Abstract
Recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) is an aggressive disease with limited predictive biomarkers, often leading to ineffective treatments and unnecessary toxicity. Circulating tumor DNA (ctDNA) provides a promising real-time, non-invasive tool for monitoring disease activity. In this study, we analyzed 137 plasma samples from 16 patients with R/M HNSCC receiving immune checkpoint blockade (ICB), using a tumor-informed, highly sensitive next-generation sequencing assay (RaDaR, NeoGenomics). Serial ctDNA monitoring was performed at baseline and throughout treatment, and its association with clinical outcomes, including disease control, three-year overall survival (OS), and progression-free survival (PFS), was evaluated through univariable and multivariable analyses. ctDNA negativity during treatment was significantly associated with improved disease control (OR 21.7, 95% CI 1.86–754.88, p = 0.0317), three-year OS (HR 0.04, 95% CI 0.00–0.47, p = 0.0103), and PFS (HR 0.03, 95% CI 0.00–0.37, p = 0.0057). Early increases in ctDNA levels correlated with disease progression. Our findings suggest that ctDNA negativity, regardless of PD-L1 expression, ICB regimen, or line of therapy, is a strong predictor of favorable outcomes in R/M HNSCC.
Similar content being viewed by others
Introduction
Recurrent and/or metastatic head and neck squamous cell carcinoma (R/M HNSCC) is an aggressive cancer. Only a minority of patients benefit from the current standard-of-care options, including first-line therapies, which for most patients consist of immune checkpoint blockade (ICB) with or without concurrent cytotoxic chemotherapy. Pembrolizumab, either alone or combined with chemotherapy, significantly improves survival compared to cetuximab plus chemotherapy in R/M HNSCC patients, in particular those with a PD-L1 combined positive score (CPS) ≥ 1 (median 12.3 months versus 10.3 months) and CPS ≥ 20 (median 14.9 months versus 10.7 months). However, objective response rates (ORR) remain suboptimal. While CPS correlates with response (e.g., ORR of 4.5% versus 14.5% for monotherapy, and 31% versus 34% for combination therapy in CPS <1 versus CPS 1–19 subgroups), the vast majority (66–95%) of patients fail to respond to ICB-based therapy1,2,3. Unsurprisingly, the prognosis remains poor, highlighting a clear unmet need to develop biomarker-informed therapeutic approaches for patients with R/M HNSCC3. Despite its clear limitations as a predictive biomarker in R/M HNSCC4, PD-L1 is currently the only FDA-approved predictive biomarker to aid clinical decision-making in R/M HNSCC1. Additionally, as a static biomarker5, PD-L1 is only useful for predicting outcomes at the time of treatment initiation and cannot assist in clinical decision-making once a patient has started treatment. This underscores the need for dynamic, non-invasive biomarkers that can provide ongoing insights into disease status, enabling clinicians to tailor treatment decisions in real time.
Patients with R/M HNSCC resistant to ICB and surgically salvageable disease who either previously declined surgical approaches or, due to cytoreduction from ICB-based therapy, have lesion(s) that have become amenable to surgical resection, may experience better local control with the application of salvage local therapy4,6. A non-invasive biomarker measured serially during therapy has the potential to augment the assessment of disease status to aid clinicians in personalizing the addition of localized treatment modalities (i.e., surgery or radiotherapy) to systemic therapy, moving on to a new line of systemic therapy, or potentially providing a treatment holiday in the event of positive response to treatment.
Liquid biopsy with tumor-informed ctDNA is a modality to monitor disease that is complementary to routine imaging methods currently used for assessing R/M HNSCC treatment response. This minimally invasive, real-time approach detects circulating tumor DNA (ctDNA) in clinically accessible biofluids, offering potential utility for treatment response assessment in the metastatic setting7,8,9,10,11. ctDNA detection using next-generation sequencing (NGS) has strong predictive and prognostic potential across various solid tumors treated with ICB12,13,14. Specifically, a decrease in ctDNA levels after one cycle of ICB has been associated with improved OS and progression-free survival (PFS) in multiple solid tumors15. Importantly, ctDNA changes after one cycle of ICB have shown strong prognostic potential for both OS and PFS in HNSCC using tumor-naive and tumor-informed ctDNA detection methods11,16. Similar results have been observed in patients with non-small cell lung cancer (NSCLC)17,18,19, colorectal cancer15, urothelial cancer20, and human papillomavirus (HPV)-associated cancers such as cervical21 and anal squamous cell carcinoma22.
Beyond estimating survival, ctDNA detection has also shown promising results in predicting disease progression in patients with HNSCC undergoing ICB. In a cohort of fourteen patients, tumor-informed ctDNA changes correlated with disease status, with decreasing ctDNA levels during treatment for responders and increasing levels for non-responders16. However, the approaches utilized in this study had a limit of detection of 0.01% (measured in estimated variant allele frequency [%eVAF]), limiting the ability to detect microscopic residual disease. ctDNA assays with improved sensitivity are needed to improve the accuracy of ctDNA monitoring. These data underscore the potential for measurement of early on-treatment ctDNA dynamics to augment the estimation of anti-tumor response to ICB for patients with R/M HNSCC.
Because PD-L1 is both a weak predictive biomarker in HNSCC at the time of diagnosis and is not of utility in monitoring response to ICB, there is a critical need to develop and validate novel non-invasive and effective biomarkers. ctDNA detection approaches have shown promise but have been limited in their sensitivity. We therefore elected to perform this prospective single-institution study in which we applied a tumor-informed, tenfold more sensitive (Limit of Detection, LoD95: 0.0011%) NGS liquid biopsy assay23,24 to assess ctDNA dynamics in patients with R/M HNSCC as a biomarker of response to ICB-based therapy. We hypothesized that (1) ctDNA becoming undetectable during treatment would be associated with improved OS and PFS, while (2) persistent ctDNA detection would identify patients who will progress on ICB for R/M HNSCC.
Results
Patient baseline characteristics
One hundred thirty-seven blood samples were collected between January 2021 and December 2022, with a median of 6.5 samples per patient (range: 3–20) from sixteen patients with R/M HNSCC being treated with ICB therapy. The median age was 62 years old (range: 52–91). The oral cavity was the most common anatomic subsite (8/16, 50%), and most patients received pembrolizumab monotherapy (11/16, 69%). Most patients had a CPS ≥20 (11/16, 69%). Among patients with virally-driven tumors, three patients had HPV-associated oropharyngeal carcinoma (OPC), one patient had HPV-associated nasopharyngeal carcinoma (NPC), and one patient had Epstein-Barr Virus (EBV)-associated NPC.
Plasma ctDNA testing results
The overall ctDNA detection rate across all samples was 57% (78/137) (Fig. 1A, B). The median %eVAF was 0.1399 (range: 0.0007–7.0738) (Table S1). Patient baseline characteristics are summarized in Table 1. 88% (14/16) of patients had a pre-ICB plasma sample (considered the baseline sample for this study) available for analysis (Patients 02, 03, 06, 08, 09, 10, 13, 14, 15, 18, 19, 20, 25, 26) with 12/14 (85.7%) being ctDNA positive (Patients 02, 03, 06, 08, 09, 10, 13, 14, 19, 20, 25, 26; median %eVAF: 0.28, range: 0.004–1.8). The two patients with negative baseline ctDNA had regionally or distant metastatic oral cavity SCC: Patient-15 had an enlarging 2.5 × 1.5 cm lung mass, and Patient-18 had a 4.1 × 5.5 × 5.7 cm cystic neck mass.
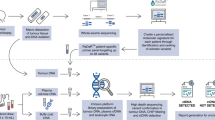
A Pretreatment FFPE tumor tissue from patients with HPV-independent HNSCC undergoing ICB therapy was obtained. DNA was extracted specimens with >20% of tumor component, and WES was performed to identify somatic mutations used to create personalized assays (RaDaR) for each patient. Plasma was obtained before and during ICB treatment, and each plasma sample was assessed for ctDNA detection. B Swimmer plot shows longitudinal monitoring of ctDNA, treatment, and response. CR complete response, PD progressive disease, PR partial response, SD stable disease. Patients 6, 8, 10 and 14 received a combination of immunotherapy plus chemotherapy. Patient 7 received Pembrolizumab plus ALX148 (anti-CD47).
The cohort consisted predominantly of patients belonging to LB-RECIST response Group 1 (detectable ctDNA that remains detectable after therapy; n = 9; Patients 02, 03, 06, 07, 09, 13, 14, 19, 26) or Group 2 (detectable ctDNA that became undetectable after therapy; n = 4; Patients 08, 10, 20, 25). One patient was classified as Group 3 (undetectable ctDNA at baseline that becomes detectable after therapy; Patient-15), and two patients were classified as Group 4 (undetectable ctDNA that remains undetectable after therapy; Patients 18, 23). Additionally, based on the quantitative response criteria categories, most patients showed ctDNA progressive disease (CPD; n = 8; Patients 02, 03, 07, 09, 13, 14, 15, 26), which is defined as an increase of >10% in eVAF or de novo ctDNA detection. The four patients belonging to LB-RECIST Group 2 were defined as having experienced a ctDNA complete response, defined as ctDNA clearance after initial detection25,26 (Table 2). The median follow-up time was 19 months (range: 1.5–42.6). When defining response by RECIST 1.1 or clinical assessment (if RECIST was not evaluable), there were two patients with best ORR of complete response (12%, CR), four patients with partial response (25%, PR), two patients with stable disease (12%, SD), and eight patients with progressive disease (50%, PD) (Table 2). The median time to achieve ctDNA negativity was 2.5 months (range 0.7–20.6), and the median time for ctDNA detection after clearance was 7 months (range 1.3–9.3). Three patients died from progressive disease soon after starting ICB therapy (Patients 09, 13, 26). For the remaining thirteen patients, a median of 1 (range 0 to 5) subsequent line of treatment was received (including systemic therapy and salvage or palliative local therapy) following disease progression on ICB therapy. A median of four ICB infusions were administered (range 2–35), and a median of 13 months (range 1.3–27) elapsed from ICB initiation until death from any cause.
Eight patients (Patients 02, 03, 06, 07, 08, 10, 14, 15) had samples available after stopping ICB. Seven halted ICB due to PD, whereas one patient stopped ICB due to adverse events but showed no evidence of recurrence (Patient-08). Among those who developed PD, three (Patients 03, 06, 15) proceeded to multimodality therapy and demonstrated a decline in ctDNA to undetectable levels. By contrast, the remaining four patients with disease not amenable to salvage local therapy (Patients 02, 07, 10, 14) exhibited an increase in ctDNA levels immediately after stopping ICB. Notably, in the patient who had achieved a complete response from ICB (Patient-08), ctDNA levels remained undetectable following cessation of ICB. Lead time from ctDNA clearance to corresponding imaging response could not be accurately assessed because blood sample collection for ctDNA analysis and imaging assessment only coincided for a small selection of patients.
ctDNA negativity predicts disease control
Among the four patients who experienced a ctDNA CR (LB-RECIST Group 2; Patients 08, 10, 20, 25), one had a RECIST 1.1 CR (Patient-08), and three had RECIST 1.1 PR (Patients 10, 20, 25) (Fig. S1, Table S2). Importantly, those patients experienced ctDNA clearance due to ICB. Two of those four patients (Patients 08, 20) experienced immune-related adverse events (colitis and pneumonitis) after 1 and 1.5 years on ICB, respectively. In univariable analyses, we found that neither ICB regimen, CPS, nor line of therapy were predictive of ctDNA clearance. Early on ICB therapy, the sensitivity and specificity of ctDNA negativity to predict an overall response (CR or PR) were 75% and 100%, respectively. Patients without pre-ICB sample available (n = 2) and with persistently negative ctDNA levels (n = 1) were also included in the analysis. For this analysis, ctDNA was assessed after a median of two infusions (range 1 to 7) and within a mean of 13 days (range –4 to 142) of radiological (n = 14) or clinical (n = 2) confirmation of RECIST 1.1 assessment. Interestingly, two patients (Patients 06, and 19) were classified as having stable disease (SD) despite a positive ctDNA result (Table 3).
We performed logistic regression analyses to assess undetectable ctDNA as a predictor of treatment response ([1] ORR: CR/PR versus SD/PD; and [2] disease control: CR/PR/SD versus PD). In univariable analysis, undetectable ctDNA at any time during the study period (either pre-treatment or on-treatment) was predictive of a 21-fold increase in odds of achieving disease control (CR, PR, or SD) (95% CI 2.04–558.55, p = 0.0236). This association remained significant in multivariable analyses controlling for PD-L1, ICB regimen, and line of therapy (OR 21.7, 95% CI 1.86–754.88, p = 0.0317). To ensure these results were not driven by the two patients in our cohort belonging to LB-RECIST Group 4 (patients who never had detectable ctDNA), we performed a sensitivity analysis by excluding Patients 18 and 23 and observed that this finding persisted (univariable OR 15, 95% CI 1.37–408.3, p = 0.04; multivariable OR 14.5, 95% CI 1.06–560.5, p = 0.07).
Undetectable ctDNA predicts improved overall and progression-free survival
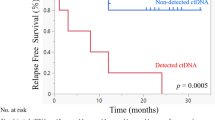
Across all patients, the median OS was 19.9 months, and the median PFS was 2.7 months (Fig. 2A, B) from ICB initiation. In univariable analyses, we found no difference in OS or PFS when stratified by ICB regimen, CPS, recurrence type (locoregional versus distant versus both), primary anatomical site, or virus (HPV or EBV) status. In our cohort, five patients received combined treatment (Patients 06, 07, 08, 10, 14). Four patients received ICB combined with chemotherapy (Patients 06, 08, 10, 14), and one received a combination of ICB and an experimental anti-CD47 antibody therapy (Patient-07). We analyzed the survival and response outcomes for those subpopulations and found no difference among groups for OS (HR 1.87, 95% CI 0.49–7.13; p = 0.35), or PFS (HR 0.93, 95% CI 0.28–3.04; p = 0.91). However, ctDNA negativity was found to be a significant predictor of improved OS and PFS. In univariable analyses, patients who had undetectable ctDNA at any point during the study period, including patients who were always negative during the study period (Patients 18, and 23; LB-RECIST Group 4), had better OS (HR 0.10, 95% CI 0.02–0.48, p = 0.001) and PFS (HR 0.17, 95% CI 0.04–0.69, p = 0.006). In multivariable analyses controlling for CPS, ICB regimen, viral status, and recurrence type, the association remained significant for both OS (HR 0.04, 95% CI 0.00–0.47, p = 0.0103) and PFS (HR 0.03, 95% CI 0.00–0.37, p = 0.0057) (Fig. 2C, D). We again performed a sensitivity analysis to ensure these results were not driven by patients who were always ctDNA negative during the study period by excluding these two patients (Patients 18 and 23) and found that the findings persisted (univariable HR for OS 0.17, 95% CI 0.03–0.86, p = 0.0321; multivariable HR for OS 0.05, 95% CI 0–0.06, p = 0.0175; univariable HR for PFS 0.23, 95% CI 0.06–0.94, p = 0.0411; multivariable HR for PFS 0.05, 95% CI 0–0.61, p = 0.019). Additionally, when limiting our analysis to patients who achieved ctDNA negativity exclusively through ICB, we observed a consistent association with improved outcomes. Three-year overall survival (OS) was 75% compared to 20% in those who did not clear ctDNA (median OS: 28 versus 22 months; p < 0.02, Log-Rank test). Similarly, three-year PFS was 50% versus 0% (median PFS: 9 versus 3 months; p < 0.003, Log-Rank test) (Fig. S2).
A Overall survival for the entire cohort. B Progression-free survival for the entire cohort. C Overall survival based on ctDNA negativity at any point during the study period based on multivariable analysis. D Progression-free survival based on ctDNA negativity at any point during the study period based on multivariable analysis.
ctDNA yielded comparable prognostic capability obtained with imaging-based prediction of OS. When response was determined by radiographic (RECIST 1.1) or clinical (when imaging was unavailable) assessment, an objective response (CR or PR) was associated with improved OS (HR 0.12, 95% CI 0.01–0.98, p = 0.04) while disease control (CR, PR, or SD) trended toward improved OS (HR 0.28, 95% CI 0.07–1.64, p = 0.08).
Early %eVAF increase is a key marker for disease progression
We evaluated the association between changes in ctDNA levels from P1 to P2 (after a median of 1 dose of ICB) and best ORR using penalized logistic regression to account for the small sample size. We assessed various ctDNA dynamics parameters—(1) absolute change in %eVAF, and thresholds of both (2) >20% and (3) >50% change in ctDNA levels from the pre-treatment to first on-treatment measurement after a median of one cycle (data not shown)—and found a strong association between absolute change in ctDNA (increase in ctDNA from P1 to P2) and disease progression at first treatment response assessment after starting ICB (Fisher’s exact test p = 0.0047; Phi coefficient = 0.85) (Fig. 3A). Among patients with an increase in ctDNA levels from P1 to P2 (Patients 02, 03, 09, 13, 14, 15, 25, 26), 87% (7/8) of patients experienced disease progression at their first treatment response assessment. In contrast, none of the patients with a decrease in ctDNA levels (Patients 06, 08, 10, 19, 20) experienced PD at first treatment response assessment. Of note, three patients (07, 18, and 23) were not included in the analysis because either no pretreatment sample was available for analysis or both P1 and P2 samples were negative.
A Distribution of best objective response rate (CR/PR/SD versus PD) stratified by changes in ctDNA levels between P1 and P2. B Forest plot showing that an increase in ctDNA levels from P1 to P2 is independently associated with disease progression, regardless of CPS (0 or <1, 1–19, and ≥20) and treatment regimen (monotherapy versus combination therapy).
To further evaluate the independent association while accounting for potential confounders, we performed multivariable logistic regression using Firth’s penalized likelihood approach. After adjusting for CPS and treatment regimen (ICB vs chemo/ICB), an increase in ctDNA levels from P1 to P2 remained significantly associated with disease progression as best ORR (adjusted OR 28.4; 95% CI 1.74–7613.5; p = 0.016). Neither CPS (adjusted OR 4.65; 95% CI 0.001–3.89; p = 0.37) nor treatment regimen (adjusted OR 1.23; 95% CI 0.003–338.8; p = 0.93) were significantly associated with disease progression (Fig. 3B).
Discussion
This study evaluated the clinical utility of a tumor-informed WES-based liquid biopsy assay in predicting ORR, OS, PFS, and PD in patients with R/M HNSCC receiving ICB-based therapy. Using serial ctDNA monitoring, we found ctDNA negativity to be strongly associated with survival outcomes and disease control, regardless of CPS, ICB regimen, and line of therapy. Furthermore, we found a strong association between an early increase in %eVAF and disease progression, regardless of CPS or treatment regimen.
In our cohort, patients were ctDNA negative through one of three ways: (1) patients who never had detectable ctDNA (LB-RECIST Group 4), (2) after ICB therapy (LB-RECIST Group 2), or (3) following salvage multimodality therapy (i.e., surgery or radiation) after progression on ICB (LB-RECIST Group 2). Having an undetectable ctDNA level conferred both an OS and PFS advantage irrespective of when the transition to becoming ctDNA undetectable occurred, including patients whose ctDNA was undetectable at baseline (OS HR 0.05, PFS HR 0.05). We also found that an increase in %eVAF early on treatment was strongly associated with disease progression at the time of first treatment response evaluation after starting ICB as determined by radiographic or clinical assessment. Taken together, these findings suggest that a tumor-informed liquid biopsy assay may aid clinicians in identifying patients with R/M HNSCC who might benefit from treatment escalation or next-line therapy, although this approach would need to be validated in future prospective studies.
Our study recapitulates findings reported in other solid tumor types, including NSCLC, uveal melanoma, and microsatellite-unstable colorectal cancer15,17,18,27. In these studies, the molecular response, as measured by serial ctDNA monitoring, was associated with radiographic response28. For example, in a phase 2 clinical trial evaluating ctDNA in NSCLC, molecular response to ICB and chemotherapy showed a sensitivity of 82% and a specificity of 75% compared to RECIST 1.1 criteria as the gold standard28. Our data indicated that this ctDNA assay is associated with comparable test characteristics, with a slightly lower sensitivity of 75% and a slightly higher specificity of 100% in HNSCC, noting that these results should be interpreted in the context of a limited sample size. Importantly, in contrast to the cohort presented here, molecular response was assessed in the NSCLC cohort after three cycles of ICB, and the median time to ctDNA negativity was 2.1 months compared with a median of two doses of ICB and 2.5 months in our cohort, respectively.
Acknowledging the limitations of small sample size despite an impressive average number of samples collected per patient, we felt it prudent to verify that the association between ctDNA dynamics and ORR, OS, and PFS was not driven by the small subset of patients whose ctDNA was undetectable for the entire study period (LB-RECIST Group 4; Patients 18 and 23). In this sensitivity analysis, we re-ran all analyses, excluding these two patients, and observed similar results. The reason for undetectable ctDNA in these two patients is not clear but could be related to a low overall disease burden relative to other patients in the study, a low tumor proliferation rate, or low shedding tumor29. Additionally, one patient (Patient-23) had already received seven infusions before the first sample was available, potentially explaining persistent ctDNA negativity. Overall, these data align with prior reports that indicate having undetectable ctDNA at any time (LB-RECIST Groups 2 and 4) is associated with improved survival14,28. Using the LB-RECIST classification system during our analysis helped to ensure a robust and systematic assessment of these data and highlighted the utility of such a classification system to aid in a structured and comprehensive approach to analyzing ctDNA data sets. This is particularly important as ctDNA monitoring becomes even more commonplace in both experimental and standard of care clinical practice. However, as the LB-RECIST classification system has not been prospectively validated, it is important to recognize its limitations. For example, evolving assay sensitivity can bias this classification system as discordant results from the same blood sample can occur when using different methods of ctDNA detection30. Therefore, harmonizing the collection and interpretation of ctDNA data across studies will strengthen the rationale for incorporating ctDNA monitoring into clinical practice across cancer types, disease settings, and treatment regimens.
Based on the results presented here, serial, tumor-informed, WES-based ctDNA monitoring has the potential to provide patients with prognostic information that is more accurate than the summary statistics typically reported by major clinical trials. This approach could also guide clinicians in tailoring subsequent treatment options for patients who fail to achieve undetectable ctDNA during ICB. Importantly, the turnaround time for all samples was 49 days. However, for prospective studies, NeoGenomics has a turnaround time of 30 days for WES plus panel design and qualification and 7–10 days for follow-up timepoints, highlighting the clinical utility of this approach for patients recieving systemic therapy.
Evidence suggests that escalation with multimodality interventions in the absence of molecular response can improve survival outcomes. For instance, although the SABR-COMET trial did not use ctDNA to determine eligibility, it demonstrated that stereotactic ablative radiotherapy in oligometastatic disease confers an overall survival advantage in multiple solid tumor types, including breast, lung, colorectal, and prostate cancers31. Incorporating serial ctDNA monitoring could serve as a valuable tool to better select patients with metastatic disease for local therapies, thereby optimizing treatment strategies and outcomes.
In addition to assessing the binary outcome of detectable/undetectable ctDNA, we also sought to characterize the predictive capability of ctDNA dynamics. We assessed various ctDNA dynamics parameters—absolute change in %eVAF after a median of one cycle, and thresholds of both >20% and >50% change in ctDNA levels from the pre-treatment to first on-treatment measurement (data not shown)—and found an association between an absolute increase in %eVAF early on treatment and disease progression. Our findings—ctDNA response was associated with improved outcomes, and rising ctDNA from P1 to P2 was associated with progressive disease—recapitulate data from Bratman et al. who demonstrated an association between early ctDNA dynamics and treatment response. In that study, decreasing ctDNA from baseline to after 3 cycles of pembrolizumab was associated with an increased chance of objective response of CR or PR (OR 28.74, 95% CI 3.51–253.04) in a large cohort of patients with a mixture of advanced solid tumors (n = 94, HNSCC n = 14)14, while any rise in ctDNA levels during surveillance above baseline was associated with rapid disease progression and poor survival14. Although ctDNA monitoring shows promise as a predictor of PD, it remains an imperfect test. For one patient in this cohort (Patient-20), ctDNA was detected six days after imaging detection of PD, yet it was undetectable 15 days prior to imaging confirmation of PD. This finding underscores the importance of interpreting ctDNA results as complementary to, rather than a replacement for, current imaging approaches, thereby reducing the risk associated with false negative ctDNA results or the inability of even highly sensitive assays to detect rapidly evolving disease progression25,29.
Our study has multiple limitations. First, a limited cohort size and the heterogeneity of the patient population (e.g., ICB regimen, a mixture of virus-associated and virus-independent tumors, CPS), limit the interpretation of our findings. Second, because samples were collected during routine clinical visits, our study did not apply a fixed schedule for sample collection. This limitation should also be interpreted with the context that the optimal timing of serial ctDNA measurements for different systemic therapy regimens (e.g., ICB versus chemotherapy plus ICB) or tumor types is not known29. Third, all clinical data were annotated retrospectively, possibly introducing retrospective bias. Lastly, although we applied a proprietary technology to characterize the serial ctDNA dynamics by defining an absolute change in the abundance of eVAF% as clinically significant, the minimum clinically meaningful change in ctDNA levels between samples in patients with HNSCC has not yet been clearly defined29.
While this study does not establish the definitive clinical utility of personalized ctDNA in R/M HNSCC, our findings support the integration of ctDNA analysis in two key contexts for future research. First, to evaluate the potential benefit of a treatment holiday for patients who achieve ctDNA negativity, and second, to explore the role of either a change in systemic therapy if ctDNA negativity is not achieved, or the transition to multimodality therapy (i.e., radiation and/or surgery) with or without systemic therapy for patients who remain ctDNA-positive but may be candidates for an intensified therapeutic approach aimed at achieving ctDNA clearance.
In summary, using serial ctDNA monitoring, we found ctDNA negativity to be strongly associated with disease control and improved survival, and rising ctDNA between the pre-treatment and first on-treatment timepoints to be associated with progressive disease in patients with R/M HNSCC treated with ICB. This study supports the role of ctDNA as a real-time biomarker to inform future prospective studies aimed at evaluating both treatment intensification and de-intensification approaches to improve survival and patient quality of life.
Methods
Patient recruitment
Patients were eligible for this study if they started treatment for R/M HNSCC (oropharyngeal, oral cavity, nasopharyngeal, sinonasal, laryngeal, or hypopharyngeal anatomical primary sites, as well as unknown primaries) at Massachusetts General Hospital Cancer Center (MGHCC) with systemic anti-neoplastic therapy containing ICB between January 2021 and December 2022. Archival FFPE tumor tissue was reviewed and confirmed by a head and neck pathologist (A.S.F). Sixteen patients were enrolled in this study. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board (DFCI 18–653). Written informed consent was obtained from all patients.
Clinical specimens
Serial blood samples were prospectively collected before, during, and after ICB treatment when blood was being drawn for clinical purposes, whenever possible, between January 2021 and December 2022. Due to the inherent nature of clinical care, blood for ctDNA analysis was not collected on a fixed schedule across all patients in this observational study. Blood samples were drawn in EDTA tubes, and plasma was obtained after two rounds of centrifugation (10 min at 1600 RCF and 3000 RCF). Plasma and buffy coat were then aliquoted and stored at –80 °C. Blood samples collected before and during treatment were available for prospective plasma ctDNA testing, while clinical data were annotated retrospectively following plasma testing completion. ORR was investigator-assessed using RECIST 1.1 or by retrospective review of physical examination data in clinical notes when imaging was unavailable (Table S2). To ensure clear communication of each patient’s ctDNA kinetics, we classified patients according to the liquid biopsy response evaluation criteria in solid tumors (LB-RECIST) proposed by Gouda et al.25 in which four key patterns of ctDNA kinetics can be observed. OS, PFS, and ORR outcomes were evaluated, and patients were followed for clinical outcomes until a censor date of August 16th, 2024.
Tumor-informed ctDNA detection assay (RaDaR) workflow and QC metrics
Formalin-fixed paraffin-embedded (FFPE) archival tissue sections from either tumor biopsies (n = 2) or surgical resection specimens (n = 14) were sent to NeoGenomics Laboratories, Inc. (Durham, NC) and processed for DNA extraction and whole-exome sequencing (WES)32. WES was performed to a median read depth of 306x (range: 112x–607x) (Fig. 1A). Personalized panels were successfully designed for all patients, capturing a median of 48 variants (range: 43–50) and released for plasma ctDNA testing with a median of 45 variants confirmed during panel QC (range: 19–49). Tumor-level key WES quality control metrics, the number of variants in each personalized panel, and those remaining after panel QC, as well as complete panel-specific variant information and their status in both tumor DNA and buffy coat control DNA, are provided in Tables S1–S3, respectively.
Cell-free DNA (cfDNA) was extracted from a median plasma volume of 3.6 mL (1.3–5.3 mL). Personalized panels were applied to serial plasma samples to assess ctDNA status before, during, and after treatment. Control genomic buffy coat DNA was included to identify and filter out confounding germline mutations and variants arising from clonal hematopoiesis of indeterminate potential (CHIP), thus eliminating false positive calls during plasma analysis. cfDNA and buffy coat control DNA were extracted at NeoGenomics Laboratories, Inc. (Durham, NC) using a solid-phase reverse immobilization magnetic bead protocol on a Hamilton Microlab STAR automated platform as previously described32. ctDNA testing was performed using a median of 7100 amplifiable copies (range: 1000–20,000 copies) (Table S1) and a median depth of 233,350 reads per variant per sample (range: 82,597–740,848) (Table S4). The ctDNA status of all 137 samples tested across the 16 patients included in the study is shown in Fig. 1B, alongside additional clinical information.
Statistical analysis
This study assessed ctDNA negativity at any point during treatment as an independent predictor of treatment response and survival. To assess clinical and ctDNA predictors of best ORR according to RECIST 1.1, we used univariable and multivariable logistic regression models to compute odds ratios (OR) with 95% confidence intervals (95% CI). To assess the association between ctDNA and both OS and PFS, Kaplan–Meier analysis was applied using the Log-rank test. We also performed univariable and multivariable Cox regressions and computed hazard ratios (HR) and 95% CI to assess the association between ctDNA negativity at any point during the study and OS and PFS regardless of the cause of ctDNA negativity (ICB or salvage intervention). The association between the increase in ctDNA levels (%eVAF) from timepoint 1 (P1) to timepoint 2 (P2) and disease progression was assessed using Fisher’s Exact test with Phi coefficients. A median of one cycle of ICB was administered in this timeframe (eleven patients received one cycle of ICB before the second ctDNA measurement, two patients received two cycles of ICB before the second ctDNA measurement, and three patients were excluded from this analysis because either they had no pre-treatment samples or never had a sample positive for ctDNA). We additionally performed multivariable logistic regression using Firth’s penalized maximum likelihood estimation. This approach was selected to mitigate the small sample size bias. The multivariable model included ctDNA dynamics (P1-P2: increase versus decrease), CPS (categorized as 0 or <1, 1–19, and ≥20), and treatment regimen (monotherapy versus combination therapy). All values were considered statistically significant if α < 0.05. Analyses were performed using R Statistical Software (v4.3.1, R Core Team 2023), GraphPad (v10.1.2), or Python (v3.11.4). In R, the aod package (v1.3.3) was used to perform logistic regression, and the survival (v3.5.5) and survminer (v0.4.9) packages were used for survival analyses, which included both Kaplan–Meier analysis and Cox proportional hazard model analysis.
Data availability
Data is provided within the manuscript or supplementary information files. The code used in this manuscript is publicly available: https://github.com/druiztorre/ctDNA_HNSCC_ICB_NPJ.git.
Code availability
The underlying code used for this study is publicly available: https://github.com/druiztorre/ctDNA_HNSCC_ICB_NPJ.git.
References
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019).
Mehra, R. et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer 119, 153–159 (2018).
Harrington, K. J. et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J. Clin. Oncol. 41, 790–802 (2023).
Cohen, E. E. W. et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 7, 184 (2019).
Lesterhuis, W. J. et al. Dynamic versus static biomarkers in cancer immune checkpoint blockade: unravelling complexity. Nat. Rev. Drug Discov. 16, 264–272 (2017).
Moon, D. H. & Sher, D. J. Oligometastasis in head and neck squamous cell carcinoma. Int. J. Radiat. Oncol.*Biol.*Phys. 114, 803–811 (2022).
Sivapalan, L. et al. Liquid biopsy approaches to capture tumor evolution and clinical outcomes during cancer immunotherapy. J. Immunother. Cancer 11, e005924 (2023).
Lapin, M. et al. Comprehensive ctDNA measurements improve prediction of clinical outcomes and enable dynamic tracking of disease progression in advanced pancreatic cancer. Clin. Cancer Res. 29, 1267–1278 (2023).
Thompson, J. C., Scholes, D. G., Carpenter, E. L. & Aggarwal, C. Molecular response assessment using circulating tumor DNA (ctDNA) in advanced solid tumors. Br. J. Cancer 129, 1893–1902 (2023).
Liu, Z. et al. Construction of a risk stratification model integrating ctDNA to predict response and survival in neoadjuvant-treated breast cancer. BMC Med. 21, 493 (2023).
Taylor, K. et al. Circulating tumour DNA kinetics in recurrent/metastatic head and neck squamous cell cancer patients. Eur. J. Cancer 188, 29–38 (2023).
Zhang, Q. et al. Prognostic and predictive impact of circulating tumor DNA in patients with advanced cancers treated with immune checkpoint blockade. Cancer Discov. 10, 1842–1853 (2020).
Lee, J. H. et al. Longitudinal monitoring of ctDNA in patients with melanoma and brain metastases treated with immune checkpoint inhibitors. Clin. Cancer Res. 26, 4064–4071 (2020).
Bratman, S. V. et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat. Cancer 1, 873–881 (2020).
Kansara, M. et al. Early circulating tumor <scp>DNA</scp> dynamics as a pan-tumor biomarker for long-term clinical outcome in patients treated with durvalumab and tremelimumab. Mol. Oncol. 17, 298–311 (2023).
Hanna, G. J. et al. Personalized ctDNA for monitoring disease status in head and neck squamous cell carcinoma. Clin. Cancer Res. OF1–OF8 https://doi.org/10.1158/1078-0432.CCR-24-0590 (2024).
Ricciuti, B. et al. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC). J. Immunother. Cancer 9, e001504 (2021).
Thompson, J. C. et al. Serial monitoring of circulating tumor DNA by next-generation gene sequencing as a biomarker of response and survival in patients with advanced NSCLC receiving pembrolizumab-based therapy. JCO Precis. Oncol. 510–524 https://doi.org/10.1200/PO.20.00321 (2021).
Goldberg, S. B. et al. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin. Cancer Res. 24, 1872–1880 (2018).
Powles, T. et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 595, 432–437 (2021).
Kang, Z., Stevanović, S., Hinrichs, C. S. & Cao, L. Circulating cell-free DNA for metastatic cervical cancer detection, genotyping, and monitoring. Clin. Cancer Res. 23, 6856–6862 (2017).
Huffman, B. M. et al. Biomarkers of pembrolizumab efficacy in advanced anal squamous cell carcinoma: analysis of a phase II clinical trial and a cohort of long-term responders. J. Immunother. Cancer 12, e008436 (2024).
Gale, D. et al. Development of a highly sensitive liquid biopsy platform to detect clinically-relevant cancer mutations at low allele fractions in cell-free DNA. PLoS ONE 13, e0194630 (2018).
Plagnol, V. et al. Analytical validation of a next generation sequencing liquid biopsy assay for high sensitivity broad molecular profiling. PLoS ONE 13, e0193802 (2018).
Gouda, M. A. et al. Liquid biopsy response evaluation criteria in solid tumors (LB-RECIST). Ann. Oncol. 35, 267–275 (2024).
Gouda, M. A. et al. Longitudinal monitoring of circulating tumor DNA to predict treatment outcomes in advanced cancers. JCO Precis. Oncol. 6, e2100512 (2022).
Cabel, L. et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann. Oncol. 28, 1996–2001 (2017).
Anagnostou, V. et al. ctDNA response after pembrolizumab in non-small cell lung cancer: phase 2 adaptive trial results. Nat. Med. 29, 2559–2569 (2023).
Wyatt, A. W. et al. Plasma ctDNA as a treatment response biomarker in metastatic cancers: evaluation by the RECIST Working Group. Clin. Cancer Res. 30, 5034–5041 (2024).
Sanz-Garcia, E. et al. Multimodal detection of molecular residual disease in high-risk locally advanced squamous cell carcinoma of the head and neck. Cell Death Differ. 31, 460–468 (2024).
Palma, D. A. et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J. Clin. Oncol. 38, 2830–2838 (2020).
Flach, S. et al. Liquid BIOpsy for MiNimal RESidual DiSease Detection in Head and Neck Squamous Cell Carcinoma (LIONESS)—a personalised circulating tumour DNA analysis in head and neck squamous cell carcinoma. Br. J. Cancer 126, 1186–1195 (2022).
Acknowledgements
RaDaR assays were provided in kind for this study by NeoGenomics Laboratories, Inc. (Durham, NC). NeoGenomics Laboratories, Inc. employees (C.P., A.C., C.M., L.G) did not participate in the design and sample collection of this study, yet they participated in the writing, review, and editing.
Author information
Authors and Affiliations
Contributions
D.L.F. conceptualized the investigation, provided supervision, and led the funding acquisition. Formal data analysis, including visualization, was performed by D.A.R.T., R.D.M., M.E.B.; D.A.R.T. and J.M. led the sample selection and shipping. J.M. and V.E. processed and stored the blood samples. A.S.F. identified the tumor on FFPE sections. D.A.R.T., R.D.M., T.R., M.P., J.C.P., S.L.S., C.P., L.J.W. and D.L.F. helped with review and editing. C.P., A.C., C.M. and L.G. processed WES samples and created the panel for variant detection in plasma samples. C.M. aided with data visualization. D.A.R.T. and R.D.M. wrote the original draft, and all authors performed critical reviews and revisions.
Corresponding author
Ethics declarations
Competing interests
Daniel L. Faden receives research funding from NeoGenomics and receives salary support from NIH 5K23DE029811, R03DE03550, and 5R21CA267152. Amber Chevalier, Lisa Gates, Christodoulos Pipinikas, and Clodagh Murray were NeoGenomics employees for the duration of the study. The other authors declare no Competing Financial or Non-Financial Interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ruiz-Torres, D.A., Merkin, R.D., Bryan, M.E. et al. Personalized circulating tumor DNA dynamics inform survival and response to immune checkpoint blockade in recurrent/metastatic head and neck cancer. npj Precis. Onc. 9, 298 (2025). https://doi.org/10.1038/s41698-025-01084-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-01084-4
This article is cited by
-
Personalized ctDNA analysis for detection of residual disease and recurrence in surgically treated HNSCC patients
npj Precision Oncology (2026)
-
Immune biomarkers for head and neck cancer
Cancer Immunology, Immunotherapy (2025)
-
Circulating tumour cells and circulating tumour DNA in head and neck cancer: from molecular insights to clinical impact
Cancer and Metastasis Reviews (2025)