Abstract
Hepatocellular carcinoma (HCC) is a prevalent and deadly cancer. Gut microbiota affect tumor immunity and immunotherapy efficacy, but the exact mechanisms are unclear. A study compared gut microbiota in HCC patients and healthy controls (HC) using 16S rDNA analysis and metabolomics. It found higher levels of Bifidobacterium and a positive correlation between Bifidobacterium and isobutyric acid in HC group. Animal models showed that the combination of HC fecal matter and αPD-1 treatment reduced tumor volume more effectively than the combination of HCC fecal matter and αPD-1. The combination of Bifidobacterium or isobutyrate with αPD-1 also decreased tumor size. In vitro, isobutyrate-stimulated CD8+T cells increased IFN-γ secretion, suppressed liver cancer cells, and downregulated the JAK/STAT3 pathway. Combined treatments increased CD8+T cells and IFN-γ levels and reduced JAK/STAT3 signaling in the tumor microenvironment. This suggests that Bifidobacterium and isobutyrate can inhibit tumor growth, offering insights into gut microbiota-host interactions and potential strategies to overcome HCC immunotherapy resistance.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in humans. In recent years, the global morbidity and mortality rates have continued to rise, resulting in at least 600,000 deaths per year and being the third leading cause of cancer-related deaths worldwide, leading to enormous economic losses1. Many liver cancer patients are diagnosed at an advanced stage, missing the opportunity for traditional surgery and chemotherapy. Currently, the benefits of immunotherapy for advanced HCC have been well established. The main class of drugs is immune checkpoint inhibitors (ICIs), particularly those that block PD-1 or PD-L1. These have been tested in large clinical trials either as monotherapy or in combination. ICIs have emerged as an effective treatment for HCC patients, capable of rescuing the immune system and suppressing tumor growth, and have become an integral part of systemic therapy for HCC2,3,4,5. However, some patients have been observed drug resistance or inefficacy in clinical applications6,7. Research have found that neither treatment using αPD-1 in advanced HCC reached overall survival points. Compared with advanced HCC patients treated with sorafenib, pembrolizumab did not meet the preset biphasic endpoints and the median progression-free survival period to improve overall survival. Another trial comparing nivolumab as a first-line treatment for patients with unresectable HCC also did not meet a preset overall survival point8,9,10,11,12. It has also been reported that anti-programmed cell death protein 1 (αPD-1) is used in the advanced HCC patients, but it has been shown to be ineffective and to induce adaptive resistance to immunosuppressive agents in a certain number of HCC patients13. Therefore, researchers are now striving to explore combination strategies for ICIs to enhance the clinical tolerance of ICIs in HCC treatment. Ayelet Sivan et al. have found that mice with different gut microbiota have the differences in tumor growth and tumor immunity, which can be eliminated after combined cage or fecal microbiota transplantation (FMT) in melanoma immunotherapy. The effect of Bifidobacterium administered orally on tumor suppression is comparable to that of αPD-1 therapy, and combined treatment can almost eliminate tumor growth, indicating that intervention in gut microbiota may enhance the αPD-1 therapy efficacy14. In another study, SPF mice had increased IFN-γ+CD8+T cells compared to GF mice in their gut tissues. These results indicate that the number of IFN-γ+CD8+T cells in the host is variable, and IFN-γ+CD8+T cells can be induced by gut microbiota, and this induction is reversible15. In addition, Erez N. Baruch et al. conducted FMT in patients with metastatic melanoma refractory to patients with αPD-1 therapy, and subsequently observed partial response16. After FMT, CD8+T cell infiltration in tumor and intestinal tissue was increased, which affected the immunotherapy efficacy. These studies suggest that gut microbiome influences can alter immunotherapy efficacy through immune pathways. Moreover, Miller SJ et al. found that short-chain fatty acids (SCFAs) produced by gut microbiota could inhibited the expression of histone deacetylases (HDACs) in T cells of mouse colon tissues, promoting T cell differentiation in mice17,18. Based on previous studies, researchers have shown significant changes in gut microbiota in patients with HCC19,20,21,22. However, the mechanism of gut microbiota-mediated CD8+T cell recruitment in the tumor microenvironment (TME) promoting tumor cell apoptosis in HCC, is still unclear. In this study, our goal is to explore the mechanisms of crosstalk between the gut microbiota and the host, and to provide a theoretical foundation for enhancing the anti-cancer effects in the immunotherapy of patients with HCC.
Methods
Sample collection
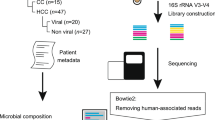
Stool samples from patients with HCC and healthy volunteers were collected. After the strict criteria, feces of 29 patients with HCC (HCC group) and 29 healthy samples (HC group) were included in our study, as shown in Fig. 1a, approved by the Ethics Committee of the Fujian Cancer Hospital (K2021-118-01).
a Experimental procedure. Fecal samples from healthy individuals and liver cancer patients were collected for 16S rDNA and metabolomics analysis. b Percent of community abundance at the family level between the two groups. Percent of community abundance at the phylum (c), family (d), and genus (e) level between the two groups. Sob index (f), ace index (g), smithwilson index (h), and simpsoneven index (i) in alpha diversity test at the OTU level between two groups. j PCoA in beta diversity at the OTU level. Community heatmap analysis at the genus (k) and phylum level (l). Wilcoxon rank-sum test was used. n = 29 per group. HCC Hepatocellular Carcinoma, HC Healthy control.
Inclusion criteria for HCC patients are as follows: ①Age ≥ 18 years; ② Primary liver cancer confirmed by pathology; ⑤ No prior therapy, including ICIs. Inclusion criteria for healthy controls: Age ≥ 18 years. Exclusion criteria for both groups are as follows: ①Allergic or autoimmune diseases; ②Diabetes; ③Cardiovascular disease; ④Psychological disorders; ⑤History of gastrointestinal surgery; ⑥Drugs or supplements being taken within the past 2 weeks, including antibiotics, probiotics, proton pump inhibitors, lactulose, oral anti-diabetic drugs, statins; ⑦Personal habits, including alcohol consumption, cigarette smoking, dietary habit. The clinical features of the included volunteers are shown in Supplementary Table 1.
After the strict criteria, 6 fecal samples from immunotherapy non-responders (NR) and 6 responders (R) were included in our study, inclusion criteria are ①Age ≥ 18 years; ②No allergic components; ③No use of antibiotics or probiotics in the past 2 weeks; ④Pathologically diagnosed primary liver cancer; ⑤First-time ICI treatment. The evaluation of αPD-1 treatment generally requires at least 2 cycles. If potential pseudoprogression is suspected after the first treatment, a re-evaluation is typically conducted at least 4 weeks later. If potential pseudoprogression is suspected after the first treatment, a re-evaluation is typically conducted at least 4 weeks later. Using the RECIST (Response Evaluation Criteria in Solid Tumors) standard, tumor size is assessed through CT or MRI scans to determine if the tumor has shrunk. A response to αPD-1 treatment is indicated if the tumor burden is reduced by more than 30% on imaging. The fecal samples were stored at −80 °C for following study. This research was reviewed and approved by the Ethics Committee of the Fujian Cancer Hospital under reference NO. K2021-118-01. Informed consent was obtained from all participants prior to their inclusion in the study. The study conforms to recognized ethical standards, including the Declaration of Helsinki, the US Federal Policy for the Protection of Human Subjects (45 CFR 46), All participants in the study have signed informed consent forms.
Animal experiment
6-weeks-old C57BL/6 J male mice (Slack Co., LTD., China) were fed adaptively for 1 week, and the circadian rhythm was 12 h: 12 h and the temperature was kept at 22 ± 2 °C and the relative humidity 55 ± 10%. AVMN (1 mg/mL ampicillin, 1 mg/mL neomycin, 1 mg/mL metronidazole, and 0.5 mg /mL vancomycin, Aladdin, China) was fed in sterile water for −7 to 2 days. The tumor volume was measured every 2 days according to the formula (length × width2 × 0.5) after day 0. Tumor cells were injected at 0.1 mL per mouse subcutaneously on day 0. FMT, Bifidobacterium sodium isobutyrate (S.I, Macklin, China) and αPD-1 (BioXcell, USA) were treated on day 4. Frozen faeces were suspended in TS broth (Hope Bio-Technology Co., LTD., China), Fecal samples from 29 individuals in the HCC group and 29 individuals in the HC group were each mixed by taking 50 mg from each individual within their respective groups, and filtered by a 100 μm cell filter for FMT (50 mg faeces in 250 μL per mouse) on day 4. S.I is dissolved in sterile water (150 mM) for drink23. αPD-1 was injected intraperitoneally once every 3 days (200 μg per mouse)15. Bifidobacterium_pseudocatenulatum and Bifidobacterium_longum (Bif., Chinese Academy of Medical Sciences, China) with Bifidobacterium medium (Haibo Biotechnology Co., LTD., China) were cultured in an anaerobic bag (Biotechnology Co., LTD., China) at 36 °C for 24 h and suspended in PYG medium (Hope Bio-Technology Co., LTD., China). Mice were given each strain mixed 1 to 1, a total about 1–2 × 108 CFU in 250 μL medium by gavage per mouse15. After these, the mice were harvested, and the samples were frozen at −80 °C. The specific experimental procedures are as shown in Fig. 4a.
This experimental animal study was reviewed and approved by the Ethics Committee of Experimental Animal Center of Fujian Medical University under approval No. IACUC FJMU 2022-0798. All experiments were conducted in compliance with the World Medical Association (WMA) Statement on Animal Use in Biomedical Research and adhered to the European Union Directive 2010/63/EU for the protection of animals used for scientific purposes. Additionally, the study followed the guidelines for experimental design and care in pharmacology.
16S rDNA analysis
Bacterial DNA was extracted from samples and stored at −80 °C. Specific primers (F primer: 5′-ACTCCTACGGGAGGCAGCAG-3′; R primer: 5′-GGACTACHVGGGTWTCTAAT-3′) were used for amplification24. High-throughput sequencing was performed using the Illumina Nextseq platform (Illumina, USA). Raw DNA fragments were merged using FLASH32 and assigned to each sample based on unique barcodes. Sequences were clustered into operational taxonomic units (OTUs) using the QIIME Uparse software (version 1.9.1). Alpha diversity indices were calculated using Mothur software (v.1.30.2). The OTU was classified based on the Silva database (Release 132). In diversity analysis, sob, shannon, smithwilson, and simpsoneven indices were calculated25,26.
ELISA assay
The ELISA kit (Elabscience, China) was used to detect serum IFN-γ level. In brief, samples and standards were added to the sample and standard wells. The plate was covered with a membrane and incubated at 37 °C for 1.5 h. The liquid in the wells is discarded and washed. Biotinylated antibody solution was added to each well, covered with a membrane, then incubated at 37 °C for 1 h. Enzyme conjugate working solution was added to each well, and incubated for 30 min. Substrate solution was added to each well, and incubated at 37 °C for 15 min in the dark. Stop solution was added to each well to terminate the reaction. The OD value was immediately measured at the corresponding wavelength using an enzyme microplate reader (Thermo, USA).
Metabolomics analysis
Briefly, samples were taken and placed in a tube, and a diameter grinding bead was added.The sample solution was ground for 6 min in a frozen tissue grinder, and then extracted by low temperature ultrasound for 30 min. The supernatant was transferred to a sample vial with an insert for LC-MS/MS analysis using an UHPLC-Q Exactive HF-X system (Thermo, USA). After that, the raw data were imported into the software Progenesis QI (Milford, USA) for baseline filtering, integration, peak recognition, peak alignment, retention time correction, and finally a data matrix of retention time, mass-to-charge ratio and peak intensity is obtained. Simultaneously, the MS and MSMS mass spectrometry data is cross-checked with metabolite databases such as the Human Metabolome Database (HMDB; www.hmdb.ca/) and Metlin (https://metlin.scripps.edu/) using personalized libraries to acquire metabolite information. Subsequently, the ropls package (Version 1.6.2) in R was utilized to conduct analysis on the data matrix. Metabolic pathway annotation was conducted using the KEGG database (https://www.kegg.jp/kegg/pathway.html) and iPath3.0 (http://pathways.embl.de), and differential metabolites were obtained.
Cell experiment
After resuscitation, Hepa1-6 cells (Chinese Academy of Sciences, China) were washed with PBS (Beyotime, China) and then resuspended. 89% DMEM (Gibco, USA), 10% FBS (Gibco, USA), and 1% P/S (Gibco, USA) were used for culturing with 5% CO2 at 37 °C. After the cells were overgrown, trypsin (Gibco, USA) was added, and the complete medium was neutralized with an equal volume for 1:2 passage culture. The cells were resuspended with PBS, counted, and a cell count of 1 × 108/mL was obtained for injection.
The mouse spleen was ground, and PBS was added. After passing through a 70 µm cell strainer, the cell suspension was slowly added to Lympholyte-M cell separation medium (Cedarlane, Canada) in a 1:1 ratio. Centrifugation was carried out at 800 g for 30 min at 20 °C, with an acceleration setting of 2 and a deceleration setting of 0. After centrifugation, the mononuclear cell layer was aspirated and subjected to red blood cell lysis using lysis buffer (Beyotime, China). CD8+ T cells were isolated using CD8+ T cell separation beads (Miltenyi, Germany). The CD8+ T cells were then cultured in a medium containing 10% fetal bovine serum, 1% penicillin/streptomycin (P/S), and 89% RPMI-1640, supplemented with 100 U/mL IL-2 (MCE, USA). Cells were stimulated with different concentrations of S.I for varying times. After washing with PBS, 4000 CD8+T cells per well were seeded into a 96-well plate containing Hepa1-6 cells pre-seeded for 12 h at a density of 4000 cells per well and co-cultured without direct contact for 24 h. IFN-γ, CCK-8, and western blot assays were then conducted. The JAK1 overexpression plasmid (JAK1-OE) and the negative control plasmid (NC) were constructed by Genechem (Shanghai, China) and transfected into Hepa 1-6 cells using jetPRIME reagent (Polyplus, France) strictly following the manufacturer’s instructions.
H&E staining
Tissue slices were stained by an automatic staining machine (Leica, Germany). The slices were fixed with 95% ethanol for 2 min and hematoxylin (Beyotime, China) for 5 min. The slices were then placed in the differentiation solution (Beyotime, China) for 2 min, soaked in weak ammonia for 3 min, washed in deionized water for 5 min, and stained with eosin for 5 min. Next, the slices were then washed, then soaked in 70%, 80%, and 90% ethanol solutions for 1 min, respectively. Finally, the slices were washed twice with anhydrous ethanol for 1 min and then washed with xylene for 1 min, immersed in neutral sesame oil, and scanned with a scanner (3DHISTECH, Hungary).
Cell Counting Kit-8 Assay (CCK-8)
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8, Beyotime, China). Briefly, 4000 cells were plated in each well of a 96-well plate. Following this, 100 μL of culture medium was mixed with CCK-8 reagent and incubated for 1 h at 37 °C in a 5% CO₂ atmosphere. The absorbance was then measured at 450 nm using a microplate reader (Sunrise Ltd., China).
Western blot
Total cell protein was extracted using RIPA buffer (Beyotime, China), and the protein concentration was quantified with an Enhanced BCA Protein Assay Kit (Beyotime, China). For protein separation, a 10% SDS-PAGE gel (Beyotime, China) was prepared. Electrophoresis was performed initially at 80 V for 30 min, followed by an increase to 120 V for 60 min. The separated proteins were transferred to a 0.22 μm PVDF membrane (Millipore, Germany).
The membrane was blocked with QuickBlock Blocking Buffer (Beyotime, China) for 30 min while shaking, and then washed with TBST buffer (Sango Biotech, China) containing Tween-20 for 15 min at room temperature. Primary antibodies were diluted 1:1000 (JAK1, 3332; pY1034/1035-JAK1, 3331; STAT3, 9139; pTyr705-STAT3, 9131; CST, USA) and incubated overnight at 4 °C with gentle agitation. Following another wash with TBST, the membrane was treated with HRP-conjugated secondary antibodies, also diluted 1:1000 (Beyotime, China), for 60 min at room temperature. Finally, multiple washes were conducted, and protein bands were visualized using an ECL detection reagent (Beyotime, China).
Immunohistochemistry
The slides were put in the drying oven (Yiheng Scientific Instruments Co., Ltd., China) and dewaxed at 63 °C for 1 h, dewaxed in xylene twice for 15 min each, 100% alcohol twice for 7 min each, 90% alcohol for 5 min, 80% alcohol for 5 mins, and 70% alcohol for 5 min. Then, the slides were placed in the antigen retrieval machine for repair, then placed in distilled water to cool for more than 10 min. The primary antibodies (JAK1, 3344, CST, 1:100; pY1034/1035-JAK1, PA5-104554, Thermo, 1:100; STAT3, 30122, Pormad, 1:200; pTyr705-STAT3, 381552, Zen-bioscience, 1:100; PARP, CY6850, Abways, 1:100; Cyclin D1, AF1183, Beyotime, 1:100) were incubated overnight at 4 °C. The slides were removed from the refrigerator and then rinsed with PBS, then placed in the automatic immunohistochemistry instrument (DAKO, Denmark) to run the blocking, secondary antibody binding (Servicebio, China), DAB (Beyotime, China) staining, hematoxylin (Beyotime, China) staining for 1 min, immersed in hydrochloric acid alcohol, rinsed with running water, dried, and sealed with neutral resin. The tissue slices were observed under a low power microscope, and divided into weak positive, medium positive and strong positive. Light yellow represented the weak positive (Score 1), brownish-yellow signified the medium positive (Score 2), and brownish denoted the strong positive (Score 3). First of all, after observing the tissue slices under a low power microscope in the whole field, three fields with different staining intensity were selected for interpretation under the high power microscope. If the protein is localized in the nucleus, 100 cells were counted randomly in each field, and the percentage of positive cells in the 100 cells was recorded as X1%. By the same principle, the percentage of positive cells in the other two fields was calculated as X2% and X3%, and the final staining positive rate is the average of X1%, X2%, and X3%. If the protein is localized in cytoplasm or membrane, three different staining intensity fields were selected to estimate their positive rates and average values.
Immunofluorescence
Briefly, the tissue slices were placed in an oven (Yiheng Scientific Instrument Co., Ltd., China) and baked at 63 °C for 1 h. Deparaffinization was carried out in a fully automated staining machine (LEICAST5020, LEICA, Germany). The deparaffinization time and method were the same as before. The citrate acid antigen retrieval solution (Servicebio, China) was diluted to the working solution. The microwave (Galanz, China) was adjusted to high power for 3 min to boil, and then the slides were placed in the microwave. The microwave power was adjusted to low heat for continued antigen retrieval for 15–20 min. Hydrogen peroxide (Agilent Technologies, China) was applied to the slides for 10 min. Afterward, the slides were then washed with TBST (Servicebio, China), removed and placed in a wet box. Blocking buffer was added, and the slides were incubated for 10 min. The diluted primary antibody working solution (CD8, GB15068; Servicebio, China; IFN-γ, CPA1575, Cohesion Biosciences, UK) was added. Secondary antibody (HRP-labeled IgG, Servicebio, China) was added, and the slides were incubated at room temperature for 10 min. Subsequently, opal dye diluent (PerkinElmer, USA) was added, and the slides were incubated at room temperature for 10 min. Subsequent staining procedures were repeated until all markers were labeled. DAPI working solution (Servicebio, China) was added. Finally, Scanning was then performed (3DHISTECH, Hungary).
Statistical method
Analyses were conducted utilizing SPSS (version 20.0). Graphical representations were created using GraphPad Prism (version 8.0) and R (version 3.3.1). P < 0.05 was deemed to indicate statistical significance.
Results
The alterations and Bifidobacterium depletion in the gut microbiota of patients with liver cancer
16S rDNA analysis was performed on fecal samples from 29 healthy individuals and 29 patients with HCC, including 3009028 sequences in total, with 1507023 sequences in the HC group and 1502005 sequences in the HCC group. The average length of the sequences was 408.2779395 for the HC group and 412.5553846 for the HCC group, respectively. The Good’s coverage indices for the HCC and HC groups were 99.865% ± 0.0414% and 99.866% ± 0.0462%. At the family level, the composition of intestinal microbiota between the two groups was shown in Fig. 1b. The abundance of Firmicutes and Actinobacteriota were increased in the HC group compared with the HCC group, while the Bacteroidota and Proteobacteria were increased in the HCC group compared to the HC group (Fig. 1c). At the family level, the abundance of Lachnospiraceae, Ruminococcaceae and Bifidobacteriaceae was higher in the HC group compared to the HCC group, while Streptococcaceae and Enterobacteriaceae were higher in the HCC group compared with the HC group (Fig. 1d). At the genus level, the abundance of Blautia, Bifidobacterium, Faecalibacterium, Fusicatenibacter, and Eubacterium_hallii_group were higher in the HC group than the HCC group, while Streptococcus, Ruminococcus_torques_group, and Bacteroides were less in the HC group compared with the HCC group (Fig. 1e). Notably, the sob and ace indices in the HC group were significantly lower than those in the HCC group, indicating that the gut microbial richness in the HC group was significantly lower than that of the HCC group. However, the smithwilson and simpsoneven indices in the HC group were higher than those in the HCC group (Fig. 1f–i). PCoA analysis revealed that PC1 was 12.52% and PC2 10.83% at the OTU level (R2 = 0.0557, P = 0.001, Fig. 1j). Using R language (version 3.3.1) and the vegan package, heatmaps and hierarchical clustering analysis were used at the genus and phylum levels (Fig. 1k, l). We found the significant differences in gut microbiota between the HC and HCC groups, such as Lactobacillus, Klebsiella, and Bifidobacterium. Furthermore, to analyze the changes at multiple levels, LEfSe multi-level tree and LDA result discrimination tables (Fig. 2a, b) were drawn, and LDA value > 3 was used to measure the impact of species on the differences. We found that gut microbiota with the significant changes in the HC group (Fig. 2b). These results indicate the differences in gut microbiota between the HC and HCC groups. Furthermore, we found that Bifidobacterium and Ruminococcaceae significantly higher in the HC group, while Lactobacillus and Prevotellaceae were significantly higher in the HCC group (Fig. 2c). We also found the significant differences in Bifidobacterium_pseudocatenulatum_DSM_20438__JCM_1200__LMG_10505 (Fig. 2d) and Bifidobacterium_longum (Fig. 2e).
a LEfSe multilevel species level tree from the phylum to species level (LDA > 3). b LDA discrimination result table (LDA > 3). The differences in gut microbiota at the family level (c), and species level about Bifidobacterium. pseu (d) and Bifidobacterium. long (e). The date (d, e) were presented as mean ± s.d. n = 29 per group.
Isobutyric acid is associated with Bifidobacterium between the patients with liver cancer and healthy individuals
To investigate the relationship between gut microbiota and their associated metabolites, we identified 2173 metabolites in positive ion mode and 1319 in negative ion mode. In the Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) for cations, the variance explained by orthogonal component 1 was 10.20%, and by component 1 was 6.62% (Fig. 3a), with Q² values of −0.4671 and 0.593, respectively (Fig. 3b). For anions, orthogonal component 1 explained 7.36% of the variance, and component 1 explained 5.98% (Fig. 3c), with Q² values of -0.3503 and 0.601 after permutation testing (Fig. 3d). A volcano plot analysis revealed significant differences between the R and NR groups, with 662 cations up-regulated and 34 down-regulated, along with 304 anions up-regulated and 51 down-regulated in the NR group (Fig. 3e, f). Isobutyric acid was identified as a significantly differential metabolite (P < 0.01, Fig. 3g). Further correlation analysis demonstrated a strong positive correlation between g_Bifidobacterium and isobutyric acid (P < 0.001, Fig. 3h).
Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA, a) and permutation testing analysis (b) of positive ion mode and OPLS-DA analysis (c) and permutation testing analysis of negative ion mode (d) in metabolites between the two groups. HCC Hepatocellular Carcinoma, HC Healthy control. The difference in the metabolites using positive (e) and negative ion mode (f). g The changes in the metabolic between the two groups (VIP > 1, P < 0.05). h Correlation analysis between differential metabolites and intestinal microbes. n = 29 per group.
Bifidobacterium or isobutyrate administration with immunotherapy significantly suppress tumor growth in mice with liver cancer
To investigate the impact of the gut microbiota and metabolites on immunotherapy, we found that the tumor volume in mice treated with HC feces was significantly decreased than with HCC feces group. The tumor volume in the HC feces combined αPD-1 treatment group was significantly decreased than that in the HCC feces group and in the HCC combined αPD-1 treatment group (Fig. 4b). Furthermore, In AVMN mice, which were deprived of gut microbiota, αPD-1 treatment failed to suppress tumor growth, as the tumor volume remained the same as in AVMN mice that did not receive αPD-1 treatment, effectively rendering the treatment ineffective. However, the combination of Bifidobacterium gavage and αPD-1 treatment significantly reduced tumor volume compared to either αPD-1 treatment or Bifidobacterium treatment alone (Fig. 4c). Furthermore, in animal experiments using Bifidobacterium or the related metabolite S.I. in combination with αPD-1, it was observed that the effect of S.I. was comparable to Bifidobacterium treatment: AVMN mice treated with S.I. gavage showed significantly reduced tumor volumes compared to untreated AVMN mice. When S.I. gavage was combined with αPD-1 treatment, tumor volumes were significantly smaller than those in the groups receiving either αPD-1 treatment or S.I. alone (Fig. 4d). These results indicate that following fecal transplantation from HCC or HC donors into AVMN mice, different tumor growth rates were observed. In combination with αPD-1 treatment, mice with HC fecal transplants showed slower tumor growth, highlighting the role of gut microbiota in immunotherapy. Additionally, the ineffectiveness of αPD-1 treatment in gut microbiota-deprived liver cancer mice further underscores the critical role of gut microbiota in αPD-1 therapy. The reduction in tumor volume following treatment with Bifidobacterium or S.I. indicates that both Bifidobacterium and the related metabolite S.I. can enhance the efficacy of liver cancer immunotherapy. Next, H&E staining showed that there were more tumor cells in the HCC group than in the HC group, the same as in the HCC immunotherapy group compared with the HC immunotherapy group. Tumor cells were significantly reduced in the combined Bifidobacterium or S.I immunotherapy group compared with the untreated group (Fig. 4e). In addition, to determine tumor cell proliferation, Ki67 staining was used and showed that the AVMN group and αPD-1 treatment had more positive cells than other groups. Positive cells in the HCC group were more than that in mice treated with the HC faces, or HCC combined immunotherapy group, and we found the HCC faces combined αPD-1 therapy had the more positive cells than the HC faces combined αPD-1 therapy. Compared with those treated with Bifidobacterium or S.I alone, the Bifidobacterium or S.I combined with αPD-1 group had a reduced number of positive cells (Fig. 4f). Furthermore, through Cyclin D1 and PARP immunohistochemical staining, we found that the Cyclin D1 or PARP staining positive cells in the HCC group were higher than in the HC group, and higher in the HCC immunotherapy group than in the HC immunotherapy group. After treatment with Bifidobacterium or S.I, the positive cells were reduced, and the Bifidobacterium or S.I combined with αPD-1 treatment had the lowest positive cells (Supplementary Fig. 1a, b).
a The process of animal experiment. 6-week-old C57BL/6J male mice were acclimated for one week. On day 0, tumor cells were injected. From day −7 to day 2, AVMN (1 mg/mL ampicillin, 1 mg/mL neomycin, 1 mg/mL metronidazole, and 0.5 mg /mL vancomycin, for drink) was administered. From day 4 to day 16, Fecal microbiota transplantation (FMT, 50 mg per mouse), Bifidobacterium (Bif, 1–2 × 108 CFU), isobutyrate (150 mM for drink), and/or αPD-1 therapy (200 μg per mouse) were conducted. On day 18, the mice were sacrificed for sampling. b–d The changes in the tumor volume among all groups. n = 8 per group. e Tumor tissues stained with H&E. f Tumor tissues stained with Ki67. g–i The changes in the IFN-γ value in the serum among all groups. n = 8 per group. One-way ANOVA test was used.
Bifidobacterium or isobutyrate enhance IFN-γ+CD8+T cell counts in the tumor immune microenvironment and increase serum IFN-γ levels in mice with immunotherapy
Many studies have reported that tumor immunity is mainly regulated by IFN-γ27,28. To investigate whether the Bifidobacterium or relevant metabolites mediate immune responses, we found that serum IFN-γ level in the HC group treated with αPD-1 was significantly higher than that in the HC group, HCC group, and HCC combined with αPD-1 treatment group. Additionally, IFN-γ level in the HC group was significantly higher than that in the HCC group, and HCC mice treated with αPD-1 had the higher value than those untreated with αPD-1 (Fig. 4g). Furthermore, the IFN-γ level in mice treated with Bifidobacterium combined with αPD-1 was also significantly higher compared with mice treated with none, Bifidobacterium, or αPD-1. The Bifidobacterium alone treated group had the higher level than that in the untreated group (Fig. 4h). Similarly, the IFN-γ level in the S.I combined with αPD-1 treated group was than significantly higher compared with mice in the untreated group, S.I or αPD-1 alone treated group (Fig. 4i). Mice treated with Bifidobacterium or S.I alone had higher IFN-γ levels than mice treated with αPD-1 alone, indicating that the gut microbiota and related metabolites play an important role in the effectiveness of immunotherapy.
Based on the differences in serum IFN-γ levels, we speculated that the gut microbiota or metabolites may mediate immune activation effects in immunotherapy. CD8+T cells are the main cells producing IFN-γ in vivo29. Next, to further determine whether there are changes in IFN-γ+CD8+ T cell infiltration in the TME among the groups, immunofluorescence staining for CD8+ and IFN-γ was performed, and we found that the percentage of IFN-γ+CD8+T cells was significantly more in the HC group than in the HCC group, more in the HC or HCC combined αPD-1 therapy than in the HC or HCC alone treatment, and more in the HC combined immunotherapy group than in the HCC combined group (Fig. 5a; Supplementary Fig. 2a). Finally, we found that the percentage of IFN-γ+CD8+T cells in the Bifidobacterium treatment was significantly more than that in the AVMN alone immunotherapy group, more in the Bifidobacterium combined immunotherapy group than in the Bifidobacterium alone treatment group (Fig. 5b), more in the S.I treatment group than in the AVMN alone immunotherapy group, and more in the S.I combined immunotherapy than in the S.I or αPD-1 treatment (Fig. 5c; Supplementary Fig. 3a). We found that changes in the IFN-γ level were generally consistent with the above trends in CD8+T cells among the groups.
a–c Statistical diagram of IFN-γ+CD8+T cell immunofluorescence staining experiment in each group. n = 3 per group. d In vitro experimental procedure. Following treatment of CD8+T cells with 0, 1.5, 3, 6, and 12 mM S.I for 24 (e), 48 (f), and 72 h (g), cells were washed with PBS, then co-cultured non-contact with Hepa1-6 cells for 24 h, and the concentrations of IFN-γ in the culture medium were quantified. n = 3 per group. h After treating CD8+T cells with 0, 1.5, 3, 6, and 12 mM S.I for 24, 48, and 72 h, washing with PBS, and co-culturing with Hepa1-6 cells for 24 h, CCK-8 was used to assess tumor cell proliferation. Statistical analysis was performed on the data, n = 6 per group. i CD8+T cells were treated with PBS or 6 mM S.I for 48 h, washed with PBS, and then co-cultured non-contact with Hepa 1-6 cells (pre-adhered for 12 h) for 24 h. Western blot analysis was performed to detect JAK1/STAT3 pathway proteins in CD8+T cells or Hepa 1-6 cells. j Western blot images of JAK1/STAT3 pathway proteins in Hepa1-6 cells. k Western blot images of JAK1/STAT3 pathway proteins in CD8+T cells. l After treating CD8+T cells with PBS or 6 mM S.I for 48 h, washing with PBS, and co-culturing with Hepa 1-6 cells treated with JAK1 plasmid (pre-adhered for 12 h) for 24 h, Western blot analysis was performed to evaluate the expression of JAK1/STAT3 pathway proteins in Hepa1-6 cells. n = 3 per group. The date were presented as mean ± s.d. One-way ANOVA test was used.
Suppression of JAK1/STAT3 pathway in the tumor immune microenvironment after Bifidobacterium or isobutyrate combined with immunotherapy
Studies have found activation of the JAK1/2 kinase pathway in melanoma cells lacking IFN-γ signaling, suggesting a potential link between IFN-γ levels and the JAK1 pathway30. Therefore, we further confirmed through in vitro experiments the effect of the Bifidobacterium-related metabolite isobutyrate on the JAK1 pathway in CD8+T cells and tumor cells. We found that IFN-γ+CD8+T cells and IFN-γ levels in the serum and tissues were altered after Bifidobacterium or S.I combined with αPD-1 treatment, indicating that immune responses may be induced by S.I. In vitro experiments showed that the Bifidobacterium-associated metabolic product S.I can induce IFN-γ secretion from CD8+T cells after 48 h of treatment with 6 mM, leading to a reduced proliferation rate of liver cancer cells. This confirms the direct activation effect of S.I on CD8+T cells (Fig. 5d–h) while downregulating the JAK1/STAT3 pathway in tumor cells without affecting CD8+T cells (Fig. 5j, k). Finally, experiments with OE-JAK1 overexpressing tumor cells validated that S.I downregulates p-JAK1, p-STAT3, and cyclin D1 within the JAK1/STAT3 pathway (Fig. 5l). Next, to investigate the JAK1/STAT3 pathway in vivo, immunohistochemical staining was performed on tumor tissues, and we found that there were no significant changes in JAK1 and STAT3 levels among the groups (Fig. 6a–c, g–i; Supplementary Figs. 4a, 6a). However, we observed significant increases in p-JAK1 expression in the HCC group compared with the HC group, in the HCC combined with αPD-1 group compared with the HC combined with αPD-1 group. Besides, P-JAK1 expression was significantly higher in the αPD-1 group compared with the S.I combined with αPD-1 group or Bifidobacterium combined with αPD-1 group (Fig. 6d–f; Supplementary Fig. 5a). Similarly, p-STAT3 expression was significantly increased in multiple comparisons. When compared with the HC group, p-STAT3 expression was higher in the HCC group. Additionally, the HCC group had higher p-STAT3 expression compared to HCC immunotherapy group. Furthermore, The AVMN group also had the higher p-STAT3 expression compared to the Bifidobacterium combined with αPD-1 group, and the Bifidobacterium group. Finally, the αPD-1 group had the higher p-STAT3 when compared with the Bifidobacterium or S.I combined with αPD-1 group, and the S.I group alone (Fig. 6j–l; Supplementary Fig. 7a).
The levels of intestinal Bifidobacterium and isobutyrate in responders to liver cancer immunotherapy were significantly higher than those in non-responders
To confirm the clinical relationship between Bifidobacterium and isobutyrate with the response to immunotherapy, we conducted 16S rDNA sequencing and metabolomic analysis on fecal samples from patients who responded (R) or did not respond (NR) to αPD-1 therapy (Fig. 7a). The results showed that compared to the NR group, the R group had significantly higher α-diversity (Shannon index, P < 0.01, Fig. 7b) and significantly altered β-diversity (PCA, R = 1.00, P < 0.01, Fig. 7c), indicating a substantial remodeling of gut microbiota in the two groups. Furthermore, the dysbiosis index was significantly higher in the NR group compared to the R group (Fig. 7d). Additionally, analysis revealed a significantly increased abundance of Bifidobacterium in the feces of patients in the R group compared to the NR group (Fig. 7e). Metabolomic analysis using OPLS-DA (Fig. 7f) and permutation testing (R2X = 0.99, R2Y = 0.99, Q2 = 0.97, Fig. 7g) further demonstrated significant differences in metabolic profiles. Notably, isobutyrate levels were significantly higher in the feces of the R group compared to the NR group (Fig. 7h). Correlation analysis showed a significant positive correlation between Bifidobacterium abundance and isobutyrate levels in the gut microbiota of patients undergoing liver cancer immunotherapy (r = 0.667, P < 0.05, Fig. 7i). These findings suggest that assessing Bifidobacterium abundance or isobutyrate levels in the gut microbiota may serve as predictive biomarkers for the response to immunotherapy in liver cancer patients. This provides a potential clinical approach for pre-treatment evaluation and intervention to enhance the efficacy of immunotherapy in liver cancer.
a The process of experiment. Fecal samples from responders and non-responders to αPD-1 immunotherapy for liver cancer were collected for 16S rDNA and metabolomic analyses. b Shannon index analysis at the OTU level. c PCoA analysis at the OTU level. d Microbial Dysbiosis Index (MDI) analysis. e Statistical analysis of Bifidobacterium abundance between the two groups. f OPLS-DA of metabolomic data between the two groups. g Permutation testing analysis of metabolomic data between the two groups. h Analysis of isobutyric acid levels between the two groups. n = 6 per group. i Correlation analysis between Bifidobacterium abundance and isobutyric acid levels, n = 12 per group, Pearson test used. For (e, h), data are presented as mean ± s.d., and unpaired t-tests were used. R responders, NR non-responders.
Discussion
Nowadays, ICIs has significantly improved the efficacy of HCC, and HCC patients who respond to ICIs show prolonged survival. However, some patients who initially respond to ICIs may develop resistance. The potential reasons for HCC patients’ resistance to ICIs are complex. Among them, recent studies have shown that the immunotherapy were regulated by shaping gut microbiota in the host15,31,32,33,34. However, how the gut microbiota regulates anti-tumor immunity is still not fully understood. Therefore, a thorough understanding of gut microbiota in HCC treated with ICIs holds significant implications for effective treatment strategies that could benefit more patients and improve prognosis35,36. In our study, we first observed the significant changes in the sob, ace, smithwilson, and simpsoneven indices in clinical fecal samples compared to healthy controls. Notably, the sob and ace indices were significantly lower in the HCC group compared to the HC group, indicating a significantly lower richness in HCC patients. Conversely, the smithwilson and simpsoneven indices were higher in the HC group than in the HCC group, indicating a significantly higher evenness in healthy controls. Previous reports on the changes in α-diversity between HCC patients and healthy controls have been inconsistent. For example, Zhi-gang Ren et al. have found that no significant differences in shannon, simpson, or invsimpson indices between HC and HCC patients at early-stage in gut microbiota37. Futhermore, Zheng et al. reported no differences in ace, chao, simpson, or shannon indices between HC and HCC patient38. In our study, the changes in the sob, ace, smithwilson, and simpsoneven indices suggest significant alterations in α-diversity between HC and HCC groups. Furthermore, PCoA analysis at the OTU level provided the distinct clustering on gut microbiota between the two groups, confirming the altered gut microbiota in the HCC group. Subsequently, we observed significant reductions in Bifidobacterium_pseudolongum and Bifidobacterium_longum in HCC group. This finding is consistent with the report by Song Q et al., who associated Bifidobacterium_longum with non-alcoholic fatty liver disease-associated hepatocellular carcinoma (NAFLD-HCC)39.
To investigate the link between gut microbtoia and metabolites on the fecal samples, we first discovered that a strong correlation between Bifidobacterium and isobutyrate acid depletion in HCC patients. Xiang Zhang et al. found that cholesterol-induced NAFLD-HCC is associated with gut microbiota dysbiosis. There is a notable clustering on gut microbiota composition across different stages in HCC, from steatosis to fatty hepatitis to HCC, and Mucispirillum, Desulfovibrio, Anaerotruncus, and Desulfovibrionaceae abundances were increased. However, Bifidobacterium was absent after mice fed with a high-fat/high-cholesterol (HFHC) diet. Dietary cholesterol alters the metabolisms induced by gut bacteria, leading to increased taurine-conjugated bile acids and decreased 3-indolepropionic acid40. The metabolites produced by gut microbiota can enter the liver via the portal circulation and influence immune environment in the liver as an indirect mechanism by which gut microbiota affects HCC development41. Research has shown that the gut microbiota synthesizes many metabolites, and some may influence cancer development and systemic immune responses42,43. Studies demonstrated that bacterial extracts from HCC patients can induce an immunosuppressive T-cell phenotype when added to peripheral blood mononuclear cells (PBMCs) from healthy individuals in vitro, suggesting a mechanism of action for microbial metabolites and gut ecological imbalances promote the immunosuppressive environment44. SCFAs are effective immune regulatory properties and the most abundant in the colon, which are produced by bacterial fermentation from dietary fiber45. Research has found that butyrate can induce more CD8+T cells activation46,47. In our study, we observed significantly higher isobutyric acid levels in the feces from HCC patients compared to healthy individuals. However, the effects of isobutyric acid on immunomodulatory in HCC have not been reported.
Due to the complex interaction between gut microbiota and the host, animal models in vivo were conducted to reveal the mechanism of gut microbiota and metabolites on immunotherapy. In our study, we found that more CD8+T cells appeared in Bifidobacterium or S.I combined with αPD-1 treatment group in the TME, highlighting the important role of Bifidobacterium and Bifidobacterium-related metabolites in immunotherapy. Moreover, serum IFN-γ level was were measured in multiple groups and found that the changes in CD8+T cell counts in tumor tissue among groups were consistent with serum IFN-γ levels, suggesting that CD8+T cells may exert anti-cancer effects through IFN-γ. Hong-xing Shen et al. found activation of the JAK1/2 kinase pathway in IFN-γ signaling-deficient melanoma cells, indicating a potential link between IFN-γ level and the JAK1 pathway30. Also, JAK can induce STAT3 target genes expression, which encode proteins that drive tumor proliferation48. The JAK/STAT3 signaling also plays a crucial role in the growth and development of various cancers by influencing the TME and anti-tumor immunity. In order to investigate the JAK/STAT3 signaling pathway in the TME, immunohistochemistry was used and found mice treated with Bifidobacterium or S.I combined with αPD-1 treatment resulted in less p-JAK1 and p-STAT3 expression in the TME. These results indicate that Bifidobacterium downregulates phosphorylated JAK/STAT3 proteins through S.I in the host, leading to decreased tumor cell proliferation and volume reduction. Studies have revealed that the JAK/STAT3 signaling pathway drives tumor cell proliferation, survival, invasion, and metastasis while suppressing anti-tumor immune responses, often correlating with poor patient prognosis49,50,51,52. Therefore, targeting the JAK/STAT3 pathway in cancer patients holds promise for therapeutic benefits by directly inhibiting tumor cell growth and stimulating anti-tumor immunity53. STAT3 exhibits the strongest correlation with promoting tumor growth and immunosuppression54. The levels of phosphorylated and/or activated STAT3 have been shown to correlate with poor clinical outcomes in several types of cancer55.
In summary, in the HCC immunotherapy, Bifidobacterium in the gut hydrolyzes and ferments polysaccharides into S.I, which activates CD8+T cells to secrete IFN-γ. These activated cells then accumulate in the TME through the circulation, suppress the JAK/STAT3 signaling pathway in tumor cells, and promote tumor cell apoptosis, ultimately enhancing the efficacy of immunotherapy, which offers theoretical support for intervening in immunotherapy resistance in HCC.
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences (GSA-Human: HRA009217, HRA009417) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human.
References
Cronin, K. A. et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 124, 2785–2800 (2018).
Ellis, P. M., Vella, E. T. & Ung, Y. C. Immune checkpoint inhibitors for patients with advanced non-small-cell lung cancer: a systematic review. Clin. Lung Cancer 18, 444–459.e441 (2017).
Yau, T. et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 23, 77–90 (2022).
Finn, R. S. et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III Trial. J. Clin. Oncol. 38, 193–202 (2020).
Qin, S. et al. A Phase 3 Study of Pembrolizumab versus Placebo for previously treated patients from asia with hepatocellular carcinoma: health-related quality of life analysis from KEYNOTE-394. Liver Cancer 13, 389–400 (2024).
Prieto, J., Melero, I. & Sangro, B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 12, 681–700 (2015).
Kudo, M. Immune checkpoint inhibition in hepatocellular carcinoma: basics and ongoing clinical trials. Oncology 92, 50–62 (2017).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017).
Zhu, A. X. et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 19, 940–952 (2018).
Yau, T. et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J. Hepatol. 71, 543–552 (2019).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Yau, T. et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 30, v874–v875 (2019).
Mazzolini, G. D. & Malvicini, M. Immunostimulatory monoclonal antibodies for hepatocellular carcinoma therapy. trends and perspectives. Medicina 78, 29–32 (2018).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Tanoue, T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565, 600–605 (2019).
Baruch, E. N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371, 602–609 (2021).
Park, J. et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8, 80–93 (2015).
Miller, S. J., Zaloga, G. P., Hoggatt, A. M., Labarrere, C. & Faulk, W. P. Short-chain fatty acids modulate gene expression for vascular endothelial cell adhesion molecules. Nutrition 21, 740–748 (2005).
Schwabe, R. F. & Greten, T. F. Gut microbiome in HCC - Mechanisms, diagnosis and therapy. J. Hepatol. 72, 230–238 (2020).
Schneider, K. M. et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat. Commun. 13, 3964 (2022).
Rajapakse, J. et al. Unveiling the complex relationship between gut microbiota and liver cancer: opportunities for novel therapeutic interventions. Gut Microbes 15, 2240031 (2023).
Huo, R. et al. Altered gut microbiota composition and its potential association in patients with advanced hepatocellular carcinoma. Curr. Oncol. 30, 1818–1830 (2023).
Kang, X. et al. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8(+) T cells. Gut 72, 2112–2122 (2023).
Liu, C. et al. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 100, 1421–1426 (2016).
Edgar, R. C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998 (2013).
Stackebrandt, E. & Goebel, B. M. Taxonomic Note: a place for DNA-DNA reassociation and 16s rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44, 846–849 (1994).
Cao, L. L. et al. Multivalent tyrosine kinase inhibition promotes T cell recruitment to immune-desert gastric cancers by restricting epithelial-mesenchymal transition via tumour-intrinsic IFN-γ signalling. Gut 72, 2038–2050 (2023).
Boulch, M. et al. Tumor-intrinsic sensitivity to the pro-apoptotic effects of IFN-γ is a major determinant of CD4(+) CAR T-cell antitumor activity. Nat. Cancer 4, 968–983 (2023).
St Paul, M. & Ohashi, P. S. The roles of CD8(+) T cell subsets in antitumor immunity. Trends Cell Biol. 30, 695–704 (2020).
Shen, H. et al. Selective suppression of melanoma lacking IFN-γ pathway by JAK inhibition depends on T cells and host TNF signaling. Nat. Commun. 13, 5013 (2022).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Vétizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Pu, X., Wu, L., Su, D., Mao, W. & Fang, B. Immunotherapy for non-small cell lung cancers: biomarkers for predicting responses and strategies to overcome resistance. BMC Cancer 18, 1082 (2018).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Ren, Z. et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 68, 1014–1023 (2019).
Zheng, R. et al. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med. 9, 4232–4250 (2020).
Song, Q. et al. Bifidobacterium pseudolongum-generated acetate suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma. J. Hepatol. 79, 1352–1365 (2023).
Zhang, X. et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 70, 761–774 (2021).
Shetty, S., Lalor, P. F. & Adams, D. H. Liver sinusoidal endothelial cells - gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 15, 555–567 (2018).
Levy, M., Blacher, E. & Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 35, 8–15 (2017).
Zitvogel, L., Daillère, R., Roberti, M. P., Routy, B. & Kroemer, G. Anticancer effects of the microbiome and its products. Nat. Rev. Microbiol. 15, 465–478 (2017).
Behary, J. et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 12, 187 (2021).
Tan, J. K., McKenzie, C., Mariño, E., Macia, L. & Mackay, C. R. Metabolite-Sensing G Protein-Coupled Receptors-Facilitators of Diet-Related Immune Regulation. Annu. Rev. Immunol. 35, 371–402 (2017).
He, Y. et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 33, 988–1000.e1007 (2021).
Luu, M. et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 12, 4077 (2021).
Chang, Q. et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia 15, 848–862 (2013).
Kusaba, T. et al. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol. Rep. 15, 1445–1451 (2006).
Chen, Y. et al. STAT3, a poor survival predicator, is associated with Lymph Node Metastasis from Breast Cancer. J. Breast Cancer 16, 40–49 (2013).
Macha, M. A. et al. Prognostic significance of nuclear pSTAT3 in oral cancer. Head. Neck 33, 482–489 (2011).
Ludwig, H., Nachbaur, D. M., Fritz, E., Krainer, M. & Huber, H. Interleukin-6 is a prognostic factor in multiple myeloma. Blood 77, 2794–2795 (1991).
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Yu, H., Pardoll, D. & Jove, R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009).
Cimica, V., Chen, H. C., Iyer, J. K. & Reich, N. C. Dynamics of the STAT3 transcription factor: nuclear import dependent on Ran and importin-β1. PLoS ONE 6, e20188 (2011).
Acknowledgements
This work was supported by Fujian Province Science and Technology Innovation Joint Fund Project (2024Y9591), the National Natural Science Foundation of China (82102468), the Natural Science Foundation of Fujian Province (2022J011043, 2021J01439, 2023J011298, 2023J011244), Excellent youth project of Fujian Cancer Hospital (2021YNYQ09), Startup Fund for scientific research, Fujian Medical University (2022QH1150), and Fujian Science and Technology Innovation Joint Fund (2021Y9217). We would like to express our gratitude to the Laboratory Animal Center of the Fujian Provincial Center for Disease Control for providing the animals and facilities essential for this research.
Author information
Authors and Affiliations
Contributions
R.H., Y.C., and Z.Z.X. designed the experiments; J.Y.G., Z.X., H.Y.X., S.H.X., M.Y.C., and Z.S.C. collected clinical samples; R.H., Q.G.X., Y.Q.Y., and Y.J.L. performed animal models; G.J.S., Y.P.X., Z.L.C., Y.Y.L., W.T.X., J.W., and S.J.H. conducted experiments in vitro; Y.L.C. and P.S. analyzed and interpreted the data; R.H. and K.R.Y. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huo, R., Xu, Qg., You, Yq. et al. Bifidobacterium boosts anti-PD-1 effectiveness through JAK pathway in hepatocellular carcinoma. npj Precis. Onc. 9, 251 (2025). https://doi.org/10.1038/s41698-025-00960-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-00960-3