Abstract
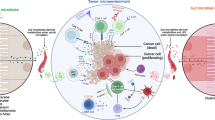
In many solid tumours, including gastric cancer (GC), the beginning and progression of the tumour are closely correlated with the tumour microbiome. Here, we show the changes in the gastric microbiota and their influence on immune regulation and the promotion of GC progression through 16s rRNA sequencing and single cell RNA sequencing. Streptococcus lutetiensis (S. lutetiensis) was found to be enriched in the tumour tissues of GC patients. Further analysis using single-cell sequencing and flow cytometry showed that S. lutetiensis notably affects the antitumour immunity by suppressing IL17 signalling and reducing the population of CD8+IL17A+ tissue-resident memory T (TRM) cells by activating Nrf2-mediated oxidative stress response. Mouse models confirm S. lutetiensis promotes GC progression by impairing immune responses in CD8+IL17A+TRM cells, suggesting it as a potential GC prognosis indicator and immunotherapy target, highlighting the microbiome's role in cancer progression.
Similar content being viewed by others
Background
Globally, gastric cancer (GC) is the third most common cause of cancer-related mortality and the fifth most common type of cancer1,2. Categorized as a Class I carcinogen, Helicobacter pylori (H. pylori) represent a major risk factor for GC. Intestinal metaplasia (IM), atrophic alterations, and gastritis are the results of H. pylori infection3. However, only 1% - 3% of people infected H. pylori will go on to develop GC, suggesting that other aetiologic factors such as environmental or host genetic factors, seem to play roles in gastric carcinogenesis4,5. host genetic (e.g. IL-1β, IL-10, TNF-α), and environmental factors.
Emerging evidence suggests the presence of a non-H. pylori microbiome within the gastric mucosa in a significant proportion of cases. It is a reasonable hypothesis that dysregulation of this microbiome may contribute to the progression of gastric cancer6,7. Nevertheless, whether alterations in the gastric microbiome affect the tumour immune microenvironment and promote GC progression remains unexplored.
Tumour-infiltrating lymphocytes (TILs) contain a distinctive population of memory T cells, designated as tissue-resident memory (TRM) cells, which are essential for tumour immune surveillance8,9. These cells are situated in peripheral tissues and do not circulate in the bloodstream, serving a crucial function in safeguarding human epithelial tissues against tumours and infectious diseases9,10,11,12,13. The majority of human tumour-infiltrating T cells display a TRM phenotype, characterised by the expression of CD69 and CD103. The cytotoxic factors granzyme B and perforin are released by T cells in response to CD103 activation, which strengthens the T cells' antitumour immunological response13. Recent research has suggested that CD8+TRM cells in human lung tumours secrete IFN-γ and IL17, which target specific Th17/Tc17 cells involved in the differentiation of Th17/Tc17 subsets14. However, studies have revealed the dual nature of TRM cells, as some express NK inhibitory receptors such as CD160, which paradoxically can promote tumour progression15.
To explore the gastric microbiome, we detected unique changes in S. lutetiensis concentrated in GC tissues and described the human GC microbiome make-up. S. lutetiensis accelerates gastric carcinogenesis. Mechanistically, we revealed that S. lutetiensis can downregulate the expression of IL17A and promote GC by inhibiting CD8+IL17A+ TRM cells. Our findings suggest that S. lutetiensis inhibits the expression of IL17A and indirectly downregulates the IL17 signalling pathway by reducing the number of CD8+IL17A+ TRM cells. This discovery may offer new insights into diagnostic and immunotherapy strategies for GC.
Methods
Cell
The MFC cells were cultured in RPMI 1640 media supplemented with 10% fetal bovine serum (FBS), 1% penicillin, and 1% streptomycin, and maintained at 37 °C with 5% CO2. HGC-27 and HEK293T cell were cultured in DMEM medium supplemented with 10% FBS, 1% penicillin, and 1% streptomycin, and maintained at 37 °C with 5% CO2. The culture medium was replaced every 24 h. Cells were passaged upon reaching 70%-80% confluency, and cells in the logarithmic growth phase were selected for further experimentation.
Mice
The 6–8 week 615 mice and NSG (NOD.Cg-PrkdcscidIL2rgtm1Wjl/SzJ) mice were purchased from Hangzhou Ziyuan Experimental Animal Technology Co., Ltd. Mice were housed in specific pathogen free (SPF) conditions, dark/light cycles: 12-h light/12-h dark (150–300 lux), ambient temperature 20–26°C and humidity 40%-70%, ventilated four times per hour. We did not use any wild animals. Sex and gender were not considered in our study design. Studies did not include samples collected from the field. All the animal studies were approved by the Animal Ethics Committee (Ethics approval No. DL2021033), Wuxi People's Hospital Affiliated to Nanjing Medical University, China. The maximal tumour weight was not exceeded 10% weight of the chosen animal as stipulated by the Ethics Committee of Wuxi People's Hospital Affiliated to Nanjing Medical University. Besides, euthanasia was implemented under some circumstances: such as the expectation of death, extremely weak physiology conditions, and tumour burden that leads to ulcer and abnormal behaviour. Tumours were finally harvested for flow cytometry analysis and further pathological analysis.
In vitro human CD8+ TRM cell culture conditions
Human peripheral blood mononuclear cells (PBMCs) from healthy donors were cultured in RMPI-1640 medium supplemented with 10% FBS, 1% penicillin– streptomycin, 1% non-essential amino acids, 1% GlutaMAX, 1mM sodium pyruvate, 0.1M HEPES and 50 μ M β -mercaptoethanol in the presence of human IL-2 (100U/ml). Anti-human CD3 (1μg/ml) and anti-human CD28 (1μ g/ml) monoclonal antibodies were used to activate PBMCs for 3 days. Purified human T cells were activated and expanded in the CD8+TRM cell culture conditions. Media was supplemented with IL-23 (50ng/mL), IL-1β (50ng/mL), TGFβ1 (5ng/mL) and IL-6 (40ng/mL). For antigen-specific CD8+TRM cell, PBMCs were stimulated by fragments of HGC-27 cells, which induced antigen-presenting cells and activated CD8+T cells. Antigen-specific CD8+T cells were subsequently enriched and cultured under conditions that promote their differentiation into CD8+ TRM cells.
Culture and counting of S. lutetiensis
S. lutetiensis was cultured at 37 °C in lactobacilli media under aerobic conditions. Plate counting was conducted to determine the CFU/mL under aerobic circumstances using a representative culture stock and a medium containing 1% agarose.
Human samples acquisition and processing
All patients participated with informed consent approved by the Institutional Ethics Committee of The Affiliated Cancer Hospital of Nanjing Medical University (2022-037) and the Affiliated Wuxi People's Hospital of Nanjing Medical University (KY21166). For 16s rRNA sequencing, tissue samples were collected from 33 patients with chronic gastritis and 100 patients with GC. These samples were then stored in liquid nitrogen. Furthermore, 35 tumour tissue samples from GC patients who had undergone gastrectomy were recently extracted and subjected to flow cytometry analysis.
Enzyme-Linked Immunosorbent Assay (ELISA)
The proteins of Human CD8+TRM cells were processed for human total superoxide dismutase (SOD), malondialdehyde (MDA) and glutathione (GSH) ELISA kit as per manufacturer’s instructions (Supplemental Table 2). The optical density (OD) was read at 450 nm and 570 nm with the spectrometer.
Dual-luciferase reporter assay
The promoter sequence of the human IL17A gene was cloned into the GV534-Luc plasmid to generate the chimeric GV534-Luc-IL17A reporter construct, which was verified by DNA sequencing. The IL17A promoter luciferase plasmid was co-transfected into HEK293T cells with the GV712-3HA-RORγt and CV702-3FLAG-Nrf2 plasmid. After transfection for 48 h, firefly luciferase activity was measured and normalized to Renilla luciferase activity in the same cell lysates using a double luciferase reporter gene assay kit.
Chromatin Immunopreci pitation (CHIP)-qPCR
CD8+ TRM cells were harvested and processed for CHIP with SimpleChIP® Enzymatic Chromatin IP Kit as per manufacturer’s instructions. Anti-Nrf2 antibody was used to pull down the Nrf2 binding DNA. Target DNA fragments were quantified by qPCR with ARE-R2 primers (Forward: 5'-GAGTGTCCCCATGCAAGACTG-3', Reverse: 5'-AGAGAGGGTGTGGCTTCATGC-3').
16s rRNA sequencing for tissues with GC and chronic gastritis
16S rRNA sequencing was performed on tissues from GC or chronic gastritis patients (Table 1 and Supplemental Table 1). In summary, 16S rRNA was amplified using two generic primers for the V4 hypervariable region: 515F (5'-GTGCCCAGCMGCCGGTAA-3') and 806R (5'GGACTACHVGGGTWTCTAAT-3'). As directed by the manufacturer, PCR products were combined at the same density ratio and purified using the GeneJETTM gel Extraction kit (Thermo Scientific, USA). As advised by the manufacturer, libraries were created using the Ion Plus fragment library kit 48 RXNS (Thermo Scientific, Waltham, MA, USA). 400 bp single-end reads of the library were sequenced using the Ion S5TM XL platform.
16S rRNA sequencing data analysis
The raw Fastq-format files were assigned to the samples based on their unique barcodes. Cutadapt (v1.12) was used to filter the reads with low sequence quality. The quality reads were aligned to the reference Silva database, and the chimeric sequence was detected using the UCHIME v4.2 algorithm and removed16. An operational taxon (OTU) is an artificially set uniform marker for a taxon for easy analysis in phylogenetic or population genetics studies. 97% of the comparable OTUs had representative sequences that could be classified and analysed using the RDP classifier Bayesian method17. The relative abundance of bacteria at the genus level was determined by computing the community species composition of each sample at the genus level. To estimate α diversity, the Sobs, Shannon, Chao, and Ace indices were employed. Principal coordinate analysis (PCoA) was used to illustrate the beta diversity, which was evaluated using the unweighted UniFrac distance matrix and the Bray-Curtis distance matrix. The impact size of linear discriminant analysis (LEfSe) was utilized to identify several taxa linked to patient groups. Taxa were deemed significant only if they had a P-value <0.05 and an LDA value greater than 3.5. RandomForest model was used to find bacteria enriched in tumour tissues and the AUC was calculated using a logistic regression model with 10-fold cross validations. Patients were divided into S. lutetiensishigh and S. lutetiensislow groups based on whether the relative abundance of S. lutetiensis in the tumour was above or below the median value.
Flow cytometry
For the analysis of freshly excised GC tissues and the corresponding mouse tumour model, tumour tissues were minced and suspended in 7ml of tumour digestion buffer. After 30 min of digestion at 37 °C with rotation (200 rpm), the resultant cell suspension was filtered through a 70μm filter to achieve a single-cell suspension. Immune cells were isolated using density gradient centrifugation with 40% and 80% Percoll solutions. For flow cytometry (FACS) analysis, surface proteins were stained with fluorescently labelled antibodies as listed in Supplemental Table 2. The cell suspensions were treated for 5 h at 37 °C in RPMI media supplemented with phorbol 12-myristate-13-acetate, ionomycin, and brefeldin A in preparation for intracellular staining.
Following surface labelling, cells were exposed to intracellular staining using the antibodies listed in Supplementary Table 2 after being fixed and permeabilized using a Cytofix/Cytoperm kit.
Single-cell RNA sequencing
CD3+ T lymphocytes from six GC patients were freshly separated by Percoll density centrifugation (Cytiva). The cells were maintained on ice and resuspended in Dulbecco's phosphate-buffered saline (PBS) containing 0.04% bovine serum albumin (BSA). A Countess II Automated Counter (Thermo Fisher Scientific, USA) was employed to ascertain the total number of cells, following the assessment of cell viability with Trypan blue. Nuclease-free water was used to dilute the living cell suspension in single cells. In nano upgrading droplets, RNA transcripts are reverse transcribed and bar coded individually. Then, the full-length cDNA of the barcodes was amplified by PCR. The amplified cDNA was then put through the procedures outlined in the User Guide for the BD RhapsodyTM Single-Cell Analysis System (BD Biosciences, USA) for end repair, A-tailing, adaptor ligation, and sample index PCR. Single-cell libraries were prepared using the BD Rhapsody Whole Transcriptome Analysis Amplification Kit (BD Biosciences) in accordance with the manufacturer’s instructions and sequenced on an Illumina NovaSeq 6000 platform.
Single-cell RNA sequencing data processing
The raw sequencing data was pre-processed using the cwl BD Rhapsody™ WTA Analysis Pipeline to obtain a unique molecular identifier (UMI) matrix for each sample. The raw UMI matrix adjusted by recursive substation error correction (RSEC) algorithms was utilized for clustering analysis. The single-cell expression profiles were read into R environment and clustered using the MetaCell R package18. For each cell, we used four quality control (QC) measures. Cells with the following criteria were excluded: <200 expressed genes or >25% UMIs of mitochondrial genes. Batch effects were removed using the KNN method. A total of 500 bootstrap iterations were employed. Metacell splitting was conducted by clustering the cells within each metacell and splitting it if distinct clusters were identified. Metacells expressing fewer than two transcription factors were considered poor metacells and were removed from the analysis. The two-dimensional projection is calculated by means of a force-directed layout algorithm on a balanced similarity graph. Then, each T-cell cluster was annotated manually with canonical T cell markers.
Multiplex immunofluorescence (mIF)
Human and mouse gastric cancer tissues embedded in paraffin were sectioned into 4-micrometre slices. After baking the tissue slices, xylene deparaffinization, gradient ethanol hydration, and antigen retrieval, the slides were treated with 3% hydrogen peroxide for 30 min. After the slices were washed with TBST, blocking buffer was added, and the samples were incubated for 30 min. Subsequently, primary monoclonal antibodies against human CD103, human CD8, human/mouse IL17A and mouse CD8 were applied, and the slices were incubated at room temperature for 1 h, followed by washing with TBST.
The secondary antibodies were added in the dark and incubated at room temperature for 30 min, followed by the addition of Opal Fluorophore Reagent (diluted at a 1:100 ratio) and incubation for another 10 min at room temperature. After the slices were washed with TBST, they were incubated with 4′,6-diamidino-2-phenylindole (DAPI) at room temperature for 5 min and washed again with TBST. Finally, the slices were covered with anti-fade mounting medium.
S. lutetiensis-specific fluorescence in situ hybridization (FISH)
Dewaxing was performed on sections of human or mouse GC tissue. These sections were treated with 0.2 N hydrochloric acid at room temperature for 15 min. Subsequently, slides were covered with a 50 μg/ml protease K solution and incubated at 37 °C for 30 min. Next, a FISH probe targeting S. lutetiensis (sequence: 5'-CTTCCTATCTCTAGGAATAGCATCGG-3', concentration: 400nM) was applied, and the slides were incubated overnight in a humidified environment at temperatures ranging from 37–45 °C. Subsequently, the samples were incubated in prewarmed washing buffer at 37 °C for 15 min. Following a 20-min air-drying period, the slides were mounted in a dark environment using a DAPI anti-fade solution, and then examined under an MVX10 fluorescence microscope equipped with a triple-pass filter (DAPI/Green/Orange).
Construction of an in situ gastric cancer mouse model
For MFC cells growing in the logarithmic growth phase, the cell concentration was adjusted to 1×107 cells/mL. We injected 100μL of the cell suspension subcutaneously into the left underarm of 6–8-week-old 615 mice. When the subcutaneous tumour reached ~1 cm in diameter, the mice were killed, the subcutaneous tumour was removed, the surrounding capsule and necrotic tissue around the tumour were removed, and the subcutaneous tumour was cut to ~1 mm in diameter and immersed in 1640 complete culture medium. We randomly divided 6–8-week-old male mice into two groups.
On the sterile operating table, an appropriate amount of 1% pentobarbital sodium (50 mg/kg) was injected intraperitoneally for anaesthesia. The skin and peritoneum were cut layer by layer ~0.5 cm below the right rib of the midline of the abdomen, and the stomach tissue was gently removed under the left lobe of the liver. The tumour tissue block orthotopic transplantation method was used to establish the subsequent mouse gastric cancer model. A tumour block with a diameter of ~1 mm was taken. Near the great curvature of the stomach near the gastric antrum, a small hole was cut in the serous membrane of the gastric tissue. The tumour was inserted into the serous membrane using a syringe needle, a drop of biological glue was added to block the tumour tissue, and attention was given to avoid adhesion to surrounding tissues, liver, and intestines. After the biological glue solidified, the stomach tissue was returned to its place, and the abdomen was closed layer by layer.
The mice were placed in a warm environment until they woke naturally. After waking, the plants were placed into the SPF level barrier for feeding. Mice were randomly divided into a S. lutetiensis group (1×109 CFU/once every 3 days/i.g.) and a Control group. On the 17th day, the mice were sacrificed after tumour implantation in situ, and the tumorigenesis of gastric cancer in situ was observed.
Survival analysis
The signature scores of TRM clusters were calculated based on single-sample Gene Set Enrichment Analysis (ssGSEA) algorithm. For the survival analysis, patients were grouped based on the optimal cutoff value determined using the surv cutpoint function from the survival package in R.
Statistical analysis
Statistical tests were performed by R v3.6.3 (The R Foundation Vienna, Austria) and GraphPad Prism software (v10). The data points represent biological replicates and are shown as the means ± SEMs. Comparisons of two groups were performed by using two-tailed unpaired Student’s t-tests or Mann‒Whitney tests. Survival data were analysed using the log-rank test.
Results
The gastric microbiota profiles are different between GC and gastritis
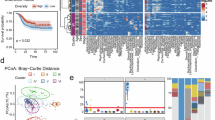
Accumulated evidence has revealed a relationship between changes in gastric mucosa and risk of GC which suggested a possible role for gastric microbiota in GC carcinogenesis. In this study we aimed to compare pattern of gastric microbiota in GC with control gastritis tissues. Through 16S rRNA sequencing, we profiled the gastric microbiota from 100 treatment-naïve GC tumours and 33 chronic gastritis specimens. The results showed that the alpha diversity was obviously greater in patients with chronic gastritis, measured by Sobs and Ace diversity (P < 0.001 and P < 0.001, respectively, Fig. 1a). Using both weighted and unweighted UniFrac phylogenetic distance matrices, beta diversity was evaluated. The findings are shown in PCoA graphs. Weighted UniFrac phylogenetic analysis revealed a significant difference in the microbiota composition between patients with GC and those with chronic gastritis (Fig. 1b, P = 0.047). Overall, the microbial diversity in GC is significantly reduced, indicating that changes in the gastric microbiome may have a significant influence on the progression of GC.
The abundance of specific microbial taxa may be related to GC
To elucidate the key taxa implicated in alterations in the gastric microbiota, differential microbial analysis was conducted. We firstly characterized the general taxonomic profiles of the microbiota in both GC patients and those with chronic gastritis. Firmicutes constituted the most prevalent phylum within the tissue specimens derived from both patient groups (Fig. 2a and Supplementary Fig. 1a–e). Combining the results of PCoA analysis and diversity analysis, it can be observed that specific bacterial communities are enriched in each group at the class, order, family, and genus levels (Figs. 1b, 2a). A linear discriminant analysis (LDA) of effect size (LEfSe) method was performed on the tissue microbiota composition in order to identify taxa with differential abundance associated with changes in the gastric microbiota. As shown in Fig. 2b, c, the results of this study demonstrated a significant divergence in the prevalence of bacterial communities between individuals diagnosed with GC and those with chronic gastritis.
a Bar plots showing the taxonomic levels of the phylum in the tissues of chronic gastritis and GC. b Cladogram representation of the differential gastric microbiota taxa between GC and chronic gastritis. For the cladogram, labels such as c1 and c2 are used to annotate specific nodes that represent branching points with a common ancestor. c The relationship between particular microbiota taxa and groups with chronic gastritis and GC as established by linear discriminant analysis (LDA) effect size (LEfSe). d The relative abundance of the top 15 genera that were found to be differentially enriched in the two clinical situations for the cohorts with chronic gastritis and GC. *Significance was determined by the Wilcoxon rank-sum test and LEfSe analysis at P < 0.05.
To further investigate these findings, we concentrated on variations in the relative abundance of the 15 species that showed substantial differences between the two groups at the species level. Significant enrichment of S. lutetiensis was observed in patients diagnosed with GC (Fig. 2d). These results suggest that S. lutetiensis may play a part in the development and spread of gastric cancer.
Intratumoural S. lutetiensis is associated with advanced TNM stage
To confirm the presence of S. lutetiensis in GC patients, we employed fluorescence in situ hybridization (FISH) and polymerase chain reaction (PCR) using specific probes and primers targeting S. lutetiensis. The findings of our study indicated that significant enrichment of S. lutetiensis was observed in patients diagnosed with GC compared to control gastritis tissues. (Fig. 3a, b). We next examined the microflora function of patients with chronic gastritis and GC to investigate the potential significance of bacterial species in predicting the clinical outcomes of GC patients. In addition, we detected a correlation between the relative abundance of S. lutetiensis and the clinicopathological characteristics of GC patients (Fig. 3c). We found that GC patients with advanced TNM stages (III-IV), which indicate poor prognosis, had more S. lutetiensis (P = 0.0142) (Fig. 3d and Fig. 2a). The 100 GC patients were divided into two groups based on the relative abundance of S. lutetiensis in the tumour. Those with a relative abundance above the median value were placed in the S. lutetiensishigh group, while those with a relative abundance below the median value were placed in the S. lutetiensislow group. The S. lutetiensislow group and the S. lutetiensishigh group did not significantly differ in H. pylori abundance, therefore potentially removing the impact of H. pylori (Supplementary Fig. 2a–c). Additionally, an area under the curve (AUC)-receiver operating characteristic (ROC) analysis of four GC-enriched species revealed an AUC of 0.89 for S. lutetiensis, along with unclassified_g Comamonas, unclassified_g Vibrio, and unclassified_g Methylobacterium (Fig. 3e and Supplementary Fig. 2d).
a Immunofluorescence with a specific FISH probe for S. lutetiensis in GC tissue. Sections were stained with a FISH probe (red) and DAPI (blue). b PCR detection of S. lutetiensis in GC tissues with high or low abundance of S. lutetiensis. c 16S functional prediction analysis of 100 GC and 33 chronic gastritis samples. *Significance was determined by the Wilcoxon rank-sum test and LEfSe analysis at P < 0.05. d Correlation analysis of S. lutetiensis abundance in operational taxonomic units (OTUs) and clinical characteristics (lymph node metastasis, vessel carcinoma embolus, Borrman type and 8th pTNM stage) of patients. *P < 0.05; **P < 0.01; ***P < 0.001. e ROC analysis of the relative abundance of taxa as a predictor of GC status. Analyses at the genus level of S. lutetiensis, unclassified_g Comamonas, unclassified_g Vibrio and unclassified_g Methylobacterium are shown. f Differences in relative abundances of KEGG pathways between high- and low-S. lutetiensis group.
KEGG enrichment analyses further indicated that S. lutetiensis downregulated the IL17 signalling pathway while upregulating the HIF-1, PI3K-Akt, and MAPK signalling pathways related to tumour metastasis (Fig. 3f). These findings suggest a positive association between S. lutetiensis and poor prognosis in GC patients, likely through the regulation of the IL17 signalling pathway.
CD8+IL17A+ TRM cells are reduced in the S. lutetiensis high GC tumours
Tumour-infiltrating T cells represent a crucial component of the antitumour immune response, with a pivotal role in the treatment of solid tumours. To understand the complex interplay between S. lutetiensis and T-cell infiltration in GC tumours, a thorough analysis of CD45+CD3+ T cells was conducted using single-cell RNA sequencing (scRNA-seq). Fourteen T-cell groups were identified within GC tumours from three patients infected with S. lutetiensis at high levels and three patients infected with S. lutetiensis at low levels (Fig. 4a). Then, we used 2D projections and heatmaps to visualize the expression of key marker genes, including CD8A, CD4, ITGAE (CD103), CXCL13, FOXP3, CD160, PDCD1, and GZMK (Fig. 4b and Supplemental Table 3). Through clustering of T-cell subsets, CD8+CD160+ TRM, CD8+IFNG+ TRM and CD8+IL17A+ TRM cells were grouped into one category. Moreover, the transcriptional characteristics of CD8+IFNG+ TRM cells and CD8+IL17A+ TRM cells were more similar (Fig. 4c). We also found that the percentage of CD8+IL17A+ TRM cells was significantly lower in the S. lutetiensishigh compared with S. lutetiensislow group (P < 0.0001) (Fig. 4d). We further analysed the expression of IL17A and found that in both CD8+IL17A+ T cells and CD4+IL17A+ T cells, the expression of IL17A was greater in the S. lutetiensislow group (Fig. 4e). These results suggest that a high abundance of S. lutetiensis is negatively associated with CD8+IL17A+TRM infiltration in GC, resulting in decreased secretion of IL17A.
a scRNA-seq data of T cells from human GC was analysed. A 2D projection plot of T-cell subpopulations is shown in (A). b Feature plots showing the differential expression of selected marker genes. c Clustering dendrogram of T cells subclusters. d Quantitative analysis of T cells fractions in the high and low groups of S. lutetiensis. e Quantitative analysis of IL17A secretion in the high and low groups of S. lutetiensis.
Antitumoral phenotypic characteristics of CD8+ TRM cells in human GC
Differential expression analysis between CD8+IL17A+ TRM cells and other subsets of CD8+ TRM cells revealed that CD8+IL17A+ TRM cells exhibit increased expression levels of genes involved in T-cell migration and activation, notably KLRB1 and CCR6 (Fig. 5a, b). This suggests a specialized role of CD8+IL17A+ TRM cells in facilitating immune cell trafficking and enhancing the activation status within the TME, likely contributing to their potent antitumour activity. Gene Ontology (GO) enrichment analysis further revealed that these DEGs are significantly associated with the regulation of cytokine‒cytokine receptor interactions and the chemokine signalling pathway, revealing the critical role of these cells in modulating immune responses within the TME.
a–c Volcano plot (a) and GO enrichment analyses (b) of up- or downregulated genes in CD8+ TRM-IL17A (right) and other CD8+ T cells (left) as determined by scRNA-seq data. Each red or blue square denotes an individual gene with a P-value < 0.05 and a fold change >1.5. Red indicates upregulation, and blue indicates downregulation. Survival analysis (c) of CD8+IL17A+ TRM cells in TCGA database. d–f Volcano plot (d) and GO enrichment analyses (e) of up- or downregulated genes in CD8+ CD160+TRM and other CD8+T cells as determined by scRNA-seq data. Each red or blue square denotes an individual gene with a P-value < 0.05 and a fold change >1.5. Red indicates upregulation, and blue indicates downregulation. Survival analysis (f) of CD8+CD160+ TRM cells in TCGA database.
Survival analysis utilizing The Cancer Genome Atlas (TCGA) data demonstrated that gastric cancer (GC) patients with a greater abundance of CD8+IL17A+ TRM cells had a better prognosis (Fig. 5c, P = 0.006, HR = 1.708). This finding underscores the prognostic value of CD8+IL17A+ TRM cells in GC, suggesting that their presence is beneficial for patient outcomes. Additionally, single-cell sequencing analysis highlighted a positive correlation between S. lutetiensis and CD8+CD160+ TRM cells in the TME, suggesting an interaction between microbial presence and specific immune cell populations. However, unlike CD8+IL17A+ TRM cells, no significant difference in prognosis was observed among GC patients based on the infiltration levels of CD8+CD160+ TRM cells (Fig. 5d–f, P = 0.34, HR = 1.197). This suggests that while CD8+CD160+ TRM cells may contribute to the immune response to GCs, their impact on patient prognosis is not as pronounced as that of CD8+IL17A+ TRM cells.
In summary, the analysis suggested that a tumour area enriched with S. lutetiensis is correlated with a decreased presence of antitumoural CD8+IL17A+ TRM cells, which are crucial for controlling GC progression. The distinct roles of CD8+IL17A+ and CD8+CD160+ TRM cells, as revealed by their transcriptomic profiles and associations with patient prognosis, highlight the complex interplay between the tumour microbiota, immune infiltration, and survival outcomes.
This information could be pivotal for developing targeted therapies that leverage the beneficial effects of specific T-cell populations within the TME.
S. lutetiensis reduced CD8+IL17A+ TRM cells via inducing oxidative stress in human GC
To confirm the observed phenotype in GC tissue, we performed mIF and flow cytometry analysis on GC samples. CD8+IL17A+ TRM cells were defined by three phenotypic characteristics: CD8 (red), CD103 (white) and IL17A (green) (Fig. 6a). We confirmed that a lower density of CD8+IL17A+ TRM cells was found in the S. lutetiensislow group than in the S. lutetiensishigh group (Fig. 6b). To further identify IL17A-producing cells, CD3+T cells from the GC were detected using flow cytometry. We observed that the overall percentage of CD8+IL17A+ TRM among all live cells (CD45+) was notably greater in the S. lutetiensislow group than in the S. lutetiensishigh group (Fig. 6c). Furthermore, in the S. lutetiensislow group, significantly more IFN-γ, Granzyme A and Granzyme B were produced in the CD8+IL17A+ TRM group than in the S. lutetiensishigh group (Fig. 6d).
a A representative example of a GC specimen stained with mIF, demonstrating the co-expression of IL17A, CD8, and CD103. b Quantification of CD8+ IL17A+TRM cells in the 22 independent patients analysed as described in (a). (c) Flow cytometry and quantification analysis of the association between S. lutetiensis and TRM cells in 35 independent cases. d Quantification of Granzyme A 、IFN-γ and Granzyme B expression on CD8+IL17A+TRM cells. (e) Flow cytometry of PI and Annexin V levels of CD8+ TRM cells after S. lutetiensis treatment. f Flow cytometry of Ki-67 levels of CD8+ TRM cells after S. lutetiensis treatment. g ELISA assay shows the levels of GSH, MDA and SOD in CD8+ TRM cells after S. lutetiensis infection. h Flow cytometry of ROS levels of CD8+ TRM cells after S. lutetiensis treatment. i, j Western blot analysis of protein levels of Nrf2, keap1, HO-1, and NQO-1 in CD8+ TRM cells at various intervals after infection with S. lutetiensis. k CD8+ TRM cells in the presence of S. lutetiensis, ML385 or vehicle control were examined for RORγt expression by flow cytometry. l CD8+ TRM cells were harvested and processed for CHIP with anti-Nrf2 antibody. The Nrf2-binding DNA was utilized for qPCR analysis to examine the interaction between the ARE-R2 motif of Rorc gene and Nrf2. m HEK293T cells were transiently transfected with IL-17A luciferase reporter vector and various concentrations of Nrf2 or RORc plasmid. After 48 h, the luciferase activity was quantified by Dual-Glo Luciferase Assay system. Data are presented as mean ± SEM. Significance was determined two-tailed Mann-Whitney U test.
To determine whether S. lutetiensis specifically affected the ability of the TRM cell populations, we performed cell proliferation and apoptosis assays in the CD8+IL17A+ TRM cells by flow cytometry and observed greatly reduced cell growth and viability when co-cultured with S. lutetiensis in vitro (Fig. 6e, f). Previous studies have shown that S. lutetiensis infection induces oxidative stress in bovine mammary epithelial cells by inhibiting the Nrf2/Keap1 signalling pathway19. Since oxidative stress results from an imbalance between the oxidative and antioxidant systems of cells and is the result of excessive production of reactive oxygen species (ROS), we measured the expression levels antioxidant and oxidative biomarkers as well as ROS in the CD8+ TRM population after S. lutetiensis treatment. The results revealed that the contents of GSH and SOD decreased, while MDA content level surged in cells treated with S. lutetiensis, leading to ROS production and eventually cell survival defect (Fig. 6g, h). Next, Nrf2, detoxifying enzymes heme oxygenase-1 (HO-1), NAD(P)H:quinone oxidoreductase-1 (NQO-1) and kelch-like ECH-associated protein 1 (Keap1) expression levels were compared between the two groups through western blot analyses to further validate changes in Nrf2 signalling pathway. As shown in Fig. 6i, j and Supplementary Fig. 3, compared with blank control group, the levels of Nrf2 protein expression in CD8+ TRM cells were increased significantly in the treatment group, whereas the Keap1 protein level was significantly below baseline. Besides, Nrf2-regulated phase-II detoxification enzymes, HO-1 and NQO-1, were both elevated over time in S. lutetiensis-infected cells. These findings suggest that S. lutetiensis can activate the Nrf2-HO-1/NQO-1 signalling pathway and increase the degree of oxidative stress. Moreover, Nrf2 acts through the regulation of antioxidant response element (ARE) binding motifs in target genes to induce or repress transcription.
Nrf2 activation play an important role in suppressing Th17 cell-mediated inflammatory IL-17A responses. Retinoic acid receptor–related orphan receptor γt (RORγt) is a transcription factor that regulates IL-17A expression. In Th17 cells, Nrf2 inhibits RORγt-dependent IL17A transactivation by binding to the ARE repressor (ARE-R2) of RORγt20. Therefore, we explored whether RORγt was involved in Nrf2-mediated regulation on IL17A transcription. Flow cytometry showed that S. lutetiensis could suppress RORγt expression in CD8+ TRM cells, which could be partially prevented by Nrf2 inhibitor ML385 (Fig. 6k). ChIP-qPCR experiments showed that Nrf2 directly binds to ARE repressor (ARE-R2) of RORγt, indicating that binding of Nrf2 to these promoters suppressed the expression levels of RORγt gene (Fig. 6l). To investigate if Nrf2 regulates IL17 transcription, we performed IL17-promoter-driven luciferase assay in HEK293T cells that were transiently transfected with IL17A luciferase reporter and Nrf2 or RORγt plasmid. Luciferase reporter analysis revealed that Nrf2 inhibited IL-17A promoter activity at the presence of ROR γ t, indicating that Nrf2 inhibited ROR γ t-induced IL-17A activation (Fig. 6m). Together, these data support a critical role of S. lutetiensis in the inhibition of IL17A expression in CD8+ TRM cells, potentially through Nrf2-mediated oxidative stress response.
S. lutetiensis promotes tumour growth and inhibits CD8+ IL17A+ TRM cell infiltration
To further explore the role of S. lutetiensis in the TME, we first sought to validate the effect of S. lutetiensis on an orthotopic gastric cancer model (Fig. 7a). Notably, S. lutetiensis was found to significantly promote tumour growth, as evidenced by a substantial increase in stomach weight in mice administered the bacterium, while their overall body weight remained consistent (Fig. 7b–d). The presence of S. lutetiensis was confirmed through fluorescence in situ hybridization (FISH) (Fig. 7e). Further analysis involving hematoxylin-eosin staining and immunohistochemical detection of tumour tissues revealed a significant increase in Ki-67 levels and a notable decrease in cleaved caspase-3 in the S. lutetiensis-treated group compared to the control group (Fig. 7f–h). Moreover, a reduction in the infiltration density of CD8+IL17A+ TRM cells was observed in tumours from S. lutetiensis-treated mice. Consistent with the results of the single-cell analysis, the secretion of cytokines such as Granzyme A and Granzyme B from CD8+IL17A+ TRM cells in the S. lutetiensis-treated group was diminished (Fig. 7i–l). Multiplex immunofluorescence staining further confirmed a significant reduction in CD8+IL17A+ TRM cells in tumour tissues following treatment with S. lutetiensis (Fig. 7m, n). Based on the above results, we developed a subcutaneous tumour model of gastric cancer to monitor tumour growth. Consistent with the results observed for in situ tumours, the growth of tumours treated with S. lutetiensis was enhanced, yet the overall weight of the mice remained unchanged (Supplementary Fig. 4).
a Experimental design of the effect of S. lutetiensis on situ model of gastric cancer (Control group, n = 5; S. lutetiensis group, n = 5). b Images of stomach tumours collected from different groups mice. The blue circles indicate the tumours. Quantification of mouse stomach weight c and body weight d in both groups. e Images of fluorescence in situ hybridization for stomach tumours in both groups. f Images of haematoxylin-eosin staining and immunohistochemical detection of Ki-67 and cleaved caspase-3 in both groups. Quantification of the proportion of Ki-67-positive cells (g) and cleaved caspase-3-positive cells in both groups (h). i Flow cytometry strategy for analysing TILs in mouse tumours. Quantification of the proportion of IL17A (j)、GZMA (k) and GZMB (l) positive cells in both groups. m Co-expression of IL17A (Green) and CD8 (Red) demonstrated by multiple immunohistochemical staining of mouse tumours. n Quantification of the proportion of CD8+IL17A+TRM cells for tumours from the two group. Data are presented as mean ± SEM. Significance was determined two-tailed Mann-Whitney U test.
To further recapitulate these findings in vivo, we transplanted HGC-27 tumour cells and effector CD8+TRM cells, with or without S. lutetiensis, in subcutaneous NSG mice model of gastric cancer. We observed that the presence of S. lutetiensis significantly accelerated tumour growth by inhibiting CD8+IL17A+TRM cells (Supplementary Fig. 5). In summary, these findings suggest that S. lutetiensis contributes to tumour growth by impeding the infiltration and reducing cytokine secretion of CD8+IL17A+ TRM cells within the gastric cancer context. This finding indicates a potential role for S. lutetiensis in modulating the immune response within the TME, thereby influencing tumour progression and providing insights into the complex interactions between microbial entities and immune regulation in cancer.
Discussion
Recent studies have shed light on the pivotal role of the microbiome in various diseases, including cancers21,22,23,24. It is believed that H. pylori infection is a necessary cause of GC25,26. However, only 1%–3% of persons infected with H. pylori go on to develop GC27,28. Recently, Fu et al. identified Streptococcus anginosus as another promoter of gastric tumourigenesis29. Several lines of evidence also suggest a potential role for non–H. pylori bacteria in GC30,31. We aimed to investigate the potential role of non-H. pylori bacteria in GC in light of these results. Here, we demonstrated that S. lutetiensis was more abundant in GC patients than in patients with chronic gastritis. The abundance of S. lutetiensis is associated with advanced TNM stage in GC patients. S. lutetiensis may inhibit the IL17 signalling pathway. Single-cell analysis revealed a negative correlation between S. lutetiensis and CD8+IL17A+ TRM cells. Furthermore, S. lutetiensis inhibited the secretion of Granzyme A, Granzyme B and IFN-γ by CD8+IL17A+ TRM cells.
S. lutetiensis belongs to the Streptococcus bovis group, which is a species of gram-positive bacteria in humans32. The bacteria were associated with oral and upper respiratory tract infections as previously described. One study also showed that Streptococcus could induce inflammation and cancer by activating COX-233,34. In patients with clear cell renal cell carcinoma, S. lutetiensis promotes cancer cell proliferation, migration and invasion through the TGFβ pathway35. The role of S. lutetiensis in GC has not been reported thus far. In this study, we observed a high abundance of S. lutetiensis in GC tumours, especially in advanced-stage GC. Notably, treatment with S. lutetiensis increased tumour growth, indicating a pivotal tumour-promoting role of S. lutetiensis.
Our study explored the association between the tumour microbiota and tumour-infiltrating T cells. The TRM cell population differed markedly between S. lutetiensishigh and S. lutetiensislow tumours. TRM cells mainly reside in peripheral tissues where they can protect against local infections and cancer36,37,38. The accumulation of TRM cells has been associated with a favourable prognosis in multiple solid tumours39,40,41. Our previous research revealed that CXCL13+CD103+CD8+ TRM cells located in tertiary lymphoid structures contribute to tumour control and are a key subset involved in the response to PD-1/PD-L1 blockade in GC42. Here, we identified a subset of CD8+ TRM cells expressing IL17A that exhibited a Tc17 phenotype and was decreased in S. lutetiensishigh s. Recent findings have indicated that CD8+IL17+ T cells expressing CD103 may promote tumour growth via IL17/22/26 signalling in GC43. However, in murine GCs, treatment with S. lutetiensis significantly decreased CD8+IL17A+ TRM cells and accelerated tumour growth, suggesting that CD8+IL17A+ TRM cells play an antitumour role. This notion is supported by previous research showing that IL17A+CD103+CD8+ TRM cells in lung tumours exhibit have increased cancer cell cytotoxicity and proliferation14. Besides, our findings suggest that S. lutetiensis may impair the CD8+IL17A+ TRM cells through activating Nrf2-mediated oxidative stress response. Elevated oxidative stress signalling and the resulting accumulation of ROS have been shown to disrupt T cell proliferation and metabolic processes44. Our ChIP‐qPCR and Luciferase reporter assay indicated that Nrf2 regulated IL-17A promoter activity in RORγt-dependent manner. This is consistent with a previous finding that the transcript levels of RORγt‐induced IL-17A were reduced by overexpressing Nrf2 in Th17 cells20. This oxidative stress-induced dysfunction via Nrf2 could explain the observed decrease of CD8+IL17A+ TRM cells in S. lutetiensishigh tumours, potentially diminishing the immune response against cancer cells in these cases.
In addition, S. lutetiensis was found to be positively correlated with CD8+CD160+ TRM cells in GC tissues. In GC, CD8+CD160+ TRM cells express low levels of PDCD1 but exhibit high AREG expression. CD160, a coinhibitory of T-cell signalling, is usually co-expressed with inhibitory receptors in exhausted tumour-reactive T cells45. In chronic lymphocytic leukemia, CD160+CD8+ T cells exhibit impaired effector functions46. In pancreatic cancer, CD160+CD8+ T cells exhibit active effector functions but limited activation potential47. Selena Viganò et al. also reported that the impaired function of CD160+CD8+ T cells is independent of PD-1 expression48. This evidence showed that CD160+CD8+ TRM cells represent a subset of dysfunctional T cells.
In addition, AREG belongs to the epidermal growth factor (EGF) family and is associated with tumour vascularization49. CD160+CD8+ TRM cells can promote tumour progression via the secretion of AREG. Targeting S. lutetiensis could be a strategy to inhibit CD160+CD8+ TRM cells.
In summary, our study indicated that S. lutetiensis is abundant in GC and is associated with advanced TNM stage. The infiltration of CD8+IL17A+ TRM cells is dependent on the level of S. lutetiensis. Targeting S. lutetiensis may be an effective therapeutic strategy for GC. However, the detailed interactions between S. lutetiensis and reduced CD8+IL17A+ TRM cells still need to be further investigated.
Data availability
The 16S rRNA sequencing data could be accessed using the primary accession code HRA002220 at the Genome Sequence Archive for Human (https://ngdc.cncb.ac.cn/gsa-human/browse/HRA002220). All other data generated in this study are available upon request from the corresponding authors.
References
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. & Lordick, F. Gastric cancer. Lancet 396, 635–648 (2020).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Wang, F., Meng, W., Wang, B. & Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 345, 196–202 (2014).
Peek, R. M. Jr. & Crabtree, J. E. Helicobacter infection and gastric neoplasia. J. Pathol. 208, 233–248 (2006).
Coker, O. O. et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 67, 1024–1032 (2018).
Marshall, B. J. & Warren, J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1, 1311–1315 (1984).
Lofgren, J. L. et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 140, 210–220 (2011).
Blanc, C. et al. Targeting Resident Memory T Cells for Cancer Immunotherapy. Front Immunol. 9, 1722 (2018).
Hogan, R. J. et al. Protection from respiratory virus infections can be mediated by antigen-specific CD4 T cells that persist in the lungs. J. Exp. Med. 193, 981–986 (2001).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrançois, L. Preferential Localization of Effector Memory Cells in Nonlymphoid Tissue. J. Immunol. 192, 2413–2417 (2014).
Woodberry, T. et al. Alpha E beta 7 (CD103) expression identifies a highly active, tonsil-resident effector-memory CTL population. J. Immunol. 175, 4355–4362 (2005).
Amsen, D., van Gisbergen, K. P. J. M., Hombrink, P. & van Lier, R. A. W. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat. Immunol. 19, 538–546 (2018).
Corgnac, S. et al. CD103(+)CD8(+) T(RM) Cells Accumulate in Tumors of Anti-PD-1-Responder Lung Cancer Patients and Are Tumor-Reactive Lymphocytes Enriched with Tc17. Cell Rep. Med 1, 100127 (2020).
Sun, H. et al. Reduced CD160 Expression Contributes to Impaired NK-cell Function and Poor Clinical Outcomes in Patients with HCC. Cancer Res 78, 6581–6593 (2018).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011).
Gao, X., Lin, H., Revanna, K. & Dong, Q. A Bayesian taxonomic classification method for 16S rRNA gene sequences with improved species-level accuracy. BMC Bioinforma. 18, 247 (2017).
Baran, Y. et al. MetaCell: analysis of single-cell RNA-seq data using K-nn graph partitions. Genome Biol. 20, 206 (2019).
Chen, P. et al. Streptococcus lutetiensis Induces Autophagy via Oxidative Stress in Bovine Mammary Epithelial Cells. Oxid. Med Cell Longev. 2022, 2549772 (2022).
Lin, X. et al. Nrf2 through Aryl Hydrocarbon Receptor Regulates IL-22 Response in CD4(+) T Cells. J. Immunol. 206, 1540–1548 (2021).
Kong, C. et al. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut 72, 1129–1142 (2023).
Karvela, A. et al. Lung microbiome: an emerging player in lung cancer pathogenesis and progression. Clin. Transl. Oncol. 25, 2365–2372 (2023).
Sepich-Poore, G. D. et al. The microbiome and human cancer. Science 371 (2021).
Villemin, C. et al. The heightened importance of the microbiome in cancer immunotherapy. Trends Immunol. 44, 44–59 (2023).
Amieva, M. & Peek, R. M. Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 150, 64–78 (2016).
Lee, Y. C. et al. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 150, 1113–1124.e1115 (2016).
Figura, N., Marano, L., Moretti, E. & Ponzetto, A. Helicobacter pylori infection and gastric carcinoma: Not all the strains and patients are alike. World J. Gastrointest. Oncol. 8, 40–54 (2016).
Ezzati Amini, E. & Moradi, Y. Association between helicobacter pylori infection and primary open-angle glaucoma: a systematic review and meta-analysis. BMC Ophthalmol. 23, 374 (2023).
Fu, K. et al. Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell 187, 882–896e817 (2024).
Peng, R. et al. Gastric Microbiome Alterations Are Associated with Decreased CD8+Tissue-Resident Memory T Cells in the Tumor Microenvironment of Gastric Cancer. Cancer Immunol. Res 10, 1224–1240 (2022).
Li, Q. & Yu, H. The role of non-H. pylori bacteria in the development of gastric cancer. Am. J. Cancer Res 10, 2271–2281 (2020).
Jin, D. et al. Dynamics of fecal microbial communities in children with diarrhea of unknown etiology and genomic analysis of associated Streptococcus lutetiensis. BMC Microbiol 13, 141 (2013).
Dresen, M. et al. Streptococcus suis Induces Expression of Cyclooxygenase-2 in Porcine Lung Tissue. Microorganisms 9 (2021).
N'Guessan, P. D. et al. Streptococcus pneumoniae induced p38 MAPK- and NF-kappaB-dependent COX-2 expression in human lung epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 290, L1131–L1138 (2006).
Chen, Y. et al. Characteristics of Gut Microbiota in Patients With Clear Cell Renal Cell Carcinoma. Front Microbiol 13, 913718 (2022).
Park, S. L., Gebhardt, T. & Mackay, L. K. Tissue-Resident Memory T Cells in Cancer Immunosurveillance. Trends Immunol. 40, 735–747 (2019).
Molodtsov, A. & Turk, M. J. Tissue Resident CD8 Memory T Cell Responses in Cancer and Autoimmunity. Front Immunol. 9, 2810 (2018).
Sasson, S. C., Gordon, C. L., Christo, S. N., Klenerman, P. & Mackay, L. K. Local heroes or villains: tissue-resident memory T cells in human health and disease. Cell Mol. Immunol. 17, 113–122 (2020).
Virassamy, B. et al. Intratumoral CD8(+) T cells with a tissue-resident memory phenotype mediate local immunity and immune checkpoint responses in breast cancer. Cancer Cell 41, 585–601. e588 (2023).
Anadon, C. M. et al. Ovarian cancer immunogenicity is governed by a narrow subset of progenitor tissue-resident memory T cells. Cancer Cell 40, 545–557.e513 (2022).
Yenyuwadee, S., Sanchez-Trincado Lopez, J. L., Shah, R., Rosato, P. C. & Boussiotis, V. A. The evolving role of tissue-resident memory T cells in infections and cancer. Sci. Adv. 8, eabo5871 (2022).
Hu, C. et al. Tertiary Lymphoid Structure-Associated B Cells Enhance CXCL13(+)CD103(+)CD8(+) Tissue-Resident Memory T-Cell Response to Programmed Cell Death Protein 1 Blockade in Cancer Immunotherapy. Gastroenterology (2023).
Sun, K. et al. scRNA-seq of gastric tumor shows complex intercellular interaction with an alternative T cell exhaustion trajectory. Nat. Commun. 13, 4943 (2022).
Yue, T. et al. SLFN2 protection of tRNAs from stress-induced cleavage is essential for T cell-mediated immunity. Science 372 (2021).
Attanasio, J. & Wherry, E. J. Costimulatory and Coinhibitory Receptor Pathways in Infectious Disease. Immunity 44, 1052–1068 (2016).
Bozorgmehr, N. et al. Expanded antigen-experienced CD160(+)CD8(+)effector T cells exhibit impaired effector functions in chronic lymphocytic leukemia. J Immunother Cancer 9 (2021).
Liu, S., Zhang, W., Liu, K. & Wang, Y. CD160 expression on CD8(+) T cells is associated with active effector responses but limited activation potential in pancreatic cancer. Cancer Immunol. Immunother. 69, 789–797 (2020).
Vigano, S. et al. CD160-associated CD8 T-cell functional impairment is independent of PD-1 expression. PLoS Pathog. 10, e1004380 (2014).
Huang, Y. W. et al. Amphiregulin Promotes Vascular Endothelial Growth Factor-C Expression and Lymphangiogenesis through STAT3 Activation in Human Chondrosarcoma Cells. Cell Physiol. Biochem 52, 1–15 (2019).
Acknowledgements
This work was supported by project grants from the National Natural Science Foundation of China [82102876 and 82203226]; Jiangsu Provincial Key Research and Development Program [BE2022770]; Basic Research Program of Jiangsu [BK20231146]; Key Laboratory of Emergency and Trauma (Hainan Medical University), Ministry of Education [KLET-201913]; Major Program of Wuxi Medical Centre, Nanjing Medical University [WMCM202308] and Jiangsu Province Postgraduate Research and Innovation Program [JX10113836].
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript. The study was designed by Y.C., Q.Z., F.Y., and F.Z.Q. Experimental work was conducted by H.Y.W., Y.H.Z., Z.N.Z., C.P.H., R.M.S., R.L and J.Y.L. Data analysis and figure generation were performed by W.H.Y., Y.H.Z., C.P.H, J.Y.L and R.P. Y.D.H. carried out pathological analysis of tissue biopsy samples. H.Y.W., Y.H.Z., and W.H.Y. were responsible for the original draft preparation, with input from all other authors. The review and editing were performed by Y.C., Q.Z., F.Y., and F.Z.Q.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, H., You, W., Zhu, Z. et al. Streptococcus lutetiensis inhibits CD8+ IL17A+ TRM cells and leads to gastric cancer progression and poor prognosis. npj Precis. Onc. 9, 43 (2025). https://doi.org/10.1038/s41698-025-00810-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-025-00810-2