (2003).
de Moraes, P. Z., Diniz, P., Spyrides, M. H. C. & Pessoa, D. M. A. The effect of pelage, background, and distance on predator detection and the evolution of primate color vision. Am. J. Primatol. 83, e23230 (2021).
Neitz, J. & Neitz, M. The genetics of normal and defective color vision. Vis. Res. 51, 633–651 (2011).
Semenov, E. P. et al. Reading performance in blue cone monochromacy: defining an outcome measure for a clinical trial. Transl. Vis. Sci. Technol. 9, 13 (2020).
Garcia-Martin, E. et al. Assessment of visual function and the neuroretina in subjects diagnosed with congenital anomaly of color vision. Opt. Express 31, 5625–5639 (2023).
Mancuso, K. et al. Gene therapy for red-green colour blindness in adult primates. Nature 461, 784–787 (2009).
Neitz, M. & Neitz, J. Curing color blindness—mice and nonhuman primates. Cold Spring Harb. Perspect. Med. 4, a017418 (2014).
Shoji, T., Sato, H., Chihara, E. & Sakurai, Y. Are middle-age blood pressure levels related to color vision impairment? The Okubo Color Study. Am. J. Hypertens. 28, 98–105 (2015).
Dall’Antonia, I., Šonka, K. & Dušek, P. Olfaction and colour vision: what can they tell us about Parkinson’s disease? Prague Med. Rep. 119, 85–96 (2018).
Garcia-Martin, E. et al. Visual function and retinal changes in patients with bipolar disorder. Retina 39, 2012–2021 (2019).
Piro, A. et al. Impairment of acquired color vision in multiple sclerosis: an early diagnostic sign linked to the greatness of disease. Int. Ophthalmol. 39, 671–676 (2019).
Chen, H. et al. New evidence of central nervous system damage in diabetes: impairment of fine visual discrimination. Diabetes 71, 1772–1784 (2022).
Vit, J. P. et al. Color and contrast vision in mouse models of aging and Alzheimer’s disease using a novel visual-stimuli four-arm maze. Sci. Rep. 11, 1255 (2021).
Koida, K. et al. Color vision test for dichromatic and trichromatic macaque monkeys. J. Vis. 13, 1 (2013).
Kelber, A., Vorobyev, M. & Osorio, D. Animal colour vision—behavioural tests and physiological concepts. Biol. Rev. Camb. Philos. Soc. 78, 81–118 (2003).
Escobar-Camacho, D., Marshall, J. & Carleton, K. L. Behavioral color vision in a cichlid fish: Metriaclima benetos. J. Exp. Biol. 220, 2887–2899 (2017).
Howe, K. et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503 (2013).
Chhetri, J., Jacobson, G. & Gueven, N. Zebrafish—on the move towards ophthalmological research. Eye 28, 367–380 (2014).
Bilotta, J. & Saszik, S. The zebrafish as a model visual system. Int. J. Dev. Neurosci. 19, 621–629 (2001).
Meier, A., Nelson, R. & Connaughton, V. P. Color processing in zebrafish retina. Front. Cell Neurosci. 12, 327 (2018).
Mackin, R. D. et al. Endocrine regulation of multichromatic color vision. Proc. Natl Acad. Sci. USA 116, 16882–16891 (2019).
Fornetto, C., Tiso, N., Pavone, F. S. & Vanzi, F. Colored visual stimuli evoke spectrally tuned neuronal responses across the central nervous system of zebrafish larvae. BMC Biol. 18, 172 (2020).
Robinson, J., Schmitt, E. A., Hárosi, F. I., Reece, R. J. & Dowling, J. E. Zebrafish ultraviolet visual pigment: absorption spectrum, sequence, and localization. Proc. Natl Acad. Sci. USA 90, 6009–6012 (1993).
Maaswinkel, H. & Li, L. Spatio-temporal frequency characteristics of the optomotor response in zebrafish. Vision Res. 43, 21–30 (2003).
Koun, S. et al. Development of an experimental model for ocular toxicity screening in zebrafish. Biochem. Biophys. Res. Commun. 559, 155–160 (2021).
Hagerman, G. F. et al. Rapid recovery of visual function associated with blue cone ablation in zebrafish. PLoS ONE 11, e0166932 (2016).
Kaiser, W. The spectral sensitivity of the honeybee’s optomotor walking response. J. Comp. Physiol. 90, 405–408 (1974).
Schaerer, S. & Neumeyer, C. Motion detection in goldfish investigated with the optomotor response is ‘color blind’. Vis. Res. 36, 4025–4034 (1996).
Orger, M. B. & Baier, H. Channeling of red and green cone inputs to the zebrafish optomotor response. Vis. Neurosci. 22, 275–281 (2005).
Nelson, R. F., Balraj, A., Suresh, T., Torvund, M. & Patterson, S. S. Strain variations in cone wavelength peaks in situ during zebrafish development. Vis. Neurosci. 36, E010 (2019).
Dingsdag, S. A. & Hunter, N. Metronidazole: an update on metabolism, structure-cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother. 73, 265–279 (2018).
Curado, S., Stainier, D. Y. & Anderson, R. M. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat. Protoc. 3, 948–954 (2008).
Pisharath, H., Rhee, J. M., Swanson, M. A., Leach, S. D. & Parsons, M. J. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech. Dev. 124, 218–229 (2007).
Jägle, H., de Luca, E., Serey, L., Bach, M. & Sharpe, L. T. Visual acuity and X-linked color blindness. Graefes Arch. Clin. Exp. Ophthalmol. 244, 447–453 (2006).
Thibos, L. N., Ye, M., Zhang, X. & Bradley, A. The chromatic eye: a new reduced-eye model of ocular chromatic aberration in humans. Appl. Opt. 31, 3594–3600 (1992).
Fraser, B., DuVal, M. G., Wang, H. & Allison, W. T. Regeneration of cone photoreceptors when cell ablation is primarily restricted to a particular cone subtype. PLoS ONE 8, e55410 (2013).
Vemala, R., Sivaprasad, S. & Barbur, J. L. Detection of early loss of color vision in age-related macular degeneration—with emphasis on drusen and reticular pseudodrusen. Invest. Ophthalmol. Vis. Sci. 58, Bio247–bio254 (2017).
Kuehlewein, L. et al. Central visual function and genotype-phenotype correlations in PDE6A-associated retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 63, 9 (2022).
Li, M. et al. Effects of acute high intraocular pressure on red-green and blue-yellow cortical color responses in non-human primates. NeuroImage Clin. 35, 103092 (2022).
Bayer, L., Funk, J. & Töteberg-Harms, M. Incidence of dyschromatopsy in glaucoma. Int. Ophthalmol. 40, 597–605 (2020).
Kassen, S. C. et al. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev. Neurobiol. 67, 1009–1031 (2007).
ARVO statement for the use of animals in ophthalmic and vision research. ARVO https://www.arvo.org/About/policies/statement-for-the-use-of-animals-in-ophthalmic-and-vision-research (2023).
The ARRIVE guidelines (animal research: reporting of in vivo experiments). ARRIVE https://arriveguidelines.org (2023).
Davison, J. M. et al. Transactivation from Gal4-VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Dev. Biol. 304, 811–824 (2007).
Yoshimatsu, T. et al. Presynaptic partner selection during retinal circuit reassembly varies with timing of neuronal regeneration in vivo. Nat. Commun. 7, 10590 (2016).
D’Orazi, F. D., Suzuki, S. C., Darling, N., Wong, R. O. & Yoshimatsu, T. Conditional and biased regeneration of cone photoreceptor types in the zebrafish retina. J. Comp. Neurol. 528, 2816–2830 (2020).
Chung, A. Y. et al. Generation of demyelination models by targeted ablation of oligodendrocytes in the zebrafish CNS. Mol. Cells 36, 82–87 (2013).
Tabor, K. M. et al. Direct activation of the Mauthner cell by electric field pulses drives ultrarapid escape responses. J. Neurophysiol. 112, 834–844 (2014).
Halpern, M. E. et al. Gal4/UAS transgenic tools and their application to zebrafish. Zebrafish 5, 97–110 (2008).
Choi, E. K., Choi, B. M., Cho, Y. & Kim, S. Myelin toxicity of chlorhexidine in zebrafish larvae. Pediatr. Res. https://doi.org/10.1038/s41390-022-02186-6 (2022).
Eom, Y. et al. Effect of titanium dioxide nanoparticle exposure on the ocular surface: an animal study. Ocul. Surf. 14, 224–232 (2016).
Fliotsos, M. J., Zhao, J., Pradeep, T., Ighani, M. & Eghrari, A. O. Testing a popular smartphone application for colour vision assessment in healthy volunteer subjects. Neuroophthalmology 45, 99–104 (2021).
Zhao, J., Fliotsos, M. J., Ighani, M. & Eghrari, A. O. Comparison of a smartphone application with Ishihara pseudoisochromatic plate for testing colour vision. Neuroophthalmology 43, 235–239 (2019).
Coblis-Color Blindness Simulator. Colblindor https://www.color-blindness.com/coblis-color-blindness-simulator (2023).
Acknowledgements
We express our gratitude to R. O. Wong (University of Washington) and T. Yoshimatsu for providing the pCG2thrb:gal4 DNA plasmid, as well as I. Jeong for the UAS:epNTR-p2a-mCherry plasmid used in this study. This work was supported by the SNUBH Research Fund (grant number 13-2020-007), by a TRC Research Grant of the Korea University Medicine and Korea Institute of Science and Technology (K2107901), by Korea University Ansan Hospital grant, by Korea University grants (K1625491, K1722121, K1811051, K1913161 and K2010921), by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (project number 1711174253, RS-2020-KD000296), by the Korea Environment Industry & Technology Institute (KEITI) through the Technology Development Project for Safety Management of Household Chemical Products, funded by the Korea Ministry of Environment (MOE) (2020002960007, NTIS-1485017544), by the Technology Development Program (S3127902) funded by the Ministry of SMEs and Startups (MSS, Korea), by the Technology Development Program (S3305836) funded by the Ministry of SMEs and Startups (MSS, Korea), by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (NRF-2021R1F1A1062017), and by ‘Technical start-up corporation fostering project’ through the Commercialization Promotion Agency for R&D Outcomes (COMPA) grant funded by the Korea Government (MSIT) (no. RS-2023-00259877). This study was funded in part by a Research to Prevent Blindness Challenge Grant to the Ophthalmology Department at Emory University; National Institutes of Health grants R01EY028450, R01EY021592, R01EY028859 and P30EY006360; The Abraham J. and Phyllis Katz Foundation; and VA RR&D grants I01RX002806, I21RX001924 and VA RR&D C9246C (Atlanta Veterans Administration Center for Excellence in Vision and Neurocognitive Rehabilitation).
Author information
Authors and Affiliations
Contributions
Y.E. conceived and designed the study, acquired data, analyzed and interpreted the data, drafted the manuscript and reviewed the final version. H.-S.P. and S.K. contributed to data acquisition, data analysis and interpretation, and reviewed the final manuscript. E.K. contributed to data interpretation and reviewed the final manuscript. H.-C.P. provided experimental equipment and reviewed the final manuscript. J.S.S., J.H.B. and J.M.N. supervised the study, contributed to data interpretation and reviewed the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Lab Animal thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eom, Y., Koh, E., Park, HS. et al. Assessment of a novel color vision optomotor response assay in zebrafish larvae with red cone ablation. Lab Anim 54, 200–206 (2025). https://doi.org/10.1038/s41684-025-01586-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41684-025-01586-5