Abstract
In the context of the global increase in early-onset tumours, investigating the global disease burden caused by early-onset pancreatic cancer (EOPC) is imperative. Data on the burden of EOPC were obtained from the Global Burden of Disease Study 2021. A joinpoint regression model was used to analyse the temporal trend of the EOPC burden, and an age‒period‒cohort (APC) model was used to analyse the influence of age, period, and birth cohort on burden trends. Globally, the number of EOPC cases increased from 24,480 to 42,254, and the number of deaths increased from 17,193 to 26,996 between 1990 and 2021. The results of the APC model showed that the burden of EOPC increases with increasing age, whereas the variations in period and cohort effects exhibited a complex pattern across different sociodemographic index regions. Consequently, the disease burden of EOPC is increasing worldwide, highlighting the need for effective interventions.
Similar content being viewed by others
Introduction
In recent years, the incidence of cancer in the young population has increased significantly because of multiple factors, such as unhealthy dietary habits, sedentary lifestyles, and environmental exposures. Typically, cancers that occur in people younger than 50 years are defined as early-onset cancers1,2. Among all tumours, early-onset tumours of the digestive system have the fastest increasing incidence, with the greatest increases in the incidences of tumours in the appendix, intrahepatic bile duct, and pancreas3. Although pancreatic cancer is relatively uncommon globally in comparison with other malignancies, it ranks among the most lethal forms of cancer4. Research has shown that early-onset pancreatic cancer (EOPC) accounts for approximately 5%-12% of all pancreatic cancers, with the proportion still increasing5,6. Compared with general pancreatic cancer, EOPC is more malignant and has a greater likelihood of metastasis6,7,8. In addition, due to social role conflicts, patients with EOPC face more difficulties, such as increased economic pressure caused by high medical costs and anxiety caused by illness9. Therefore, more attention needs to be given to the management of EOPC.
Currently, research on the disease burden of EOPC has focused on a handful of countries, particularly high-income countries with well-established cancer registries and epidemiological databases10. Previous studies used data from the 2019 Global Burden of Disease (GBD) project, providing valuable references for the study of EOPC11,12. However, we acknowledge the significant impact of COVID-19 outbreak in recent years worldwide, and the changes caused by epidemics may lead to certain time lags in our analysis of the burden of disease and may not fully reflect the latest health trends. Newer and more accurate data are therefore important for assessing the global disease burden of EOPC and for developing effective public health policies. On the other hand, the disease burden of EOPC not only varies with age but is also influenced by advances in diagnostic technology and differences in health status across generations. However, previous studies have failed to fully account for the interplay of age, period, and cohort effects. Age-period-cohort (APC) analysis is a statistical method that simultaneously examines the impact of age, period, and cohort effects, revealing the complex interactions that shape disease trends over time13,14. Through APC analysis, we can not only determine the specific contributions of individual factors to evolving disease trends but also provide a scientific foundation for the development of targeted public health interventions.
The GBD 2021 database is a comprehensive, multinational, multidisciplinary health research program that aggregates the latest health data worldwide, including information on disease incidence, mortality, disability, and risk factors15. Numerous studies utilizing the GBD database have provided substantial evidence regarding the global disease burden, which plays a pivotal role in shaping global health policies, advancing disease research, and informing public health decision-making16,17. This study described the disease burden of EOPC and analysed trends in the burden of EOPC from 1990 to 2021. In addition, we analysed the factors influencing the disease burden via APC analysis to provide new references for resource planning and health policy-making with respect to EOPC.
Results
Global level
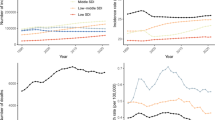
Overall, the prevalence and mortality rates of EOPC increased rapidly (Fig. 1 and Table 1). The number of cases of EOPC nearly doubled from 24,480 (95% UI, 22,957 to 26,100) in 1990 to 42,254 (95% UI, 38,708 to 45,940) in 2021. The number of deaths caused by EOPC worldwide was 17,193 (95% UI, 16,038 to 18,465), with an ASDR of 0.75 deaths per 100,000 population (95% UI, 0.70 to 0.81) in 1990. By 2021, the number of deaths caused by EOPC reached 26,996 (95% UI, 24,481 to 29,699), but the ASDR decreased to 0.65 deaths per 100,000 population (95% UI, 0.59 to 0.72) (AAPC: −0.44 (95%CI:−0.58 to −0.30), P < 0.001). Compared with the ASDR, the ASPR remained stable (1.04 cases per 100,000 population (95% UI, 0.98 to 1.11) in 1990 to 1.03 cases per 100,000 population (95% UI, 0.94 to 1.12) in 2021 (AAPC: −0.09 (95% CI: −0.34 to 0.16), P > 0.05)) (Table 1).
Compared with females, males bear a greater burden. The number of EOPC cases in males accounted for 66.0% and 65.1% of the entire burden in 1990 and 2021, respectively. Specifically, the global number of EOPC cases was 16,166 (95% UI, 14,955 to 17,406) in males and 8314 (95% UI, 7644 to 9167) in females in 1990 (Fig. 2 and Table 1). By 2021, the global number of cases was 27,518 (95% UI, 24,660 to 30,757) for males and 14,736 (95% UI, 13,530 to 15,976) for females. The observed trend in the ASPR for both sexes aligned with the stability noted in the overall ASPR of EOPC. The number of deaths caused by EOPC increased from 1990 to 2021 in males (from 11,593 to 18,251) and females (from 5600 to 8744) (Fig. 2). Nevertheless, the ASDR of EOPC decreased in both males (from 1.00 to 0.88 deaths per 100,000 population; AAPC = -0.40 (95% CI: -0.52 to -0.28), P < 0.001) and females (from 0.50 to 0.43 deaths per 100,000 population; AAPC = -0.52 (95% CI: -0.64 to -0.39), P < 0.001) between 1990 and 2021. In terms of age, both the number of cases of EOPC and the number of deaths caused by EOPC increased with age. The age group with the highest prevalence of and number of deaths caused by EOPC was the 45–49-year-old group in both 1990 and 2021 (Fig. 2).
A Prevalence of EOPC in 1990 and 2021 in the global and five SDI regions stratified by age and sex. B Death of EOPC in 1990 and 2021 in the global and five SDI regions stratified by age and sex. The bar chart shows the number of burdens in the five SDI regions by age, and the line chart shows the crude rate of EOPC by age. EOPC early-onset pancreatic cancer, SDI sociodemographic index.
Regional level
When stratified by the SDI, the highest ASPR (1.97 cases per 100,000 population [95% UI: 1.87 to 2.07]) of EOPC was observed in the high-SDI region, whereas the highest ASDR (1.09 deaths per 100,000 population [95% UI: 0.94 to 1.25]) was observed in the high-middle-SDI region in 2021 (Table 1 and Fig. 2). Between 1990 and 2021, the ASPR of the low-middle-SDI region increased the fastest (AAPC: 1.26 (95% CI: 1.18 to 1.33), P < 0.001), as did the ASDR (AAPC: 1.11 (95% CI: 1.04 to 1.18), P < 0.001) (Table 1). Notably, the ASDR decreased significantly in the high- and high-middle-SDI regions, but the ASDR still increased in the low- and low-middle-SDI regions. Among the 21 regions, the highest ASPR of EOPC was in Western Europe (2.37 cases per 100,000 population [95% UI: 2.17 to 2.58]), followed by high-income North America (2.01 cases per 100,000 population [95% UI: 1.92 to 2.11]) and Eastern Europe (1.95 cases per 100,000 population [95% UI: 1.75 to 2.17]) in 2021 (Table 1). In 14 of the 21 regions, the ASPR of EOPC increased significantly, with the fastest growth observed in western sub-Saharan Africa (AAPC: 1.99, 95% CI: 1.86 to 2.13, P < 0.001) from 1990 to 2021. East Asia is one of the most densely populated regions in the world and reported 13,332 cases of EOPC (95% UI: 10,457 to 16,574), accounting for nearly one-third of the global cases in 2021. The burden of mortality attributable to EOPC was also concentrated in East Asia (9199 [95% UI: 7166 to 11,521]). However, when counts were adjusted to age-standardized rates, the highest ASDR was observed in Eastern Europe (1.43 deaths per 100,000 population [95% UI: 1.28 to 1.59]), whereas the region with the lowest ASPR in 2021 was South Asia (0.21 cases per 100,000 population [95% UI: 0.18 to 0.25]). The ASDR of EOPC continued to increase in most regions but decreased significantly in Central Europe, high-income Asia Pacific, Western Europe, and high-income North America.
National level
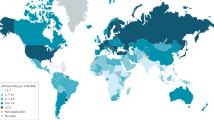
France had the highest ASPR of EOPC (4.63 cases per 100,000 population (95% UI: 3.75 to 5.69)), followed by Germany (3.68 cases per 100,000 population (95% UI: 2.99 to 4.44)) and Monaco (3.03 cases per 100,000 population (95% UI: 1.45 to 5.14)) in 2021 (Fig. 3 and Supplementary Table 1). Ukraine had the highest ASDR of EOPC (1.60 deaths per 100,000 population (95% UI: 1.06 to 2.23)), followed by Monaco (1.50 deaths per 100,000 population (95% UI: 0.73 to 2.51)) and Mongolia (1.44 deaths per 100,000 population (95% UI: 1.02 to 1.96)) in 2021. Conversely, Mozambique had both the lowest ASPR (0.09 cases per 100,000 population (95% UI: 0.05 to 0.13)) and ASDR (0.07 deaths per 100,000 population (95% UI: 0.05 to 0.12)) in 2021. Owing to its large population, China reported the highest number of cases (12,831 (95% UI: 9,956 to 16,072)) and deaths (8,887 (95% UI: 6,853 to 11,204)) globally in 2021. From 1990 to 2021, the temporal trend of EOPC presented significant variation across countries and territories. Between 1990 and 2021, the ASPR of EOPC significantly increased in 129 countries (territories), whereas it decreased in 20 countries (territories). The countries with the fastest increase in the ASPR (AAPC: 9.65 (95% CI: 7.51 to 11.83), P < 0.001) were in Turkmenistan. The countries with the fastest decrease in the ASPR (AAPC: -1.59 (95% CI: -1.78 to -1.40), P < 0.001) were in Poland. Only 40 of the 204 countries (territories) presented a decrease in the ASDR of EOPC. The country with the fastest decrease in the ASDR was Luxembourg (AAPC: -2.07 (95% CI: -2.76 to -1.37), P < 0.001).
Age-period-cohort analysis of the EOPC burden
The net drifts (%) of the global EOPC prevalence and mortality rates (per 100,000 population) were -0.07 (95% CI: -0.18, 0.03) and -0.61 (95% CI: -0.75, -0.46), respectively, indicating that the prevalence of EOPC remained stable and that the mortality rate decreased significantly (Fig. 4). In terms of specific ages and SDI regions, the results of the local drift analysis differed from those of the global trend analysis. Similar to the findings from the joinpoint regression, the mortality rates decreased in regions with high SDIs, whereas both prevalence and mortality rates continued to increase in areas with low SDIs. These findings suggest that high-SDI regions have made significant progress in cancer prevention and control, whereas low-SDI regions continue to face challenges in addressing the cancer disease burden. With the period and cohort effects controlled for, the prevalence and mortality rates of EOPC both increased with age. The period effect results revealed that the period effect of prevalence remained almost unchanged and that the period effect of death significantly decreased at the global level from 1992 to 2021 (Supplementary Fig. 1). Regions with high SDIs experienced a significant risk of increased prevalence prior to 2011. In contrast, the risk of increased prevalence in regions with low SDIs was not significant between 1992 and 2006 but exhibited dramatic increases after 2006 (Supplementary Fig. 2). For the risk of death, high-SDI areas showed a downwards trend, whereas low-SDI areas showed an increasing trend. Similar to the period effect, in regions with high SDIs, as the birth year shifted backwards, the prevalence risk initially increased significantly and then remained stable. The increased prevalence and death risk associated with EOPC continued to increase among birth cohorts in low- and low-middle-SDI regions (Supplementary Fig. 3).
Discussion
In this study, we found that both the global number of prevalent cases and deaths increased dramatically between 1990 and 2021. However, when the counts were transformed to age-standardized rates, the overall global ASPR remained stable, and the ASDR showed a downwards trend. During this period, the disease burden distribution and temporal trend of EOPC significantly differed across countries and regions.
The burden of EOPC presented a rapid increasing trend and increased with age, similar to the growth pattern of overall pancreatic cancer burden worldwide18. When the oldest EOPC patients were born, the world population was approximately 2.5 billion; the current world population is around 8 billion, which is more than three times the number in the mid-20th century19. This significant increase in the population means that more people may be at risk for pancreatic cancer, especially high-risk individuals with a family history or genetic predisposition. Moreover, some modifiable dietary habits and lifestyle changes have also contributed significantly to the increasing burden of EOPC. In terms of diet, the consumption of processed foods by the general public has increased significantly due to convenience and ease of storage. Excessive consumption of processed foods and sedentary behaviour are strongly associated with the development of pancreatic cancer20,21. Compared with middle-aged and elderly individuals, younger individuals are more significantly influenced by these factors, leading to a higher incidence of obesity and metabolic-related diseases22,23. Obesity has long been recognized as an important risk factor for many different types of tumours24. Being overweight, especially in early adulthood, is strongly associated with a younger age at diagnosis of pancreatic cancer25,26,27. With respect to sex, the burden of EOPC was greater among males than females. Putative explanations include differences in health behaviours and awareness, such as smoking and drinking habits28. Tobacco consumption is an important risk factor for pancreatic cancer, and its role in EOPC has also been widely recognized29. Worldwide, the proportion of males who smoke and drink greatly exceeds that of females30.
Previous studies have demonstrated a correlation between pancreatic cancer incidence and socioeconomic development, a pattern further corroborated by our study, which revealed that high-income regions bear the primary burden of EOPC31. The accumulation of risk factors and early screening are important reasons for the higher prevalence of EOPC in regions with higher SDIs. In terms of obesity, the prevalence of central obesity is 44.7% in high-income countries but only 30.6% in low-income countries32. In addition, high-income areas have more abundant medical resources for rapid pancreatic imaging and regular pancreatic monitoring, which may help identify individuals with EOPC. However, we noted a significant international disparity: the growth rate in the prevalence in lower-SDI countries exceeded that in higher-SDI regions. This observation highlights differences in the effectiveness of cancer prevention strategies in terms of their formulation and implementation across countries. Specifically, smoking is a major risk factor for pancreatic cancer, and bans and health education related to smoking behaviours have been shown to be effective in reducing the incidence of pancreatic cancer33. As early as 2009, the World Health Organization recommended that countries implement comprehensive smoke-free policies, but such tobacco control measures were implemented only in a small number of high- and middle-income countries. Under this initiative, the smoking rate among individuals aged 22 to 23 years decreased significantly from 74.6% in 2002 to 51.4% in the United States in 2018, highlighting the effectiveness of such policies34. However, according to the latest edition of the 2023 report on the global tobacco epidemic, the rigor of tobacco control policies in some low-income countries remains inadequate because of insufficient policy enforcement and poor public health awareness35,36. In addition, limited health expenditures significantly hamper cancer control efforts in low- and middle-income countries (LMICs). For example, in 2021, 17.4% of the gross domestic product in the United States was allocated to health, compared with only 4.23% in low-income countries such as Armenia37. Insufficient health spending restricts access to high-quality cancer screening and treatment services, causing many patients to miss critical opportunities for early detection and effective treatment38.
Pancreatic cancer is an extremely malignant cancer with a low 5-year survival rate. Surgery is currently the only effective way for pancreatic cancer patients to potentially be cured and have long-term survival39. In higher-SDI regions, advanced health care infrastructure, including imaging technologies and early detection protocols, allows for timely surgical interventions, which reduce the risk of death. In the United States, owing to the robust medical foundation and early diagnostic technology, the proportion of patients diagnosed with stage IA pancreatic ductal adenocarcinoma has increased, enabling more patients to undergo surgical resection, which has notably increased their overall survival rate40. On the other hand, radiotherapy plays a crucial role in the treatment of pancreatic cancer and is relatively common as a supplementary treatment in developed countries. Radiotherapy can not only be used as an adjuvant treatment before and after surgery to improve the surgical resection rate and pathological response but also provide palliative treatment to alleviate symptoms and improve quality of life for patients with locally advanced and metastatic pancreatic cancer41. However, compared with developed countries, LMICs often face limited treatment options because of the lack of medical resources. In particular, there is a serious shortage of radiotherapy equipment in many LMICs. According to a survey, 51 out of 137 LMICs (37.3%) currently lack radiotherapy facilities42,43. This shortage means that even when patients are diagnosed, it is difficult for them to receive standard radiotherapy, affecting their chances of receiving the best possible treatment. Furthermore, treatment options such as precision medicine, targeted therapy, and immunotherapy in high-income countries offer patients with pancreatic cancer more choices for treatment, significantly increasing their survival and quality of life44,45,46. Furthermore, it is important to consider the potential impact of the COVID-19 pandemic on the epidemiology of EOPC. Many countries reported sharp decreases in cancer diagnoses and screenings at the peak of the COVID-19 pandemic. For example, the number of newly diagnosed cancers in South Korea decreased by 3.6% in 2020 compared with 201947. The situation was similar in the UK, where approximately 3.4 million fewer key cancer diagnostic tests—such as endoscopy, CT imaging, ultrasound, and MRI—were conducted between March and August 2020 than in the same period in 2019, representing a 35% decrease48. This could have influenced the reported trends in the EOPC burden. While the decrease in the ASDR is significant, it is crucial to acknowledge that the effects of the pandemic on health care access and disease reporting may have contributed to this trend.
In recent years, the field of pancreatic cancer screening has expanded significantly, involving biomarkers, imaging omics, and genetic mutation detection49,50. Research on biomarkers such as CA19-9, circulating tumour DNA, and exosomal proteins has shown promise for the noninvasive early diagnosis of pancreatic cancer, their sensitivity and specificity remain insufficient for widespread early screening51. Artificial intelligence (AI) is increasingly revolutionizing the early diagnosis of various diseases. By leveraging deep learning to analyse imaging data (including computed tomography and endoscopic ultrasound), AI has significantly increased the detection rate of EOPC, offering new possibilities and directions for improving pancreatic cancer screening52. In addition, recent research has revealed that EOPC presents unique molecular features that distinguish it from late-onset pancreatic cancer, including CDKN2A, BRCA2, and PALB2 mutations53,54. These molecular insights provide a foundation for developing targeted screening strategies tailored to EOPC, potentially improving early detection rates in this unique subgroup. However, the relatively low incidence of pancreatic cancer in the general population makes mass screening inefficient and costly. Current guidelines recommend screening in high-risk populations, including patients with germline mutations or a family history of pancreatic cancer, mucinous cysts, new-onset diabetes, and pancreatitis55,56. In particular, patients with inherited pancreatitis caused by PRSS1 mutations are at significantly greater risk of developing pancreatic cancer and should start screening at age 4057. This finding is consistent with our finding that the risk of EOPC increases with age, with individuals aged 40 to 49 years being the primary group bearing the burden of EOPC. Therefore, in the future, 40 years of age should be considered the age at which EOPC screening is initiated, which may be helpful in reducing the burden of disease. The formulation and implementation of cancer prevention and control strategies are based on the following four key elements: the identification and intervention of risk factors, the early detection and diagnosis of cancer, cancer treatment and palliative care. Overall, the investment and effectiveness in the above four aspects are significantly better in high-income countries than in LMICs, which may be the main reason for the difference in the burden of pancreatic cancer. Therefore, promoting the implementation of cancer prevention and control policies in LMICs and narrowing the gap in these fields are important directions for reducing the burden in the future. Especially in areas with low SDIs, prioritizing early screening programs is critical for increasing the early detection of EOPC. In addition, high-income countries could offer funding, technical support, and opportunities to help low-income regions enhance screening capacity and ensure the accessibility of screening equipment and medications. Through cooperation with high-income countries and international organizations, low-income countries can learn from their successful experiences and advanced management models in cancer prevention and control and rapidly increase their level of prevention and control.
This study described the global geographic distribution and temporal trends of EOPC between 1990 and 2021 using data from the newly updated GBD 2021 database. We acknowledge that several factors may have influenced the trends observed in this study. First, the major limitation of the GBD analysis of the burden of diseases and injuries is the lack of primary data. Differences in the level of health care in different countries and regions, especially in LMICs, can lead to incomplete EOPC registration, causing bias in the burden of disease of EOPC. When data are not available, the results depend on the out-of-sample predictive validity of the model efforts. Notably, the COVID-19 pandemic had a profound impact on health care systems, diagnostic practices, and disease reporting. This may have led to delays in diagnosis and changes in disease burden estimates. These disruptions could influence the reported disease trends. Therefore, when interpreting the results of this study, caution must be exercised and the potential confounding effects of the COVID-19 pandemic must be considered. Second, there are different pathological types and clinical stages of pancreatic cancer, but we only evaluated the overall disease burden of EOPC, and the other types and stages should be investigated in future studies. Moreover, we did not assess the contribution of risk factors to the burden of EOPC; genetic susceptibility, environmental exposure, and lifestyle factors are critical contributors to EOPC.
In conclusion, the global burden of EOPC has increased over the past three decades, with notable variances between regions and countries. It is imperative to intensify prevention efforts by improving the management of EOPC, particularly in countries and regions with high disease burdens and relatively underdeveloped economies.
Methods
Data source
The GBD 2021 database provides a comprehensive and reliable assessment of the incidence rates, mortality rates, and risk factors for 371 diseases and injuries (including pancreatic cancer) in 204 countries and territories from 1990 to 202115. This study used the Global Health Data Exchange online website (https://ghdx.healthdata.org/gbd-2021) to retrieve and download raw data about EOPC.
Definition of EOPC
EOPC is defined as pancreatic cancer that occurs in young people, but there is currently no consensus on the exact age range of people affected by EOPC. The definition of EOPC varies among studies, with some considering EOPC as occurring in individuals younger than 50 years, while others set the upper age limit at 45 or even 40 years58. Considering the age structure used in the GBD 2021 database, we defined pancreatic cancer that occurred in the 15–49-year age group as EOPC. Pancreatic cancer was diagnosed according to the International Classification of Diseases (ICD) classification criteria, including the ICD-9 (157–157.9, 211.6–211.7) and the ICD-10 (C25-C25.9, D13.6-D13.7) criteria.
Measures of disease burden and the sociodemographic index (SDI)
Two measures were used to assess the burden of EOPC in this research: prevalence and mortality. The prevalence of EOPC refers to the number of existing cases in the current year, whereas mortality refers to the number of deaths caused by EOPC per year. These rates were calculated by dividing the number of cases or deaths by the size of the population. GBD estimates and corresponding 95% uncertainty intervals (UIs) for EOPC were collected for the aforementioned indicators (number of cases, prevalence rate, number of deaths, and mortality rate), for both sexes, seven age groups (15–19 years, 20–24 years, 25–29 years, 30–34 years, 35–39 years, 40–44 years, and 45–49 years), 5 SDI levels, 21 GBD-defined regions, and 204 countries and territories. Furthermore, in consideration of variations in age and sex distributions among populations across different countries and regions, we utilized age-standardized rates, including the age-standardized prevalence rate (ASPR) and age-standardized death rate (ASDR), to compare disease burdens. In this study, the GBD world population age structure was used as a reference for the calculation of age standardization rates. Specifically, the direct standardization method was applied to calculate the age-standardized rates for pancreatic cancer in the 15–49-year age group, which involves multiplying the crude rate for each age group (in 5-year intervals) by the corresponding proportion of the standard population in that group and then summing the values to obtain the overall age-standardized rate59,60.
The SDI is a summary indicator that identifies where the health-related development status of a country or region is located on the spectrum of development worldwide. The SDI considers the birth rate, educational attainment, and economic status. The SDI ranges between 0 and 1, which reflects the degree of social development; the closer the value is to 1, the higher the level of social and economic development, and the closer the value is to 0, lower the level. According to SDI values, all 204 countries/territories were divided into one of the following SDI quintiles: high, high-middle, middle, low-middle, or low.
Joinpoint regression
Joinpoint regression models are created by separating the study time into multiple parts according to the temporal distribution characteristics of a disease61,62. First, a logarithmic linear model was used for segmented regression. We used the grid search method to establish all possible joinpoints, calculated the corresponding mean squared errors (MSEs), and selected the grid point with the smallest MSE as the joinpoint. The Monte Carlo permutation test was subsequently used to select the number of joinpoints, with the number ranging between 0 and 5. Finally, we calculated the average annual percent change (AAPC) and the corresponding 95% confidence interval (CI). The AAPC is used to describe the overall change trend; when the AAPC is >0 and P is <0.05, it indicates an increase during a period63. For example, if the AAPC in the EOPC incidence for the period 1990–2021 is 2.0 (P < 0.05), the incidence of EOPC has an annual rate increase of 2.0% from 1990 to 2021. Joinpoint software is provided by the National Cancer Institute and available on the website (https://surveillance.cancer.gov/help/joinpoint).
Age-period-cohort analysis
APC models were employed to examine the impacts of age, period, and cohort on the disease burden of EOPC. Specifically, the age effects revealed how the risk profiles of the population changed with age, highlighting the natural progression of health risks associated with the maturation and ageing process. The period effects showed the influence of time-specific societal, environmental, and policy changes that uniformly affected all age groups during the study periods. The cohort effects highlight the long-term impacts of early-life experiences and exposures on the health of different birth cohorts. To meet the standards of APC analysis and present the latest data, we focused our analysis on the timeframe from 1992 to 2021. The data were subsequently analysed using the online tools (https://analysistools.cancer.gov/apc) to acquire the net drift, local drift, and relative risk (RR) of period and cohort effects. Both net drift and local drift were adjusted for in the analysis of period and birth cohort effects, reflecting linear trends in the disease burden overall and across age groups, respectively. Net drift captures the overall trend or drift in disease burden due to period effects—such as changes in environmental, social, or health care-related factors over time. This provides an indication of whether a disease burden generally increases or decreases over time, independent of specific age or period factors. Local drift focuses on more localized trends in the data, typically capturing short-term variations within particular age groups.
Software and statistical analysis
All analyses and visualizations were based on R (version 4.2.3), with P < 0.05 considered to indicate a significant difference. The original data for the world map used in this study were sourced from the National Geographical Information Resource Directory Service System (http://www.webmap.cn/main.do?method=index)64. The data were then processed and visualized using the ggplot2 package. This study was re-analyzed using published GBD 2021 data. We did not collect raw data on this manuscript and therefore no separate ethical approval was required for this study.
Data availability
The datasets analyzed during the current study are available in the Global Burden of Disease 2021 Database (https://ghdx.healthdata.org/gbd-2021/data-input-sources).
Code availability
Some or all data, models, or code generated or used during the study are available from the corresponding author by request.
References
Sung, H., Siegel, R. L., Rosenberg, P. S. & Jemal, A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health 4, e137–e147 (2019).
He, T. C. et al. Biological and clinical implications of early-onset cancers: a unique subtype. Crit. Rev. Oncol. Hematol. 190, 104120 (2023).
Koh, B. et al. Patterns in cancer incidence among people younger than 50 years in the US, 2010 to 2019. JAMA Netw. Open 6, e2328171 (2023).
Mizrahi, J. D., Surana, R., Valle, J. W. & Shroff, R. T. Pancreatic cancer. Lancet 395, 2008–2020 (2020).
Li, Y. & Zhang, X. Pancreatic cancer in young adults—an evolving entity? Am. J. Cancer Res. 13, 2763–2772 (2023).
Tingstedt, B., Weitkamper, C. & Andersson, R. Early onset pancreatic cancer: a controlled trial. Ann. Gastroenterol. 24, 206–212 (2011).
Kang, J. S., Jang, J. Y., Kwon, W., Han, Y. & Kim, S. W. Clinicopathologic and survival differences in younger patients with pancreatic ductal adenocarcinoma—a propensity score-matched comparative analysis. Pancreatology 17, 827–832 (2017).
Ordonez, J. E. et al. Clinicopathologic features and outcomes of early-onset pancreatic adenocarcinoma in the United States. Ann. Surg. Oncol. 27, 1997–2006 (2020).
Printz, C. Younger cancer survivors are more likely to face financial strain. Cancer 126, 3605 (2020).
Huang, J. et al. Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology 160, 744–754 (2021).
Li, Z. et al. Global, regional, and national burdens of early onset pancreatic cancer in adolescents and adults aged 15-49 years from 1990 to 2019 based on the Global Burden of Disease Study 2019: a cross-sectional study. Int. J. Surg. 110, 1929–1940 (2024).
Dahia, S. S., Konduru, L., Pandol, S. J. & Barreto, S. G. The burden of young-onset pancreatic cancer and its risk factors from 1990 to 2019: a systematic analysis of the global burden of disease study 2019. Pancreatology 24, 119–129 (2024).
Fosse, E. & Winship, C. Bounding analyses of age-period-cohort effects. Demography 56, 1975–2004 (2019).
Bell, A. Age period cohort analysis: a review of what we should and shouldn’t do. Ann. Hum. Biol. 47, 208–217 (2020).
Diseases, G. B. D. & Injuries, C. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2133–2161 (2024).
Pega, F. et al. Global, regional and national burdens of non-melanoma skin cancer attributable to occupational exposure to solar ultraviolet radiation for 183 countries, 2000-2019: a systematic analysis from the WHO/ILO Joint Estimates of the Work-related Burden of Disease and Injury. Environ. Int. 181, 108226 (2023).
Liu, C. et al. Global, regional, and national burden of liver cancer due to non-alcoholic steatohepatitis, 1990-2019: a decomposition and age-period-cohort analysis. J. Gastroenterol. 58, 1222–1236 (2023).
Kan, C. et al. Global, regional, and national burden of pancreatic cancer, 1990-2019: results from the Global Burden of Disease Study 2019. Ann. Glob. Health 89, 33 (2023).
Nations, T. U. Global issues population. https://www.un.org/zh/global-issues/population (2024).
Larsson, S. C. & Wolk, A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br. J. Cancer 106, 603–607 (2012).
Zhao, Z., Yin, Z., Pu, Z. & Zhao, Q. Association between consumption of red and processed meat and pancreatic cancer risk: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 15, 486–493.e410 (2017).
GBD 2015 Obesity Collaborators, et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377, 13–27 (2017).
Viner, R., White, B. & Christie, D. Type 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burden. Lancet 389, 2252–2260 (2017).
Ruze, R. et al. Mechanisms of obesity- and diabetes mellitus-related pancreatic carcinogenesis: a comprehensive and systematic review. Signal Transduct. Target Ther. 8, 139 (2023).
Stolzenberg-Solomon, R. Z., Schairer, C., Moore, S., Hollenbeck, A. & Silverman, D. T. Lifetime adiposity and risk of pancreatic cancer in the NIH-AARP Diet and Health Study cohort. Am. J. Clin. Nutr. 98, 1057–1065 (2013).
Urayama, K. Y. et al. Body mass index and body size in early adulthood and risk of pancreatic cancer in a central European multicenter case-control study. Int. J. Cancer 129, 2875–2884 (2011).
Genkinger, J. M. et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann. Oncol. 26, 2257–2266 (2015).
Naudin, S. et al. Healthy lifestyle and the risk of pancreatic cancer in the EPIC study. Eur. J. Epidemiol. 35, 975–986 (2020).
Raimondi, S., Maisonneuve, P., Lohr, J. M. & Lowenfels, A. B. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol. Biomark. Prev. 16, 1894–1897 (2007).
WHO. Prevalence of current tobacco use among adults aged ≥15 years (%). https://www.who.int/data/gho/indicator-metadata-registry/imr-details/128 (2023).
Wong, M. C. S. et al. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci. Rep. 7, 3165 (2017).
Wong, M. C. S. et al. Global, regional and time-trend prevalence of central obesity: a systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epidemiol. 35, 673–683 (2020).
Mulder, I. et al. Smoking cessation would substantially reduce the future incidence of pancreatic cancer in the European Union. Eur. J. Gastroenterol. Hepatol. 14, 1343–1353 (2002).
Barrington-Trimis, J. L. et al. Trends in the age of cigarette smoking initiation among young adults in the US from 2002 to 2018. JAMA Netw. Open 3, e2019022 (2020).
Siddiqi, K. et al. Framework convention on tobacco control 2030—a program to accelerate the implementation of World Health Organization framework convention for tobacco control in low- and middle-income countries: a mixed-methods evaluation. Nicotine Tob. Res. 25, 1074–1081 (2023).
WHO. WHO report on the global tobacco epidemic, 2023: protect people from tobacco smoke. https://www.who.int/publications/i/item/9789240077164 (2023).
Global Health Expenditure Database. World Health Organization. https://www.who.int/gho/health_financing/en/ (2024). Accessed July 25, 2024.
Batouli, A., Jahanshahi, P., Gross, C. P., Makarov, D. V. & Yu, J. B. The global cancer divide: relationships between national healthcare resources and cancer outcomes in high-income vs. middle- and low-income countries. J. Epidemiol. Glob. Health 4, 115–124 (2014).
Strobel, O., Neoptolemos, J., Jager, D. & Buchler, M. W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 16, 11–26 (2019).
Blackford, A. L., Canto, M. I., Klein, A. P., Hruban, R. H. & Goggins, M. Recent trends in the incidence and survival of stage 1A pancreatic cancer: a surveillance, epidemiology, and end results analysis. J. Natl Cancer Inst. 112, 1162–1169 (2020).
Reyngold, M. et al. Association of ablative radiation therapy with survival among patients with inoperable pancreatic cancer. JAMA Oncol. 7, 735–738 (2021).
Datta, N. R., Rogers, S. & Bodis, S. Challenges and opportunities to realize “The 2030 Agenda for Sustainable Development” by the United Nations: implications for radiation therapy infrastructure in low- and middle-income countries. Int. J. Radiat. Oncol. Biol. Phys. 105, 918–933 (2019).
Ige, T. et al. Understanding the challenges of delivering radiotherapy in low- and middle-income countries in Africa. J. Cancer Policy 35, 100372 (2023).
Wells, J. C. et al. An analysis of contemporary oncology randomized clinical trials from low/middle-income vs high-income countries. JAMA Oncol. 7, 379–385 (2021).
Park, B. K. et al. Trends in treatment patterns and survival outcomes in pancreatic cancer: a nationwide population-based study in Korea. Eur. J. Cancer 189, 112932 (2023).
Neoptolemos, J. P. et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 389, 1011–1024 (2017).
Seo, S. H., Cho, S., Yoo, S. H., Keam, B. & Shin, A. Changes in the utilization of health care services by cancer patients during the COVID-19 pandemic. Yonsei Med. J. 64, 463–470 (2023).
Greenwood, E. & Swanton, C. Consequences of COVID-19 for cancer care—a CRUK perspective. Nat. Rev. Clin. Oncol. 18, 3–4 (2021).
Yang, J. et al. Early screening and diagnosis strategies of pancreatic cancer: a comprehensive review. Cancer Commun. 41, 1257–1274 (2021).
Stoffel, E. M., Brand, R. E. & Goggins, M. Pancreatic cancer: changing epidemiology and new approaches to risk assessment, early detection, and prevention. Gastroenterology 164, 752–765 (2023).
Abe, T. et al. Gene variants that affect levels of circulating tumor markers increase identification of patients with pancreatic cancer. Clin. Gastroenterol. Hepatol. 18, 1161–1169.e1165 (2020).
Huang, B. et al. Artificial intelligence in pancreatic cancer. Theranostics 12, 6931–6954 (2022).
Tsang, E. S. et al. Delving into early-onset pancreatic ductal adenocarcinoma: how does age fit in? Clin. Cancer Res. 27, 246–254 (2021).
Ogobuiro, I. et al. Multiomic characterization reveals a distinct molecular landscape in young-onset pancreatic cancer. JCO Precis. Oncol. 7, e2300152 (2023).
US Preventive Services Task Force, et al. Screening for pancreatic cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 322, 438–444 (2019).
Canto, M. I. et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 62, 339–347 (2013).
Ilic, M. & Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 22, 9694–9705 (2016).
Eguchi, H., Kobayashi, S., Gotoh, K., Noda, T. & Doki, Y. Characteristics of early-onset pancreatic cancer and its association with familial pancreatic cancer and hereditary pancreatic cancer syndromes. Ann. Gastroenterol. Surg. 4, 229–233 (2020).
GBD 2019 Demographics Collaborators. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1160–1203 (2020).
He, H. et al. Trends in the incidence and DALYs of schizophrenia at the global, regional and national levels: results from the Global Burden of Disease Study 2017. Epidemiol. Psychiatr. Sci. 29, e91 (2020).
Kim, H. J., Fay, M. P., Feuer, E. J. & Midthune, D. N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 19, 335–351 (2000).
Irimata, K. E. et al. Guidance for selecting model options in the National Cancer Institute Joinpoint Regression Software. Vital Health Stat. 1, 1–22 (2022).
Clegg, L. X., Hankey, B. F., Tiwari, R., Feuer, E. J. & Edwards, B. K. Estimating average annual per cent change in trend analysis. Stat. Med. 28, 3670–3682 (2009).
National Geographical Information Resource Directory Service System. National Geomatics Center of China. http://www.webmap.cn/main.do?method=index (2024). Accessed June 13, 2024.
Acknowledgements
We thank the collaborators of the Global Burden of Disease (GBD) Study 2021 for their work. We thank all the individuals who contributed to the GBD 2021 for their extensive support in finding, cataloging, and analyzing data and facilitating communications.
Author information
Authors and Affiliations
Contributions
Conception and design: Z.B.T., Y.M., and Y.R.W.; Administrative support: W.G.D., J.X.Z.; Provision of study materials or patients: J.H.Z. and J.X.Z.; Collection and assembly of data: Y.P. and H.D.H.; Data analysis and interpretation: Z.B.T., Y.M., Y.R.W., and J.H.Z.; Manuscript writing: all authors; Final approval of manuscript: all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tan, Z., Meng, Y., Wu, Y. et al. The burden and temporal trend of early onset pancreatic cancer based on the GBD 2021. npj Precis. Onc. 9, 32 (2025). https://doi.org/10.1038/s41698-025-00820-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-00820-0
This article is cited by
-
Development and validation of a nomogram for predicting distant metastasis and prognosis in elderly T1–T2 pancreatic ductal adenocarcinoma patients
Discover Oncology (2026)
-
Growing burden of early-onset pancreatic cancer without increasing risk: what is the trick
npj Precision Oncology (2025)
-
Persistent Global Associations Between Gallbladder-Biliary Diseases and Pancreatic Cancer: Evidence from 204 Countries and Territories Over 32 years
Digestive Diseases and Sciences (2025)