Abstract
Currently, knowledge of changes in cardiovascular function across the menstrual cycle and how these changes may inform upon underlying health is limited. Utilizing wrist-worn biometric data we developed a novel measure to quantify and investigate the cardiovascular fluctuation (i.e. cardiovascular amplitude) within resting heart rate (RHR) and heart rate variability (RMSSD) across 11,590 participants and 45,811 menstrual cycles. Within participants, RHR and RMSSD fluctuated in a regular pattern throughout the menstrual cycle, with population RHRmin and RMSSDmax at cycle day 5, RHRmax at day 26, and RMSSDmin at day 27. Cardiovascular amplitude was attenuated (p < 0.05) in older participants and participants using birth control, suggesting the novel metric may mirror differences in hormonal fluctuations in these cohorts. Longitudinal tracking of cardiovascular amplitude may offer accessible non-invasive monitoring of female physiology and underlying health across the menstrual cycle.
Similar content being viewed by others
Introduction
In postmenarcheal, premenopausal females, disruptions to the menstrual cycle outside of pregnancy may indicate an underlying health concern, and it has been suggested that the menstrual cycle be used as a vital sign1. For instance, missed periods (i.e., secondary amenorrhea) are often related to low estrogen and progesterone levels, and low progesterone can result in premenstrual dysphoric disorder2,3. Additionally, those experiencing irregular menstrual cycles have been found to have a higher risk of coronary heart disease, cancers, and osteoporosis later in life4,5,6. Unfortunately, recognizing these cycle disruptions can be challenging as it may take several months before an individual identifies irregular or missed menses. A practitioner may also need to follow up with invasive blood tests to properly diagnose the underlying cause, further delaying the time until treatment. However, developing strategies that assist in rapidly identifying menstrual irregularities and facilitating earlier treatment should improve patient outcomes.
Menstrual cycle tracking via smartphone applications has become increasingly widespread, with over 200 million downloads of menstruation or fertility tracking applications globally7. Simultaneously, wearable technology has been consistently identified as one of the top fitness trends for several consecutive years8,9,10, with approximately one in three U.S. adults using a wearable health monitor in 202311. Since wearable technology provides continuous and accurate cardiovascular measures such as resting heart rate (RHR) and the root mean square of successive differences measure of heart rate variability (RMSSD), and these cardiovascular measures are known to vary across the menstrual cycle4,5,6,7, the combination of wearable technology and menstrual cycle tracking offers an intriguing opportunity to monitor health across the reproductive lifespan and to rapidly identify menstrual irregularities. Relatedly, recent research suggests that wearables can be used for non-invasive and automatic tracking of fertility and pregnancy status12,13. Thus, wearable technology presents an attractive lens to evaluate female physiology across the menstrual cycle, and a unique opportunity to explore how individual factors such as age, body mass index (BMI), and birth control status may influence cycle-related physiological fluctuations.
The present study examined the magnitude of variation in RHR and RMSSD across the menstrual cycle in a large, free-living cohort using a wrist-worn device with motion and photoplethysmography (PPG) capabilities. To investigate the significance and degree of cardiovascular fluctuations across the menstrual cycle, we derived a novel “cardiovascular amplitude” metric, which we subsequently leveraged to explore the influence of individual factors on fluctuations in cardiovascular metrics. We hypothesized that cardiovascular function would fluctuate in a regular and predictable pattern across the menstrual cycle, and that age, BMI, and hormonal birth control would influence the magnitude of cardiovascular amplitude. If validated, monitoring of cardiovascular dynamics via wearable devices could serve as an easily accessible and objective indicator of female physiology with the potential to improve our ability to track cycle health beyond standard journal-based methods.
Results
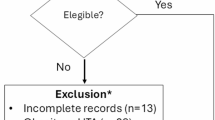
A total of 11,590 participants met the inclusion criteria for the study, as illustrated in Fig. 1. In total 9968 individuals were included in the naturally cycling cohort and 1661 in the birth control pill cohort. Data was collected from global participants, with the majority of data collected from the United States (69.9%), Great Britain (8.0%), Australia (4.2%), Germany (4.2%), Ireland (1.4%), the Netherlands (1.3%), the UAE (1.1%) and Switzerland (1.0%), with 92 other countries accounting for the remaining 8.9% of participants.
Diagram of participant exclusion criteria illustrating the number of participants excluding at each step of data refinement. Two pathways are shown illustrating the naturally cycling cohort on the left side and the birth control pills cohort on the right side. In total 23,395 participants were assessed for eligibility; 9968 participants were included in the naturally cycling cohort and 1661 participants were included in the birth control pill cohort.
Eligible participants recorded 1,241,929 days of data and 45,811 unique menstrual cycles during the study period. Participant demographics and cardiovascular and menstrual cycle overview data are provided in Table 1. The mean duration of all menstrual cycles was 27.42 days (±2.16 days), with menstrual bleeding recorded on average for 4.66 days (±1.05 days).
Population model
Analysis of the population-level GAMM of naturally cycling participants revealed a significant relation (p < 0.001) between the day of the menstrual cycle and metric offset from the cycle mean for both RHR (Fig. 2a) and RMSSD (Fig. 2b) in both unadjusted and adjusted models, confirming a robust association between menstrual cycle day and these cardiovascular metrics. RHR offset from cycle mean decreased during the beginning of each menstrual cycle, reaching a nadir nearest to day 5 (day 4.81, RHR offset: −1.83 BPM), after which RHR rose to its highest point at approximately day 26 (day 26.44, RHR offset +1.64 BPM) and maintained a positive offset through the conclusion of the menstrual cycle. The dynamics of the RMSSD offset as a function of the day of the menstrual cycle followed an inverse pattern to RHR with a maxima nearest to day 5 (day 4.81, RMSSD offset + 3.57 ms) and a nadir nearest day 27 (day 27.13, RMSSD offset −3.22 ms). All additional variables included in the adjusted model defined were significant (p < 0.05; Table 2).
The partial dependence plot of the day of the menstrual cycle (D) from the population-level GAMM modeling the offset from the cycle-mean RHR and RMSSD in the naturally cycling cohort. Adjusted models are represented by the black dashed line, unadjusted models by the grey dotted line. The) models for RHR offset values are displayed in (a), models for RMSSD offset are displayed in (b). A red dashed line indicates the mean end of menstrual bleeding. Boundary lines indicate the 95% CI for estimates.
Individual cardiovascular amplitude measures
As established above, the minimum expected point in the population model for RHR offset occurred at approximately day 5 of the menstrual cycle, and the maximum occurred at approximately day 26. As day 26 is within 3 days of the end of the menstrual cycle, the mean of the final 7 days of each cycle was used to provide equivalent data in each cycle. Therefore, the cycle cardiovascular amplitude for RHR was defined as the mean value on days 2–8 (7-day mean centered on day 5) subtracted from the mean value of the final 7 days, and RHRamp was defined as each participant’s mean cycle cardiovascular amplitude across all eligible cycles. The average RHRamp was 2.73 BPM, and 93.6% of participants demonstrated a positive RHRamp value. The cycle amplitude measure for RMSSD was defined as the mean value on days 2–8 (7-day mean centered on day 5) less the mean value from the final 7 days. The average RMSSDamp was 4.65 ms, and 80.6% of participants demonstrated a positive RMSSDamp value.
Association of Age and BMI with Cardiovascular Amplitude
The results from the GLMs suggest a negative association between age and RHRamp (β = −0.04 95%CI[−0.05– −0.04], p < 0.001 and RMSSDamp (β = − 0.09 95%CI[− 0.11 – −0.07], p < 0.001 (Fig. 3; Table 3). Notably, while the individual’s baseline value of the given metric (i.e. RHR or RMSSD) was a significant factor in the cardiovascular amplitude, the relationship was not linear. Rather than a linear relationship between cardiovascular amplitude and the individual’s baseline metric, RHRamp and RMSSDamp peak when baseline values are at the 50th–75th percentile (RHRamp knot 3: β = 2.76 95%CI[1.28− 4.24], p < 0.001, RMSSDamp knot 3: β = 7.04 95%CI[4.35–9.73], p < 0.001). BMI in this analysis was not significantly associated with RHR, but there was a statistically significant association between BMI and RMSSDamp (β = 0.03 95%CI[0.00–0.06], p = 0.026).
The partial dependence of age is plotted for (a) RHRamp and (d) RMSSDamp and the partial dependence of the four spline terms of baseline RHR is plotted for RHRamp (b) and the four spline terms of baseline RMSSD is plotted for RMSSDamp (e). The partial dependence of BMI is plotted for (c) RHRamp and (f) RMSSDamp. Boundary lines indicate the 95% confidence interval for estimations.
Birth control cohort comparison
To investigate the relationship between birth control usage and cardiovascular amplitude, a sub analysis calculating individual cardiovascular amplitude was carried out in a cohort of confirmed birth control pill users (n = 1661), a population model of the birth control cohort is included in Supplementary Note 1. For RHRamp, the mean value was 2.73 bpm (±1.95) for those naturally cycling and 0.28 bpm (±1.94) for participants using birth control pills. For RMSSDamp, the mean value was 4.65 ms (±6.90) for those naturally cycling and −0.51 ms (±6.70) for participants using birth control pills (Table 4). There is a statistically significant difference between the two cohorts in the mean values of both RHRamp (p < 0.001) and RMSSDamp (p < 0.001) (Fig. 4). The results remained consistent in a small (n = 3322) age-matched subset of both cohorts (RHRamp (p < 0.001) and RMSSDamp (p < 0.001). Results for the age-matched subset are included in Supplementary Note 2.
Histogram plots for the distributions of RHRamp values are shown in (a) and the distributions of RMSSDamp values are shown in (b). In both (a) and (b), the naturally cycling cohort is displayed in light gray with the cohort mean represented by the dashed (---) line, while the birth control pill cohort is displayed in dark gray with the cohort mean represented by the dotted (…) line.
Discussion
The purpose of this study was to examine the magnitude of the fluctuations in RHR and RMSSD across the menstrual cycle, which led to the derivation of a novel metric based on the cardiovascular amplitude of RHR and RMSSD. Secondarily, we sought to determine whether individual factors known to influence cycle-related variability in reproductive hormones (i.e., age, BMI, and birth control) may also be associated with variability in cardiovascular function. Our primary findings are that cardiovascular amplitude fluctuated in a regular and predictable pattern across the menstrual cycle and that fluctuations for RHR and RMSSD were tempered by increased age, hormonal birth control use, and increased BMI.
Consistent with our hypothesis, RHR and RMSSD fluctuated substantially and with consistent patterns across the menstrual cycle, both at the population and individual levels. More specifically, in this large cohort of naturally cycling and regularly menstruating females, the lowest RHR and highest RMSSD values were observed at the end of menstruation (i.e. days 3–7), and the highest RHR and lowest RMSSD values were observed in the late luteal phase and conclusion of the menstrual cycle (i.e. days 25–27). This pattern of menstrual cycle-related fluctuation in RHR and RMSSD metrics is comparable to previous studies from our group14, and others15,16, and is likely explained by cycle-related fluctuations in reproductive hormones15. Notably, there is an expanding market for objective, home-based cycle tracking methods such as urine and saliva testing to measure hormone levels. Unfortunately, these tests can be costly, burdensome, and are not always accurate17,18. Our findings in a large cohort of free-living individuals add to the existing literature in highlighting the possible value of considering wearable-derived cardiovascular metrics as an easily accessible and reliable indicator of female reproductive hormones across the menstrual cycle.
We observed significantly lower RHRamp and RMSSDamp in females reporting birth control pill use compared to those naturally cycling. Notably, the typical offsets from baseline during menstruation were relatively similar for participants naturally cycling and taking birth control but diverged significantly during the second half of the menstrual cycle (i.e., late luteal phase). We hypothesize that the significant fluctuations in cardiovascular parameters in naturally cycling participants may be due to progesterone increases in the late luteal phase, which are dampened in individuals on hormonal birth control19. This observation is consistent with previous findings from our group in females using combined oral contraceptives14. Taken together, these findings demonstrate significant attenuation of menstrual cycle-related cardiovascular variation by birth control, and highlight a major difference in cardiovascular physiology across the menstrual cycle in females who are naturally cycling compared with birth control users.
Reduced cardiovascular amplitude was also observed in females with higher body mass index and of older age. This reduction in cardiovascular amplitude in older females is consistent with findings demonstrating a gradual decline in anti-müllerian hormone, progesterone, and estrogen (i.e., reproductive hormones) among females throughout their reproductive life and approaching their last menstrual period14,20,21. With respect to body mass, individuals with abnormal BMI at either extrema are more likely to have anovulatory cycles, irregular menses, and hormonal imbalances22. Research has shown that premenopausal females with an elevated BMI exhibit reduced levels of estrogen and anti- müllerian hormone22,23. It is possible that the attenuated RMSSDamp and RHRamp in participants with abnormal BMI is connected to these underlying differences in hormonal health. Further research is warranted to better understand the association between the novel cardiovascular amplitude values derived in this study and direct measures of hormonal health.
Prior research exploring RMSSD across the menstrual cycle is limited. However, the High Frequency power (HF) frequency measure of HRV, which is strongly correlated with RMSSD and parasympathetic function, also exhibits a decrease in power during the mid-luteal phase of the menstrual cycle24. HF power levels are at their highest during the high-estrogen follicular phase, and at their lowest during the high-progesterone mid-luteal phase, however, whether these changes are ascribed to levels of estrogen, progesterone, or, more likely, a combination of each, is debated25,26,27. Examining the link between parasympathetic activity and menstrual cycle conditions investigations of individuals with premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD) found stronger phase to phase differences within the HF component of HRV were found in those with severe PMS, while a reduction in overall parasympathetic activity was observed in those with PMDD27. Autonomic Nervous System dysfunction has also been proposed as a driving factor in female fertility disorders due to a lack of sympathetic shift in the luteal phase28. This connection to menstrual cycle related disorders and fertility warrants further investigation into the change in RMSSD and RMSSDamp in regards to the diagnosis and detection of PMS, PMDD, infertility, or other conditions.
Our findings should be interpreted in the context of our study’s strengths and limitations. First, we restricted our sample to include only regularly menstruating females. Thus, the generalizability of our findings to females with irregular menstrual cycles is unclear. Second, we relied on self-reported data concerning menstruation status and birth control methods, which can be subject to human error. Third, we did not have access to relevant sociocultural variables such as race/ethnicity and socioeconomic status that may influence reproductive physiology and cardiovascular function29. Additionally, our study would have benefited from direct hormonal measurements as well as a more thorough characterization of our cohort, inclusive of information regarding potential reproductive and/or cardiovascular health conditions as well as more detailed information regarding the use of hormonal contraception (e.g., duration of use, dose, brand, etc.), all of which may influence cycle-related physiology24. These weaknesses are balanced by the size of our dataset: over a million days of data and nearly 50,000 cycles from more than 10,000 regularly menstruating females, making our analysis among the largest to appear in the scientific literature to date. Additionally, our data collection featured state-of-the-art wearable technology that has been validated against gold-standard electrocardiogram and polysomnography measures and has been found to have a low degree of bias and low precision errors30,31.
In conclusion, we present cardiovascular amplitude as a novel method for quantifying the magnitude of cardiovascular fluctuations across the menstrual cycle in a large cohort of free-living menstruating individuals. Cardiovascular amplitude measures were tempered by increasing age, BMI, and birth control use. These findings add to a small but growing body of literature in support of changes in cardiovascular function across the menstrual cycle that mirror and may be causally related to fluctuations in reproductive hormones. Though further research is needed, the novel cardiovascular amplitude metric, derived through wearable technology, demonstrates an accessible and objective indicator of changes in female physiology within and across menstrual cycles, with the potential to inform upon underlying health.
Methods
Data collection
Daily data used for analysis were collected from study participants between January 2, 2022, and October 2, 2023, using a wrist-worn wearable device, the WHOOP Strap (Whoop, Inc., versions 3.0 or 4.0; Boston, MA, USA), which continuously measures vital signs, including RHR and RMSSD, RHR and RMSSD were calculated by the WHOOP cloud-based analytics platform during non-wake periods of the primary sleep episode24. The WHOOP cardiovascular and sleep measures have been validated in a general population against gold-standard electrocardiogram and polysomnography measures with a low degree of bias and low precision errors30,31. Respondent demographics including birthday, gender, height, and weight were available via study participants’ existing WHOOP profiles and were used to calculate participants’ age and BMI. Participants reported data regarding birth control type, birth control start date, and birth control stop date – if the use was discontinued – via an on-demand survey in the WHOOP app; data regarding menstruation status were self-reported daily via a survey in the WHOOP mobile application. Since data were not identifiable and were stored on a secure server, this study was determined to be exempt according to 45 CFR 46.104(d) by Salus IRB (https://www.versiticlinicaltrials.org/salusirb) and a full waiver of Informed Consent was granted as data collection involved minimal risk.
Inclusion criteria
Participants who self-identified as female, were aged 18 or older at the time of data collection, had an active WHOOP membership, had previously reported use (or non-use) of hormonal birth control, and manually logged menstruation within the WHOOP mobile application were eligible for inclusion. In accordance with established guidelines for menstrual cycle research32, participants that reported menstrual cycles of irregular length (i.e., less than 21 or greater than 35 days)33, menstrual bleeding longer than 7 days34, or had fewer than 95% days of wear across a minimum of 2 consecutive menstrual cycles were excluded from the analysis. Given the known influence of hormonal birth control on hormone levels and cardiovascular dynamics, the primary analysis was restricted to participants reporting no regular birth control use or the exclusive use of barrier methods of birth control (i.e. naturally cycling) at the time of data collection14,35. A secondary analysis compared key results within naturally cycling participants with results for participants who reported the use of hormonal-based birth control pills. Participants could contribute data to both cohorts if they experienced a change in birth control status during the data collection period. All study participants consented to the use of their data for purposes of scientific research at the time of enrollment and could withdraw consent at any time.
Data processing
RHR and RMSSD were normalized to within-user and within-cycle values by calculating the offset in absolute units from the cycle mean value with outliers removed. Outliers were defined as values above quartile 3 + 1.5*interquartile range (IQR) or below quartile 1–1.5*IQR for a given user and cycle. Following established guidelines, a menstrual cycle was defined as the time between the first self-reported menstrual bleeding and the onset of the next self-reported menstrual bleeding following a time of no reported menstrual bleeding36.
Statistical analysis
To assess population-level trends in RHR and RMSSD across the menstrual cycle, a generalized additive mixed effect model (GAMM) was constructed for each metric using the “gam” package in R37. The variables examined for inclusion in the model as independent variables were the number of days since the onset of the menstrual cycle (D), the subject’s age in years (age), the subject’s daily kilojoule expenditure (kJ) from the prior day, whether the date was a Saturday or Sunday (weekend)38, BMI in kg/m2 (BMI), and a unique participant identifier (pID). The following equations give the unadjusted and adjusted models for the applied GAMM:
UNADJUSTED
ADJUSTED
Where yi represents the offset from cycle mean of the metric (RMSSD or RHR), f1(D), f3(age), and f4(kJ) are smooth, non-linear functions of the previously defined variables D, age, and kJ respectively, and f2(pID) is the within-participant random effect associated with the variable pID.
The partial dependence plots for the number of days since the onset of the menstrual cycle (D) from the population GAMM models were used to determine the day within a standard menstrual cycle in which an individual’s RHR or RMSSD could be expected to reach a peak or nadir. The observed population peak and nadir were determined by the nearest day to the maxima and minima of the partial dependence plot in the population-level GAMM, respectively. A cycle amplitude for each participant-cycle was calculated as the difference between the 7-day mean centered on the expected peak and the 7-day mean centered on the expected nadir (Fig. 5). For instance, if the population model suggested a maximum on day 4 and a minimum on day 14, the cycle cardiovascular amplitude value for each participant-cycle was determined by subtracting the mean of days 11–17 from the mean of days 1–7. To establish individual cardiovascular amplitude, the mean of the cycle amplitudes from all of the participants’ eligible menstrual cycles was calculated. A 7-day mean was used to mitigate the impact of day-of-week effects24. If the maxima or minima were fewer than 3 days from the beginning or end of the cycle, the 7-day period was shifted to ensure a 7-day window of data was available for each subject. The derived participant cardiovascular amplitude values are denoted as RMSSDamp and RHRamp for RMSSD cardiovascular amplitude and RHR cardiovascular amplitude values, respectively.
Visual exploration of the assignment of individual menstrual cycle measures of cardiovascular amplitude. The ‘Population Model’ frame illustrates the population GAMM trend of Resting Heart Rate offset across the menstrual cycle. The population minimum and maximum are identified, and the surrounding days are shaded, with the grey shaded region encompassing the range of the expected minimum values and the blue shaded range encompassing the range of the expected maximum values. The second frame, ‘Individual Cycle’ illustrates the application to a single menstrual cycle. The individual resting heart rate offset values are represented by open circles. The dashed lines in each shaded region correspond to the mean values of resting heart rate offsets in the grey and blue shaded regions respectively. In the final pane, ‘Cycle Cardiovascular Amplitude’, the values from the prior panel are subtracted from one another, with the mean offset of the expected maximum minus the mean offset of the expected minimum representing the final cardiovascular amplitude metric.
To understand the relationship between known mediators of hormonal health and the novel cardiovascular amplitude metric, generalized linear models (GLM) were fit with the participant’s age (age), underlying cardiovascular metric value (RHR and RMSSD respectively), and BMI (BMI) as covariates to estimate values of RHRamp and RMSSDamp. Natural spline functions with four knots were selected to model RHR and RMSSD data, while age and BMI were modeled linearly. The general formulae of the GAMM for RHRamp (Eq. 3) and RMSSDamp (Eq. 4) can be expressed as follows:
The cardiovascular amplitude metrics were also calculated for a cohort of participants who reported the use of birth control pills. The cardiovascular amplitude values for this cohort were calculated using the days for the expected minima and maxima derived from the naturally cycling population model. The cardiovascular amplitudes of naturally cycling participants were then compared to the cardiovascular amplitudes of the cohort of participants using hormonal birth control pills. In a supplementary analysis, the two cohorts were matched age using the ‘MatchIt’ package in R to create comparable populations (Supplementary Note 2)39. All data were expressed as mean ± standard deviation (SD) and differences were tested by a two-tailed t-test unless otherwise reported. All analyses were carried out in either Python (version 3.8) or R (version 4.2.2)40. Significance was set a priori at 0.05. The visualizations of the model outputs were generated using the sjPlot package41.
Data availability
The data that support the findings of this study are not publicly available due to intellectual property concerns of WHOOP, Inc. Data may be made available upon request to the company via research@whoop.com for researchers who meet the criteria for access to confidential data.
Code availability
The underlying code for this study is not publicly available for proprietary reasons. Code for this study was written using Python (version 3.8) and R (version 4.2.2).
References
American Academy of Pediatrics, Committee on Adolescence, American College of Obstetricians and Gynecologists & Committee on Adolescent Health Care. Menstruation in Girls and Adolescents: Using the Menstrual Cycle as a Vital Sign. Pediatrics 118, 2245–2250 (2006).
Shufelt, C. L., Torbati, T. & Dutra, E. Hypothalamic Amenorrhea and the Long-Term Health Consequences. Semin. Reprod. Med. 35, 256 (2017).
Stefaniak, M. et al. Progesterone and Its Metabolites Play a Beneficial Role in Affect Regulation in the Female Brain. Pharmaceuticals. 16, 520 (2023).
Solomon, C. G. et al. Menstrual Cycle Irregularity and Risk for Future Cardiovascular Disease. J. Clin. Endocrinol. Metab. 87, 2013–2017 (2002).
Cirillo, P. M., Wang, E. T., Cedars, M. I., Chen, L.-M. & Cohn, B. A. Irregular menses predicts ovarian cancer: Prospective evidence from the Child Health and Development Studies. Int. J. Cancer 139, 1009–1017 (2016).
Adams Hillard, P. J. & Nelson, L. M. Adolescent girls, the menstrual cycle, and bone health. J. Pediatr. Endocrinol. Metab. 16, 673–681 (2003).
Bull, J. R. et al. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digital Med. 2, (2019).
Thompson, W. R. Worldwide Survey of Fitness Trends for 2023. ACSMs Health Fit. J. 27, 9 (2023).
Thompson, W. R. Worldwide Survey of Fitness Trends for 2022. ACSMs Health Fit. J. 26, 11 (2022).
Thompson, W. R. Worldwide Survey of Fitness Trends for 2021. ACSMs Health Fit. J. 25, 10 (2021).
Study reveals wearable device trends among U.S. adults. NHLBI, NIH https://www.nhlbi.nih.gov/news/2023/study-reveals-wearable-device-trends-among-us-adults#:~:text=Almost%20one%20in%20three%20Americans,Health%20Information%20National%20Trends%20Survey.
Goodale, B. M. et al. Wearable Sensors Reveal Menses-Driven Changes in Physiology and Enable Prediction of the Fertile Window: Observational Study. J. Med. Internet Res. 21, e13404 (2019).
Grant, A. & Smarr, B. Feasibility of continuous distal body temperature for passive, early pregnancy detection. PLOS Digital Health 1, e0000034 (2022).
Sims, S. T., Ware, L. & Capodilupo, E. R. Patterns of endogenous and exogenous ovarian hormone modulation on recovery metrics across the menstrual cycle. BMJ Open Sport Exerc Med 7, e001047 (2021).
Moran, V. H., Leathard, H. L. & Coley, J. Cardiovascular functioning during the menstrual cycle. Clin. Physiol. 20, 496–504 (2000).
Brar, T. K., Singh, K. D. & Kumar, A. Effect of Different Phases of Menstrual Cycle on Heart Rate Variability (HRV). J. Clin. Diagn. Res. 9, CC01 (2015).
Lequin, R. M., van den Boogaard, A., Vermeulen, J. & Danhof, M. Progesterone in saliva: pitfalls and consequent implications for accuracy of the determination. Clin. Chem 32, 831–834 (1986).
Stanczyk, F. Z., Mandelbaum, R. S. & Lobo, R. A. Potential pitfalls of reproductive direct-to-consumer testing. F&S Rep. 3, 3–7 (2022).
Alvergne, A. & Lummaa, V. Does the contraceptive pill alter mate choice in humans? Trends Ecol. Evol. 25, 171–179 (2010).
Kelsey, T. W., Wright, P., Nelson, S. M., Anderson, R. A. & Wallace, W. H. A validated model of serum anti-müllerian hormone from conception to menopause. PLoS One. 6, 7 (2011).
Santoro, N. & Randolph, J. F. Jr. Reproductive Hormones and the Menopause Transition. Obstet. Gynecol. Clin. North Am. 38, 455 (2011).
Imterat, M., Agarwal, A., Esteves, S. C., Meyer, J. & Harlev, A. Impact of Body Mass Index on female fertility and ART outcomes. Panminerva Med. 61, 58–67 (2019).
Freeman, E. W. et al. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil. Steril. 87, 101–106 (2007).
Shaffer, F. & Ginsberg, J. P. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health 5, 258 (2017).
de Zambotti, M., Nicholas, C. L., Colrain, I. M., Trinder, J. A. & Baker, F. C. Autonomic regulation across phases of the menstrual cycle and sleep stages in women with premenstrual syndrome and healthy controls. Psychoneuroendocrinology 38, 2618–2627 (2013).
Schmalenberger, K. M. et al. Menstrual Cycle Changes in Vagally-Mediated Heart Rate Variability Are Associated with Progesterone: Evidence from Two Within-Person Studies. J. Clin. Med. Res. 9, 617 (2020).
Matsumoto, T., Ushiroyama, T., Kimura, T., Hayashi, T. & Moritani, T. Altered autonomic nervous system activity as a potential etiological factor of premenstrual syndrome and premenstrual dysphoric disorder. Biopsychosoc. Med. 1, 24 (2007).
Autonomic dysfunction may be an under-recognized cause of female fertility disorders. Med. Hypotheses 63, 172–177 (2004).
Grosicki, G. J., Bunsawat, K., Jeong, S. & Robinson, A. T. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: Social determinants of health. Prog. Cardiovasc. Dis. 71, 4–10 (2022).
Berryhill, S. et al. Effect of wearables on sleep in healthy individuals: a randomized crossover trial and validation study. J. Clin. Sleep Med. 16, 775–783 (2020).
Miller, D. J., Sargent, C. & Roach, G. D. A Validation of Six Wearable Devices for Estimating Sleep, Heart Rate and Heart Rate Variability in Healthy Adults. Sensors 22, 6317 (2022).
How to study the menstrual cycle: Practical tools and recommendations. Psychoneuroendocrinology 123, 104895 (2021).
Abnormal Uterine Bleeding. https://www.acog.org/womens-health/faqs/abnormal-uterine-bleeding.
Heavy Menstrual Bleeding. https://www.acog.org/womens-health/faqs/heavy-menstrual-bleeding.
Armean Wright, A., Fayad, G. N., Selgrade, J. F. & Olufsen, M. S. Mechanistic model of hormonal contraception. PLoS Comput. Biol. 16, e1007848 (2020).
Menstrual Cycle. https://doi.org/10.1016/B978-012373947-6.00256-7 10.1016/B978-012373947-6.00256-7.
Website. Hastie T (2023). gam: Generalized Additive Models. R package version 1.22-2, https://CRAN.R-project.org/package=gam.
Capodilupo, E. R. & Miller, D. J. Changes in health promoting behavior during COVID-19 physical distancing: Utilizing wearable technology to examine trends in sleep, activity, and cardiovascular indicators of health. PLoS One 16, e0256063 (2021).
Website. Ho D., Imai K., King G., Stuart E. (2011). MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. _Journal of Statistical Software_ *42*(8), 1-28. https://doi.org/10.18637/jss.v042.i08 .
Website. R. Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Website. Lüdecke D. (2023). _sjPlot: Data Visualization for Statistics in Social Science_. R package version 2.8.14, https://CRAN.R-project.org/package=sjPlot.
Acknowledgements
We would like to thank Laura Ware for assisting with code development and methodology review for this study. We would also like to thank Christopher Bailey and Davide Valeriani for their thoughtful feedback and review throughout the drafting process.
Author information
Authors and Affiliations
Contributions
S.R.J, E.R.C, D.M.P, and V.H.L conceived and designed the analysis plan; S.R.J cleaned and organized the data and performed the analysis; S.R.J generated the figures and tables, and S.R.J, G.J.G., and V.H.L wrote the first draft. All authors contributed to manuscript revisions, figure design, and interpretation. All authors read, edited, and approved the article.
Corresponding author
Ethics declarations
Competing interests
D.M.P., S.R.J., V.H.L., G.J.G and E.R.C. are employed by the commercial company WHOOP Inc. WHOOP is developing commercial products that build off the research presented in this manuscript and has filed a patent related to those inventions. The authors would like to declare the following patents/patent applications associated with this research: the US application number is 18/463,096. The patent name will be “ Coaching based on reproductive phases”. This patent addresses coaching related to the cardiovascular amplitude of individuals. This does not alter our adherence to npj digital medicine policies on sharing data and materials.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jasinski, S.R., Presby, D.M., Grosicki, G.J. et al. A Novel method for quantifying fluctuations in wearable derived daily cardiovascular parameters across the menstrual cycle. npj Digit. Med. 7, 373 (2024). https://doi.org/10.1038/s41746-024-01394-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41746-024-01394-0
This article is cited by
-
A longitudinal dataset of physiological, hormonal, metabolic, and self-reported menstrual health data
Scientific Data (2026)
-
Wearable-Derived Heart Rate Variability Across the Menstrual Cycle, Hormonal Contraceptive Use, and Reproductive Life Stages in Females: A Living Systematic Review
Sports Medicine (2026)
-
Platform matters -- Differences in COVID data collected from Android and iOS app users
npj Digital Medicine (2025)
-
A new metric to understand the association between heart rate variability and menstrual regularity
npj Digital Medicine (2025)