Abstract
Interhalogen compounds (IHCs) are extremely reactive molecules used for halogenation, catalyst, selective etchant, and surface modification. Most of the IHCs are unstable at room temperature especially for the iodine-monofluoride (IF) whose structure is still unknown. Here we demonstrate an unambiguous observation of two-dimensional (2D) IF bilayer grown on the surface of WSe2 by using scanning transmission electron microscopy and electron energy loss spectroscopy. The bilayer IF shows a clear hexagonal lattice and robust epitaxial relationship with the WSe2 substrate. Despite the IF is known to sublimate at −14 °C and has never found as a solid form in the ambient condition, but surprisingly it is found stabilized on a suitable substrate and the stabilized structure is supported by a density functional theory. This 2D form of IHC is actually a byproduct during a chemical vapor deposition growth of WSe2 in the presence of alkali metal halides as a growth promoter and requires immediate surface passivation to sustain. This work points out a great possibility to produce 2D structures that are unexpected to be crystallized or cannot be obtained by a simple exfoliation but can be grown only on a certain substrate.
Similar content being viewed by others
Introduction
Since one atomic layer of carbon was isolated from the bulk graphite by exfoliation as graphene1, other elements and chemical compounds, such as silicon2, germanium3, boron4, phosphorus5, hexagonal boron nitride (hBN)6, and transition metal dichalcogenides (TMDCs)7, have been extensively tested to form atomically thin planar structures over the past decade. Choosing a suitable substrate is the key to realize monolayer epitaxy out of the natural layered crystals, and is also essential to lower the formation energy of those elements to form 2D structures on the surface instead of forming 3D bulk solids8,9.
Halogens also have been predicted to form 2D structures with compressed or expanded forms when absorbed on solid surfaces in the 1980s10. Halogen adsorption is of technological importance in many fields, such as anisotropic dry etching, the catalyst for chemical reaction, oxychlorination reaction, and electrochemistry for battery operation. Iodine is the largest atom of halogens, except for astatine, and has the lowest electronegativity and largest polarizability, which makes iodine more flexible in coordinate bonding. In the 1980s, the atomic iodine layer chemisorbed on Pt single-crystal surface was studied in an ultra-high vacuum chamber with low energy electron diffraction and Auger electron spectroscopy11,12.
In the chemical vapor transport method, halogens (I2 or Br2) and halogenides are essential to transport agents to grow layered TMDCs under equilibrium condition13. The solid TMs sources can be transformed into a gaseous metal halide with the presence of halogens which can effectively lower the growth temperature during vapor transport growth. Recently, alkali metal halides (i.e., KI and NaCl) were also used as the growth promoters to synthesize numerous types of monolayer TMDCs through chemical vapor deposition7,14. Some early studies discussed the possibility of intrinsic doping of TMDCs with the halogen incorporation from the transport agents13,15 which suggests that the TMDCs might have been functionalized by the coverage of 2D halogens on the surface. Since the halogens possess strong electronegativity, the formation of 2D halogen compounds would be potentially an effective way for surface modification on the layered materials. However, up to date, the reports related to the 2D halogen compounds are few and the actual structures have not been directly assigned yet. Here in this letter, we report a direct visualization of 2D interhalogen compound, iodine monofluoride (IF), grown on the surface of WSe2 by using scanning transmission electron microscopy (STEM). The 2D IF layer was formed as a bilayer and arranged in hexagonal symmetry which was supported by a density functional theory (DFT) study. Atomic probe electron energy loss spectroscopy (EELS) was utilized for identifying the chemical composition of iodine monofluoride. This is the observation of crystalline 2D IHC which is beyond the knowledge of material science. Our findings can break through the perception and theory of the fundamental metallurgy.
Results and discussion
Synthesis and characterization of 2D iodine-monofluoride on WSe2
During a chemical vapor deposition method to grow WSe2, we added potassium iodide (KI) and Sodium fluoride (NaF) as the growth promotor. The experimental setup is shown in Fig. 1 and the growth detail is presented in the method. The grown WSe2 with the possible halogenated surface was passivated with a layer of polycarbonate immediately after the growth and gently transferred to TEM Cu quantify oil by using the well-established clean transfer technique16,17.
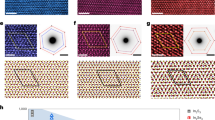
Figure 2a shows a wide area annular dark field (ADF) image of the WSe2 with extra platelet structures covering the surface. Almost 50% of the WSe2 substrate (dark contrast) is covered by the extra surface layer in brighter contrast. An EELS chemical analysis (Fig. 2b) shows the brighter contrast layer is a compound of iodine and fluorine. The W and Se peaks are shown in the low energy region around 40–50 eV. The red spectrum shows a clear iodine signal at 650 eV and fluorine edge at ~700 eV. At low energy region, iodine edge at 50 eV is overlapping with W and Se peaks which gives extra background to the WSe2 substrate spectrum. A quantitative analysis shows a wide range of the atomic ratio 0 < F:I < 4 (Supplementary Fig. 1), which suggests strong beam damage happening during the EELS experiments resulting in severe changes in the composition from the stoichiometric value. In fact, a few seconds of acquisition time for EELS always destroys the IF layers, and no longer time acquisition with a better statistic is possible. Halogens are actually known for their beam sensitives. The IF layer was originally a continuous film with a size of about 0.07 μm2 as pointed by the white arrows in a low-magnification image of Fig. 2a inset. The ADF contrast of the IF layer is slightly weaker than the overgrown WSe2 bilayer pointed by the black arrow and is distinguishable from the second layer of WSe2. In order to minimize the electron beam damage in observation at high magnification, we moved the specimen under the gun valve closed condition prior to the image acquisition. A moiré pattern is formed at IF and WSe2 overlapping area which is more clearly seen in the magnified ADF image shown in Fig. 2c. The fast Fourier transform (FFT) image shown in Fig. 2d displays the structure information of the 2D IF/WSe2 which shows a clear epitaxial relationship of the surface IF layer and the WSe2 substrate. One can see the two sets of hexagonal diffraction patterns refer to the structure of WSe2 (blue circles) and the IF layer (red circles) shown in Fig. 2d. The structure of the 2D IF layer appears hexagonal symmetry in the crystal c-axis. Note that the IF layer survives only the first scan and will go away instantly in the second. The IF layers are also sensitive to laser excitation, which makes it difficult for us to obtain their optical spectra.
a A STEM-ADF image of IF layer is grown on WSe2 substrate. The scale bar is 20 nm. b EEL spectra of the IF layer (red spectrum) and WSe2 substrate (blue spectrum) taken from the brighter layer and the dark region in (a), respectively. c A closeup of the yellow rectangle area in (a), which shows a moiré pattern of the surface IF layer with the WSe2 substrate. The scale bar is 2 nm. d An FFT pattern of the whole image in (a), where the left part marked with red and blue circles in the diffraction spots showing a clear epitaxial relationship of the surface IF layer and the WSe2 substrate.
Structural analysis of the 2D iodine-monofluoride
Most of the ever-recognized interhalogen compounds are known as molecules consisting of two different halogen atoms (i.e., fluorine, chlorine, bromine, and iodine) without elements from other groups. The gas-phase IF was firstly identified by its emission spectrum (435–690 nm) in 195118 and its laser response was also reported19,20. The interhalogen compounds are assembled in the forms of XYn, (n = 1, 3, 5 or 7, X is the heavier or less electronegative halogen, Y is the lighter or more electronegative halogen) by halogen atoms reaction at high temperature. They are very reactive and have covalent bonds in nature with diamagnetic due to the existence of bond pairs and lone pairs. Most of the X–Y bond in interhalogens is much weaker than the X–X bond in halogens, except for the F–F bond because F has the strongest electronegativity with the possible ionic charge of only −1. The IF is a powder below −58 °C and sublimates at −14 °C which cannot be isolated as a pure substance and should disproportionate rapidly into elemental iodine and iodine trifluoride at room temperature21.
The IF layer existing on WSe2 surface at room temperature is, therefore, extremely surprising. The IF layer alone is not stable in air but probably stable in air for a short time when it absorbs to the WSe2 surface. We transferred specimens in a vacuum-sealed package and minimized the exposure time before the STEM observations. Figure 3a shows the raw ADF image of the IF/WSe2 layer with a clear moiré pattern. In order to visualize the structure of the IF layer, we applied an image filtering to the FFT pattern by masking the WSe2 spots (Fig. 3b), and the inversed FFT image presenting only the IF structure is shown in Fig. 3c. The 2D IF layer clearly exhibits in hexagonal structure with homogeneous contrast. Recently IF compounds have been predicted to crystalize in the 2D planner structure at high-pressure condition22,23. Numerous possible 2D atomic configurations of IFn (n = 1,3,5, or 7) were searched globally using an evolutionary algorithm combined with DFT calculations but the IF structure was the only one found to match our experiments. The IF molecule was found relatively stable as a linear chain structure than the other formation but still cannot preserve the structure as a monolayer IF chain array. The most stable 2D IF structure is bilayer IF chain array stacking with 120° rotation as shown in Supplementary Fig. 2. The formation energy of the IF bilayer is −4.76 eV per unit IF, while it is −4.89 eV when IF epitaxy on WSe2. Figure 3e shows the atomic model of the IF layer on the WSe2 surface. The calculated unit cell constant of the IF bilayer is 4.297 Å which is 1.3 times larger than the WSe2 lattice (3.289 Å). The simulation image based on this theoretical atomic model is shown in Fig. 3d, which matches extremely well with the experimental image shown in Fig. 3a. The IF bilayer was also found 30° rotational stacking on the WSe2 surface, which suggests an again a strong epitaxial relationship with the substrate. The ADF image in Fig. 3f shows the epitaxial IF layer on the WSe2 surface consisting of 0° and 30° rotational domains highlighted by cyan and yellow lines, respectively. The IF structural orientation can be clearly seen in the FFT image in the inset and also the inversed FFT image (Fig. 3g) after the WSe2 structure been filtered out. The simulation image of 30° epitaxial IF layer on WSe2 shown in Fig. 3h again fits greatly well with the experimental image. According to our DFT calculation, the IF layer is energetically stable on the surface of 2D materials. So, it should be possible to epitaxy 2D IHCs on the surface of graphene or hBN. The reproducibility of the epitaxy IF layer on 2D materials should be high once the iodine and fluoride sources are added to the synthesis system, however, the stability of the IF layer is extremely low in the ambient condition. The preserving of the IF layer is still challenging, but we believe it can be stabilized under an inert environment.
a The intact ADF image of the IF layer on the WSe2 substrate. The scale bar is 1 nm. b The masked FFT pattern of a used for the image filtering. c An inversed FFT filtered image of the IF layer after the contrast from the WSe2 substrate been removed. The scale bar is 1 nm. d A simulated STEM-ADF image of the atomic models of the IF layer and the substrate. e A top view of the atomic model of IF bilayer on WSe2 obtained through a DFT calculation. Gray and purple rhombus indicate the unit cell of WSe2 and IF bilayer. f The ADF image of IF bilayer stacking on WSe2 with the different rotation angles 0° and 30°. Inset is the corresponding FFT, where the diffraction spots of WSe2, the 0°, and 30° rotated IF layers are highlighted by white, cyan, and yellow hexagons. The scale bar is 2 nm. g The inversed FFT filtered image of the IF layer with the WSe2 contrast been removed. The scale bar is 2 nm. h The simulated STEM-ADF image of the 30° rotated IF bilayer on WSe2. Inset is a raw ADF image of the 30° rotated IF region cropped from (f) for comparison. The scale bar is 1 nm.
Theoretical electronic properties of the 2D iodine-monofluoride
This is the direct proof of any interhalogen compound in a 2D crystal structure form. Figure 4a shows a colored ADF of the IF bilayer image after filtering out the WSe2 contrast. The STEM simulation image and the corresponding atomic model of the IF bilayer are shown in Fig. 4b, c. As we have known the ADF contrast is proportional to the atomic mass where heavier atom gives rise to brighter ADF contrast. As a result, the contrast of fluorine (Z = 9) is much weaker than the iodine (Z = 53) and is barely seen in both the experimental and the simulation images. The hexagonal atomic configuration is basically contributed by the contrast of iodine atoms. The IF is a parallelly assembled linear chain structure with a distance of 3.8 Å. The second layer of IF is stacking with an in-plane rotation of 120°. The iodine atoms in the bilayer IF form a hexagonal arrangement in the top view. Figure 4d displays the energy band profile of the IF bilayer along with high-symmetry points. The IF bilayer is calculated as a semiconductor with an indirect energy gap of ~0.19 eV if free-standing. The WSe2 is a semiconductor with a direct energy gap of ~1.64 eV (Fig. 4e), while the band structure becomes metallic when IF bilayer absorbed on WSe2 as shown in Fig. 4f. The transition from semiconductor to metal is caused by the interaction between IF bilayer and WSe2 as shown in the density of states and charge density difference analysis as shown in Fig. 4g, h. The main factor of semiconductor to metal transition is the Fermi level shift as electrons in the Se p-orbital transferred to I p-orbital. The IF epitaxial adhesion can be a robust surface modification material to improve the contact with metal electrodes in electronic devices.
a A STEM-ADF image of the IF bilayer after the contrast due to the WSe2 substrate has been removed. Bright atoms correspond to the iodine atoms (Z = 53). Fluorine atoms are hardly visible because of their smaller atomic number (Z = 9). The scale bar is 0.5 nm. b The STEM simulation image of the IF bilayer showing an excellent match with the experimentally derived atomic positions of iodine. c The corresponding atomic model of IF bilayer obtained through a DFT calculation. The top and bottom IF layers are colored by orange and blue ribbons, respectively. d–f DFT calculated band structures of free-standing IF bilayer, WSe2 monolayer, and IF bilayer on WSe2 substrate. Cusps at K in (f) are due to the asymmetric atomic positions of the IF bilayer. g Density of states analysis of IF bilayer on WSe2. Se p-states and I p-states (pointed by the green arrow) near the Fermi level contribute to the interaction between the IF bilayer and WSe2. The typical coupling between W d- and Se p-states of WSe2 is also shown just below the Fermi level. h Charge difference analysis of IF bilayer on WSe2 from separate IF bilayer and WSe2. Yellow and cyan colored regions indicate the charge accumulation and depletion, respectively. The isosurface was drawn at 5 × 10−4e Å−3.
The IF should possess a physical property intermediate between iodine and fluorine. Our report on the epitaxial growth of IF opens up another bottom-up process to assemble unstable elements in a 2D form on the surface of 2D materials. A suitable passivation layer is necessary to preserve the 2D IF layer for further applications. This can be particularly useful for gas detection, ohmic contact engineering, conductivity, and magnetic property modulation especially beneficial in the design of van der Waals heterostructures. Our findings unveil the understanding of halogen surface absorption which is helpful for the modeling of the surface halogenation process and we expect to inspire more research interests in surface physics and material science.
Methods
Material growth
The WSe2 was grown in a 1-in. quartz tube placed in a single-zone tube furnace. We used WO3 (300 mg) as the metal precursor and placed it at the center of the furnace. The growth substrate, sapphire, was placed in close proximity to the WO3 source at the downstream side. The Se (500 mg) source was placed the upstream of gas flow and heated using an independent nichrome wire heater. Alkali metal halides (KI and NaF) were added as the growth promotor. KI (0.85 mg) was placed at the edge of the tube furnace, which is 15 cm from the furnace center, while the NaF was placed 5 cm in front of the WO3. The growth process was controlled at low pressure (15 Torr) with the flow of Ar (120 sccm) and H2 (30 sccm). The quartz tube was purged with Ar and pumped to a base pressure of 0.1 Torr at 100 °C for 30 min prior to the growth. Then the furnace center was heated to 840 °C in 40 min with maintaining the pressure of 15 Torr. During the growth, the Se was heated to 250 °C and the temperature of KI at the edge of the furnace was at about 600 °C. The growth time was 15 min. After the growth, the furnace was cooled down to room temperature with a cooling rate of 0.3 °C s−1.
Scanning transmission electron microscopy and electron energy loss spectroscopy
STEM images were acquired by using an ARM200F-based UHV microscope equipped with a JEOL delta corrector and a cold field emission gun operating at 60 kV. The probe current is about 15 pA. The convergence semi-angle and the inner acquisition semi-angle are is 37 and 76 mrad. Typical ADF image was 1024 × 1024 pixels resolution captured by using 38.5 μs pixel time. The EELS core-loss spectra were taken by using Gatan Rio CMOS camera optimized for low-voltage operation. EEL spectrum was acquired by using a line scan with an exposure time of 0.1 s pixel−1.
DFT calculation
We performed structure search using the evolutionary algorithm implemented in USPEX code24,25,26 combined with Vienna ab initio simulation package27. Variable-composition 2D structure search was performed within the thickness of 0–4 Å and a total number of atoms 2–10. The initial population of IF structures was generated randomly under the 80-layer group symmetry. DFT calculations for structure searching and electronic property analyses were carried out within the projector-augmented wave method28 and the exchange-correlation part was treated using the generalized gradient approximation of Perdew–Burke–Ernzerhof functional code29. Van der Waals interaction was correlated using Grimme’s DFT-D3 method30.
For the structural searching, the plane-wave basis set was contained up to 400 eV and the Brillouin zone was sampled using 2π × 0.06 Å−1 resolution of Monkhorst–Pack mesh. Structural optimization was performed until the Hellman–Feynman force of all atoms to be less than 0.02 eV Å−1.
For the electronic property analysis, the same basis set was used, and the Brillouin zone was sampled using 8 × 8 × 1 Monkhorst–Pack meshes for the primitive unit cell of IF bilayer and WSe2 monolayer. To simulate IF bilayer on WSe2 substrate, 3 × 3 IF bilayer supercell was placed on 4 × 4 WSe2 monolayer supercell, and 3 × 3 × 1 Monkhorst–Pack mesh was used.
Data availability
All data that support the findings of this study included in this published article and its Supplemental Material are available from the corresponding author upon request.
References
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Tao, L. et al. Silicene field-effect transistors operating at room temperature. Nat. Nanotechnol. 10, 227–231 (2015).
Dávila, M. E., Xian, L., Cahangirov, S., Rubio, A. & Lay, G. L. E. Germanene: a novel two-dimensional germanium allotrope akin to graphene and silicene. N. J. Phys. 16, 095002 (2014).
Alducin, D., Myers, B. D., Liu, X. & Fisher, B. L. Synthesis of borophenes: anisotropic, two-dimensional boron polymorphs. Science 350, 1513–1516 (2015).
Li, L. et al. Black phosphorus field-effect transistors. Nat. Nanotechnol. 9, 372–377 (2014).
Dean, C. R. et al. Boron nitride substrates for high-quality graphene electronics. Nat. Nanotechnol. 5, 722–726 (2010).
Zhou, J. et al. A library of atomically thin metal chalcogenides. Nature 556, 355–359 (2018).
Li, G. et al. Epitaxial growth and physical properties of 2D materials beyond graphene: From monatomic materials to binary compounds. Chem. Soc. Rev. 47, 6073–6100 (2018).
Momeni, K. et al. Multiscale computational understanding and growth of 2D materials: a review. npj Comput. Mater. 6, 22 (2020).
Jones, R. G. Halogen adsorption on solid surfaces. Prog. Surf. Sci. 27, 25–160 (1988).
Frank, D. G., Chyan, O. M. R., Golden, T. & Hubbard, A. T. Probing three distinct iodine monolayer structures at Pt(111) by means of angular distribution Auger microscopy: results agree with scanning tunneling microscopy. J. Phys. Chem. 97, 3829–3837 (1993).
Felter, T. E. & Hubbard, A. T. L.e.e.d. and electrochemistry of iodine on Pt(100) and Pt(111) single-crystal surfaces. J. Electroanal. Chem. 100, 473–491 (1979).
Legma, J. B., Vacquier, G. & Casalot, A. Chemical vapour transport of molybdenum and tungsten diselenides by various transport agents. J. Cryst. Growth 130, 253–258 (1993).
Li, S. et al. Halide-assisted atmospheric pressure growth of large WSe2 and WS2 monolayer crystals. Appl. Mater. Today 1, 60–66 (2015).
Pisoni, A. et al. The role of transport agents in MoS2 single crystals. J. Phys. Chem. C 119, 3918–3922 (2015).
Lin, Y. C. et al. Graphene annealing: how clean can it be? Nano Lett. 12, 414–419 (2012).
Lin, Y. C. et al. Clean transfer of graphene for isolation and suspension. ACS Nano 5, 2362–2368 (2011).
Durie, R. A. The electronic emission spectrum and molecular constants of iodine monofluoride. Can. J. Phys. 44, 337–352 (1966).
Harris, D. G., Blauer, J. A. & Hurlock, S. C. Iodine monofluoride discharge laser investigation. J. Appl. Phys. 68, 3844–3848 (1990).
De Young, R. J. Lasing characteristics of iodine monofluoride. Appl. Phys. Lett. 37, 690–692 (1980).
Hoyer, S. & Seppelt, K. The structure of IF3. Angew. Chem. Int. Ed. 39, 1448–1449 (2000).
Zhang, L. et al. Crystal structure and properties of iodine monofluoride compounds at high pressure. J. Phys. Condens. Matter 32, 385404 (2020).
Luo, D. et al. A hypervalent and cubically coordinated molecular phase of IF8 predicted at high pressure. Chem. Sci. 10, 2543–2550 (2019).
Oganov, A. R. & Glass, C. W. Crystal structure prediction using ab initio evolutionary techniques: principles and applications. J. Chem. Phys. 124, 244704 (2006).
Oganov, A. R., Lyakhov, A. O. & Valle, M. How evolutionary crystal structure prediction works-and why. Acc. Chem. Res. 44, 227–237 (2011).
Lyakhov, A. O., Oganov, A. R., Stokes, H. T. & Zhu, Q. New developments in evolutionary structure prediction algorithm USPEX. Comput. Phys. Commun. 184, 1172–1182 (2013).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Acknowledgements
Y.-C.L. and K.S. acknowledge to the JSPS-KAKENHI (JP16H06333), (18K14119), JST-CREST programs (JPMJCR20B1, JMJCR20B5, and JPMJCR1993), JSPS A3 Foresight Program, and Kazato Research Encouragement Prize. G.-D.L. and S.L. acknowledge support from the National Research Foundation (NRF) of Korea grant (RIAM 2019R1A2C2005098, 2019R1A6A3A01091287, and 2017M3D1A1040688) and the Supercomputing Center/Korea Institue of Science and Technology Information with supercomputing resources (KSC-2018). P.-W.C. appreciates the project support of Taiwan Ministry of Science and Technology: Grants MOST 107-2119-M-007-011-MY2 and MOST 106-2628-M-007-003-MY3.
Author information
Authors and Affiliations
Contributions
Y.-C.Y. and P.-W.C. contributed to the material synthesis. Y.-C.L. performed STEM-EELS experiment and data analysis. S.L. and G.-D.L. carried out the DFT calculation. Y.-C.L. and K.S. designed this work and wrote the paper. S.L., G.-D.L., and P.-W.C. discussed the experimental results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, YC., Lee, S., Yang, YC. et al. Two-dimensional iodine-monofluoride epitaxy on WSe2. npj 2D Mater Appl 5, 18 (2021). https://doi.org/10.1038/s41699-021-00201-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41699-021-00201-8