Abstract
This review summarizes the current literature on the use of wearable devices for collecting physiological data in pediatric and young adult (0−25 years) oncology. Searches were conducted in MEDLINE, PubMed and Embase, focusing on pediatric and young adult patients with a cancer diagnosis, and utilizing a wearable device during and/or after treatment. Of the 77 articles that met the inclusion criteria, 61 studies primarily used wearable devices as a tool to monitor physiological changes in an interventional or observational setting. Only 16 studies integrated wearable devices as an active component of the intervention. The most reported wearable device brands were ActiGraph (19, 24.7%), FitBit (14, 18.2%), Ambulatory Monitoring Inc. (11, 14.3%) and Philips Respironics (10, 13%). This scoping review offers valuable insights into the current use of wearable devices in pediatric and young adult (0−25 years) oncology but also reveals notable gaps in the literature.
Similar content being viewed by others
Introduction
Wearable devices have rapidly diffused into consumer markets over the last decade, offering a unique opportunity to engage in managing health issues. A wearable device is a portable, non-invasive, body-adhered electronic tool designed to collect, monitor and transmit data related to physiological or behavioral parameters. These devices can monitor a wide range of physiological and activity metrics, including but not limited to, electrocardiograms (ECGs), heart rate, respiratory rate, blood oxygen saturation, and physical activity levels. Due to the capacity for real-time, continuous monitoring of patients, and the possibility of timely intervention to improve health outcomes advancements in wearable technology have generated considerable interest within the healthcare system.
In adult oncology, wearable devices have gained significant traction when used to attain data for prognostication, and rehabilitation planning1—which is unsurprising given physical activity is the most readily available and recorded wearable data2. A review of wearable devices in adult oncology identified 199 studies which utilized devices for oncology prognostication, treatment monitoring or rehabilitation3. However, implementation in clinical application and early detection using continuously monitored data (e.g. blood oxygen saturation, ECGs) is limited. The Apple Heart Study, is the largest wearable device research project to date, led by Apple and Standford, to assess the utility of these data to identify early interventions. Over 400,000 participants across the United States of America were closely monitored for irregular heart rhythms (specifically atrial fibrillation), using the Apple Watch in built optical heart rate sensor4. This groundbreaking clinical trial found that the wearable device could not only be used to identify irregular heart rhythms but also distinguished episodes of atrial fibrillation in adult participants4. This technology is now readily available to the public, allowing individuals to continuously monitor their heart rhythm in real-time and share this information with the appropriate health professionals.
In contrast, the use of wearable devices in pediatrics and young adults (0–25 years) is limited with most wearable studies using activity trackers only. The gap, and potential for intervention with wearable devices, remains for children with chronic illnesses. Wearable devices are an opportunity for improving the long-term outcomes of children with cancer and has a number of potential clinical applications. Children with cancer experience in the first 12 weeks of treatment (on average) 2.5 adverse drug reactions. The most common are nausea, fatigue, vomiting and myelosuppression requiring blood product transfusions5. Wearable technologies in pediatric and young adult oncology have the potential to monitor and manage these common treatment-related side effects by providing continuous physiological data. This can enable timely intervention, improve symptom management, and enhance quality of life.
The aim of this scoping review is to understand how wearable devices are being utilized in pediatric and young adult (0–25 years) oncology and the types of physiological data being collected. Furthermore, we aim to report on any potential benefits or inefficacies of the wearable devices.
Results
Study selection and characteristics
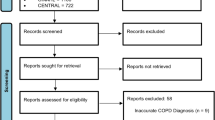
The search strategy (see methods) identified 4860 records. After duplicates were removed 3328 articles underwent title and abstract screening by two independent reviewers. Articles that did not meet the inclusion criteria were removed. Secondary selection involved the review of 239 full texts to establish article relevance. The main reasons for article exclusion during the full text review was due to the wearable device not collecting physiological and/or activity data, or the article was a conference abstract. Ultimately, 77 articles were in included in this scoping review6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82 (Fig. 1).
Many of the studies were conducted in the United States (USA) (33) or Europe (31), with 6 studies conducted in Australia, 3 in Canada, 2 in Türkiye, 1 in Japan and 1 in Taiwan. As shown in Tables 1, 79.2% of the studies utilized wearable devices primarily as data collection tools, either in observational contexts or to monitor physiological and activity-related outcomes. These applications included tracking physical activity (35.1%), sleep patterns (22.1%), symptoms or treatment-related side effects (15.6%), and other combinations such as physical activity and sleep monitoring (2.6%). In contrast, only 20.8% of the studies utilized the wearable device as part of the intervention itself. Nearly half of the studies (49.3%) were conducted while the patient was at home, 22.1% of the studies were conducted while the patient was in hospital, and 28.6% were conducted both in and out of a clinical setting. Sample sizes ranged from 2−1378 participants (median = 37 participants). Across the included studies, the aims generally fell into three categories: assessing feasibility, monitoring physiological or activity data, and using the device as part of an active intervention. These overarching aims are reflected in how we’ve categorized device use and functionality throughout the results.
While gender distribution varied across the 77 studies, females comprised of 50.5% of the overall participant population. 13 studies (16.9%) reported exclusively on patients with acute lymphoblastic leukemia (ALL), 9 studies (11.7%) reported on patients with central nervous system (CNS) tumors and 2 studies (2.6%) reported on patients with bone tumors. 52 studies (67.5%) reported on patients with any cancer diagnosis and 1 study (1.3%) did not state the exact cancer diagnosis of participants. As shown in Table 2, over half (55.8%) of the studies reported on cancer patients currently receiving treatment, 36.4% reported on patients who have completed treatment, and 7.8% of studies included a mixed cohort of patients on and off treatment. A full breakdown of study characteristics is available in Supplementary Table 1.
Wearable devices
The most reported brands of wearable devices were ActiGraph (19 studies, 24.7%), FitBit (14 studies, 18.2%), Ambulatory Monitoring Inc (11 studies, 14.3%) and Philips Respironics (10 studies, 13%). An accelerometer was the most common sensor used across 70% of the studies. Two studies connected one wearable device to another, to collect physiological information which would in turn influence the second device63,64. Table 3 lists all wearable device brands utilized across the 77 studies and their associated sensors. While many wearable device brands (e.g., Fitbit, Biofourmis) can measure multiple physiological parameters through various integrated sensors, we only report on the specific sensor modalities explicitly described within the included studies (n = 77). For full details on the devices and functionality utilized for each individual study, please refer to Supplementary Table 2.
Over half of the studies (42 studies, 55%) used wearable devices that were worn on the wrist/arm, with 18 studies (23.4%) providing participants with hip-worn devices. The remaining 17 studies used devices placed on the patient’s skin, clothes, chest or head. Most of the wearable devices utilized physical activity, step count and sleep detection capabilities (Table 4). In addition, only 11 (14.3%) studies utilized more advanced wearable device capabilities such as respiratory rate, blood oxygen, ECG, heart rhythm and eye movement. While wearable device brands and their primary functionalities are presented in separate tables to preserve accuracy, often studies made use of different functionalities within the same device. For example, Fitbit devices were used to collect step count data in some studies and heart rate data in others, depending on the device model and study aim. Supplementary Table 2 summarize these uses.
The duration of time participants were asked to wear the wearable device ranged from 5 min to 1 year, however, the majority of studies utilized the wearable devices for <1 month. Only twenty-five studies reported on the wear time adherence, with studies reporting high adherence at the start of the study, but adherence decline over time, particularly for longer studies7,13,17,18,20,21,22,23,26,27,36,37,40,41,43,48,58,65,69,75,77,78,79,81. Of the studies that reported an average wear time, the overall average was 12.35 h/day. With an overall average wear duration of 5.76 days/week based on studies that reported this data.
Twenty (26%) studies reported patients had technical difficulties with the wearable devices during the study, with the most common problem being data syncing and/or connectivity issues6,10,19,22,23,25,26,29,32,36,37,40,47,53,57,58,61,78,79,81. Four studies reported adverse effects as a direct result of the wearable device22,36,37,47, with skin irritation being the most common. Detailed data on device placement, functionality, and reported compliance metrics for each study are provided in Supplementary Table 3.
Discussion
The current scoping review summarizes the literature surrounding the use of wearable devices in pediatric and young adult (0−25 years) oncology, the types of data collected, and the benefits and limitations of the technology. We have identified the use of wearable device is of interest in pediatric and young adult oncology. However, with only 77 studies identified, it has highlighted a clear gap in the literature. In contrast, systematic reviews in adult oncology have identified 199 studies.
Our analysis reveals that the predominant wearable device used in pediatric and young adult oncology is the ActiGraph (25% of studies). The ActiGraph is a wrist-worn accelerometer used to monitor and record physical activity or sleep behavior over time, with a long-standing presence in the wearable device market. ActiGraph is also the predominant device used in adult oncology, used in around 36% of studies3. However, within adult oncology there is a wider variety of wearable device brands utilized when compared to pediatric and young adult oncology. In addition, given the size of the wearable device market, and it’s expected revenue of USD 138 billion in 202583, commercial grade devices will become more accessible for oncology research. However, only 19 (25%) studies utilized a commercially available wearable device (e.g. FitBit, Oculus/Meta and Garmin). Of the types of wearable devices used, only Phillips Respironics devices have been validated and approved for use in pediatrics and young adults as a medical device. Other brands are research grade wearable devices. Validation of wearable device data in pediatrics and young adults is technically challenging because of the changes in physiology across the age span from neonates through adolescence. This presents difficulties in interpreting wearable data in real time, particularly where clinical decisions rely on parameters that vary with age, such as heart rate, sleep, and activity patterns84,85. For example, distinguishing between sleep and daytime naps in infants and young children may complicate sleep analysis, while physical activity expectations differ greatly between toddlers and adolescents, making standardization complex. The best example of this is heart rate, starting at a median rate of 140 (beats per minute) BPM in neonates, before gradually decreasing during infancy (median 110BPM), early and late childhood (median 100BPM and 90BPM) and adolescence (median 70BPM)86. In the context of short-term studies the variability in physiological parameters across the age spectrum can be mitigated by collecting baseline data of participants prior to treatment or intervention. Whilst complex annotation for activity would be required, this could individualize comparisons without the need to monitor across the entire pediatric age range.
Within pediatric and young adult oncology, more research is required in the validation and feasibility of using commercially available products that are readily accessible on the consumer market. Testing of these products within the confines of robust and blinded clinical studies is needed to support the further implementation of wearable devices in this cohort.
Whilst 77 studies used wearable devices for data collection, few monitored emerging functionalities such as heart rhythm monitoring, ECGs, respiratory rate, temperature and blood oxygen. Only 11 studies (14%) utilized these newer functionalities. It is important to note that, given 68% of included studies involved mixed cancer cohorts, it was not possible to draw conclusions about how diagnosis type may influence device functionality or data collection choices. Moreover, very few studies (21%) looked to implement the wearable device as part of an intervention13,15,30,33,38,40,44,53,63,64,81. Of these, 7 studies (9%) explicitly stated the utilization of real-time data monitoring (respiratory rate, heart rate, temperature, blood oxygen, activity and steps), with 2 studies (3%) using this real-time respiratory rate data to influence a virtual reality device in a bio-feedback loop63,64. In the remaining five studies, real time data was either reviewed by healthcare professionals to inform patient care or used to provide immediate feedback to participants to influence their activity behavior. Despite the limited number of intervention-focused studies, wearable technologies appear to be well-received by patients and survivors, who are increasingly open to integrating these tools into their care experience87,88. One retrospective study analyzed electronic medical records to identify how many referrals to a pediatric arrythmia clinic were prompted by identification of abnormal heart rhythms using the Apple Watch device. Interestingly, in 71% of cases (n = 145) the Apple Watch electrocardiogram findings prompted the cardiology team to pursue further workup88. In addition, health organizations across the United States of America have started to integrate wearable device data directly into patient electronic medical records to utilize real-time data to assist with patient care89. These studies show the opportunities for early intervention that are consumer led rather than investigator-initiated trial lead. Pediatric and young adult patients particularly benefit from wearable devices as they enable continuous, non-invasive monitoring that can reduce hospital visits and clinical burden. They also offer the potential for early detection of physiological changes, promote self-management and engagement with their own health, and support tailored interventions that accommodate developmental and behavioral variability unique to children and adolescents36,40,81. Furthermore, real-time monitoring and feedback can empower caregivers and clinicians to make timely decisions, potentially improving clinical outcomes and quality of life.
A total of 21 studies included in this scoping review assessed feasibility6,10,19,22,23,25,26,29,30,32,36,37,40,47,53,58,61,78,79,81,90, with reported barriers to implementation including well-established issues in patient compliance, technical issues resulting in data failure, and the clinical validation of the device91,92. However, it is important to note that compliance in pediatrics is dependent on the parent/guardian and patient. No studies in our scoping review reported on parental compliance of enforcing or facilitating wearable device wear and this remains a research gap. Another factor when considering a wearable device in pediatric and young adult oncology is the placement of the device and compliance with wearing it appropriately. While wrist and hip worn devices commonly use accelerometers to measure physical activity, compliance appears to be lower with hip placement, particularly in younger children16,31,80. This suggests that device placement itself, regardless of sensor type, may influence wear compliance due to comfort and acceptability. In this context, three studies reported on the lower compliance in younger children when using hip worn devices but didn’t specify how this differed from older participants. The reported information in a study by Hooke et al. was that the device on the hip make them feel “different”, whilst Wu et al. reported two participants withdrawing from the study due to discomfort of wearing the hip-worn device.
Technical issues were a reported barrier to wearable use in children. 26% of studies reported connectivity and data syncing problems that impacted the quality and/or quantity of data available to researchers (see Supplementary Table 3 for full details). However, many studies did not provide detailed descriptions of these technical issues, limiting insight into their nature and resolution. In the context of the broader literature with respect to wearable technology, reported issues include dropped connections, signal interference, pairing problems, low battery impacting connectivity, incompatible devices, environmental factors and software glitches93,94. In assessing wearable devices in sensitive setting such as pediatric and young adult oncology, ease of use and careful study design to avoid these common technical issues is essential. Private healthcare providers with existing medical devices, such as Kaiser Permanente or Ochsner, have addressed these concerns by implementing on-site technology assistance, similar to the Apple ‘Genius Bar’, to troubleshoot any technical issues that may arise92. This could be a viable solution to overcoming technical issues but should be explored in both public and private sectors, allowing access for all patients.
While wearable technology holds significant promise in pediatric oncology, health equity must be a central consideration in its development and implementation87,95. Access to wearable technology and consistent internet connectivity is not universal and may be limited for families from lower socioeconomic backgrounds, rural areas, or marginalized communities96,97,98,99. This divide, risks excluding those who may already experience health disparities, thereby widening inequities in care and outcomes. Furthermore, health literacy, language barriers, and varying levels of technological familiarity among caregivers may influence the ability to use these tools effectively100. To mitigate this, future studies and programs should incorporate equity-focused strategies, such as, providing devices and/or data plans, offering multilingual support, and involving diverse communities in study design. This will ensure that the benefits of wearable technologies are accessible to all pediatric and young adult cancer patients.
Although 28 studies (36%) reported on the acceptability and ease of use of the wearable devices9,16,19,22,25,29,30,31,32,33,36,37,40,41,44,47,48,53,56,58,61,64,69,73,78,80,81,82, none of them reported on the thoughts, attitudes and barriers identified from consumers (defined here as pediatrics and young adults and/or their caregivers) around using the wearable device to detect diseases or treatment adverse reactions. This is likely due to minimal studies exploring the wearable device as an independent, stand-alone intervention. This highlights a current gap in the literature which indicates another potential barrier to implementation. While we acknowledge that younger pediatric patients may face developmental or communication-related challenges in articulating their experiences, it remains essential to incorporate their perspectives using age-appropriate and child-centered methods. At the same time, caregiver insights can provide valuable complementary context to better understand attitudes, barriers, and acceptance of wearable devices for treatment-related monitoring.
This scoping review offers valuable insights into the current use of wearable devices in pediatric and young adult oncology but also reveals notable gaps in the literature, particularly when compared to the substantial body of research in adult oncology. Barriers to implementation is an ongoing challenge within this area of research and needs to be explored further in the context of wearable devices being an independent intervention. This scoping review also highlights the need for more validation studies using consumer grade devices, which may make way for more accessible and viable option for research use. Moreover, modern consumer grade devices provide a unique opportunity for a rich dataset of continuous and real time data from patients, without interrupting day to day life and burdening pediatric and young adult patients19,36,44,81.
Methods
This review was conducted according to the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement – Scoping Review (PRISMA-ScR) and registered with the OSF Registry (osf.io/fsdb9). Completed PRISMA-ScR Checklist is provided in Supplementary Note 1.
Inclusion and exclusion criteria
Studies were eligible for inclusion if they involved:
-
Pediatric, adolescent and young adults aged between 0 and 25 years AND
-
Participants had a cancer diagnosis or had received a hematopoietic stem cell transplant AND
-
Included wearable devices collecting physiological and/or activity data during and/or after cancer treatment AND
-
Articles published in English, full text (or have a translated version available) from 2014 onwards.
Studies were excluded if they involved:
-
Pre-cancerous conditions
-
Invasive devices (e.g.: insulin pumps)
-
Books, chapters, conferences, editorials, comments, theses, protocols, systematic reviews and guidelines
If articles included participants both inside and outside this age range, the results needed to be clearly defined for the pediatric, adolescent and young adult cohorts, or report a median or mean age within the 0–25 year range.
Wearable technology included non-invasive devices e.g. virtual reality devices: and excluded current medical technologies such as hearing aids.
Search methods
Literature searches were conducted in June 2024 in the following electronic databases; Medline, Embase and PubMed, and restricted to the last 10 years. Medline was used as the primary database for this review. The database selection was developed in consultation with an academic librarian and focused on high-yield, subject-specific sources to ensure relevance and feasibility within the scope of the review.
The search strategy consisted of a combination of Medical exploded Subject Headings (MeSH) and various keywords to identify the literature. MeSH terms applied in the database searches included: “Wearable Technology”, “Neoplasms”, “Stem Cell Translation”, “Pediatrics”, “Adolescents”, “Young Adults”. These terms were combined in their associated cluster groups with all word variations included in the search. The search strategy was adjusted to focus on pediatric, adolescent and young adults, with search terms removed if they were not appropriate for this search.
To refine the search, the “AND” operator was applied to combine all distinct concepts and yield relevant results. This approach was consistently applied across all electronic databases used for this review, with adjustments tailored to each database’s subject thesaurus. An additional search was completed on reference lists of similar review articles to ensure all relevant literature was captured. A detailed search has been provided in Supplementary Note 2.
Article appraisal
Results were uploaded to the systematic review software Catchii. Catchii is a new web-based systematic review platform that streamlines the development of systematic reviews with a user-friendly interface on both computers and mobile devices101.
Catchii removed all duplicate articles before two reviewers independently reviewed title and abstracts against the predefined inclusion and exclusion criteria. Any discrepancies were resolved through collaborative discussion, prior to reviewers proceeding to screen the full text articles. Following the two-stage screening process, a further collaborative discussion was undertaken to reach consensus. One reviewer extracted the data from Catchii into an excel spreadsheet consisting of:
-
Study title
-
Author(s)
-
Year published
-
Country of study
-
Setting (home/hospital/both)
-
Data collection tool or Intervention (Wearable Device)
-
Study design
-
Study primary aim(s)
-
Stage of treatment (on/off/mixed)
-
Number of participants
-
Age of participants
-
Gender of participants
-
Diagnosis of participants
-
Wearable device brand
-
Wearable device name
-
Where wearable device was worn during study
-
Expected wear time of wearable device
-
Minimum wear time of wearable device for valid data?
-
How is wear time calculated?
-
Actual wear time
-
Sampling frequency of wearable device
-
Physiological and/or activity data collected via the wearable device
-
Benefits of the wearable device
-
Inefficacies of the wearable device
A second reviewer independently checked the entered data.
Data analysis
The primary outcome of this scoping review was the use of wearable devices in pediatric oncology, with the secondary outcome being its benefits or limitations. Data were analyzed using descriptive statistics to identify common themes related to these outcomes.
Data availability
The data generated and analyzed during this study are largely available within the manuscript and supplementary material. Any additional data supporting the findings of this study can be made available upon reasonable request to the corresponding author.
Code availability
This study did not involve the utilization of any custom code or algorithm.
References
Chow, R. et al. The use of wearable devices in oncology patients: a systematic review. Oncologist 29, e419–e430 (2023).
Beauchamp, U. L., Pappot, H. & Holländer-Mieritz, C. The use of wearables in clinical trials during cancer treatment: systematic review. JMIR Mhealth Uhealth 8, e22006 (2020).
Chow, R. et al. The use of wearable devices in oncology patients: a systematic review. Oncologist 29, e419–e430 (2024).
Apple. Using Apple Watch For Arrhythmia Detection. https://www.apple.com/healthcare/docs/site/Apple_Watch_Arrhythmia_Detection.pdf (2020).
Conyers, R. et al. Minimising adverse drug reactions and verifying economic legitimacy-pharmacogenomics implementation in children (MARVEL- PIC): protocol for a national randomised controlled trial of pharmacogenomics implementation. BMJ Open 14, e085115 (2024).
Braam, K. I. et al. Cardiorespiratory fitness and physical activity in children with cancer. Support Care Cancer 24, 2259–2268 (2016).
Bratteteig, M. et al. Device-measured physical activity and cardiovascular disease risk in adolescent childhood cancer survivors. A physical activity in childhood cancer survivors (PACCS) study. Front. Pediatr. 10, 977365 (2022).
Bratteteig, M. et al. Physical activity, fitness, and cardiovascular disease risk in adolescent childhood cancer survivors compared to controls: the physical activity in childhood cancer survivors study. J. Adolesc. Young. Adult Oncol. 13, 338–346 (2024).
Dalla Santa, E. et al. Feasibility of a prospective physiotherapy model of care during the intense treatment phase of childhood cancer (FITChild): a mixed methods design. Pediatr. Blood Cancer 15, e30488 (2023).
Daniel, L. C. et al. Can extending time between vital sign checks improve sleep in hematopoietic stem cell transplant patients? testing feasibility, acceptability, and preliminary efficacy. Pediatr. Blood Cancer 71, e30832 (2024).
Darezzo Rodrigues Nunes, M., Jacob, E., Adlard, K., Secola, R. & Nascimento, L. Fatigue and sleep experiences at home in children and adolescents with cancer. Oncol. Nurs. Forum. 42, 498−506 (2015).
DeNysschen, C. A., Panek-Shirley, L. M. & Zimmerman, B. Exercise with nutrition education to improve quality of life of adolescent and young adult cancer survivors: a pilot study. J. Adolesc. Young Adult Oncol. 10, 454−461 (2021).
Devine, K. A. et al. Feasibility of FitSurvivor: a technology-enhanced group-based fitness intervention for adolescent and young adult survivors of childhood cancer. Pediatr. Blood Cancer 67, e28530 (2020).
Fiuza-Luces, C. et al. Exercise intervention in pediatric patients with solid tumors: the physical activity in pediatric cancer trial. Med. Sci. Sports Exerc. 49, 223–230 (2017).
Fuemmeler, B. F. et al. Mila blooms: a mobile phone application and behavioral intervention for promoting physical activity and a healthy diet among adolescent survivors of childhood cancer. Games Health J. 9, 279–289 (2020).
Gaser, D. et al. Analysis of self-reported activities of daily living, motor performance and physical activity among children and adolescents with cancer: Baseline data from a randomised controlled trial assessed shortly after diagnosis of leukaemia or non-hodgkin lymphoma. Eur. J. Cancer Care (Engl.) 31, e13559 (2022).
Gaser, D. et al. Effects of strength exercise interventions on activities of daily living, motor performance, and physical activity in children and adolescents with leukemia or non-hodgkin lymphoma: results from the randomized controlled ActiveADL study. Front. Pediatr. 10, 982996 (2022).
Gotte, M. et al. Physical activity in 9−15 year-old pediatric cancer survivors compared to a nationwide sample. J. Cancer Res. Clin. Oncol. 149, 4719−4729 (2023).
Götte, M., Kesting, S. V., Gerss, J., Rosenbaum, D. & Boos, J. Feasibility and effects of a home-based intervention using activity trackers on achievement of individual goals, quality of life and motor performance in patients with paediatric cancer. BMJ Open Sport. Exerc. Med. 4, e000322 (2018).
Gotte, M., Seidel, C. C., Kesting, S. V., Rosenbaum, D. & Boos, J. Objectively measured versus self-reported physical activity in children and adolescents with cancer. PLoS ONE. 10, e0172216 (2017).
Graef, D. M. et al. Sleep and mood during hospitalization for high-dose chemotherapy and hematopoietic rescue in pediatric medulloblastoma. Psychooncology 12, 1847−1853 (2018).
Grimshaw, S. L., Taylor, N. F., Conyers, R. & Shields, N. Promoting positive physical activity behaviours in children undergoing acute cancer treatment: feasibility of the CanMOVE intervention. Braz. J. Phys. Ther. 28, 100577 (2024).
Grydeland, M. et al. Physical activity among adolescent cancer survivors: the PACCS study. Pediatrics 152, e2023061778 (2023).
Gundle, K. R., Punt, S. E., Mattioli-Lewis, T. & Conrad, E. U. 3rd. Can a made-for-consumer activity monitor assess physical activity in adolescents and young adults after lower extremity limb salvage for osseous tumors?. J. Pediatr. Orthop. 37, e192–e196 (2017).
Ha, L. et al. A digital educational intervention with wearable activity trackers to support health behaviors among childhood cancer survivors: pilot feasibility and acceptability study. JMIR Cancer 8, e38367 (2022).
Ha, L. et al. Patterns of physical activity and sedentary behavior in child and adolescent cancer survivors assessed using wrist accelerometry: a cluster analysis approach. Health Inform. J. 29, 14604582231212525 (2023).
Haemmerli, M., Ammann, R. A., Roessler, J., Koenig, C. & Brack, E. Vital signs in pediatric oncology patients assessed by continuous recording with a wearable device, NCT04134429. Sci. Data9, 89 (2022).
Hamari, L. et al. The effect of an active video game intervention on physical activity, motor performance, and fatigue in children with cancer: a randomized controlled trial. Bmc Res. Notes. 12, 784 (2019).
Hoag, J. A. et al. Playing with a purpose: the impact of therapeutic recreation during hospitalization. J. Pediatr. Hematol. Oncol. Nurs. 39, 6–14 (2022).
Hooke, M. C., Gilchrist, L., Tanner, L., Hart, N. & Withycombe, J. S. Use of a fitness tracker to promote physical activity in children with acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 63, 684–689 (2016).
Hooke, M. C. et al. Kids are moving: a physical activity program for children with cancer. J. Pediatr. Oncol. Nurs. 36, 379–389 (2019).
Hooke, M. C. et al. Symptoms, physical activity, and biomarkers in children at the end of Leukemia maintenance therapy. J. Pediatr. Hematol. Oncol. Nurs. 40, 386–399 (2023).
Howell, C. R. et al. Randomized web-based physical activity intervention in adolescent survivors of childhood cancer. Pediatr. Blood Cancer 65, e27216 (2018).
Jacobs, S. et al. Pilot study of massage to improve sleep and fatigue in hospitalized adolescents with cancer. Pediatr. Blood Cancer 63, 880–886 (2016).
Johnson, A., Rice, M., Turner-Henson, A., Haase, J. E. & Azuero, A. Perceived stress and the fatigue symptom cluster in childhood brain tumor survivors. Oncol. Nurs Forum 45, 775−785 (2018).
Koenig, C., Ammann, R. A., Kuehni, C. E., Roessler, J. & Brack, E. Continuous recording of vital signs with a wearable device in pediatric patients undergoing chemotherapy for cancer-an operational feasibility study. Support Care Cancer 29, 5283–5292 (2021).
Koenig, C. et al. Continuous timely monitoring of core temperature with two wearable devices in pediatric patients undergoing chemotherapy for cancer - a comparison study. Support Care Cancer 32, 188 (2024).
Krnavek, N. J. et al. Sensor-based frailty assessment in survivors of childhood cancer: a pilot study. J. Frailty Aging 10, 176–181 (2021).
Lazar, D. R. et al. Anthracycline’s effects on heart rate variability in children with acute lymphoblastic leukemia: early toxicity signs-pilot study. J. Clin. Med. 12, 7052 (2023).
Le, A. et al. A home-based physical activity intervention using activity trackers in survivors of childhood cancer: a pilot study. Pediatr. Blood Cancer 64, 387–394 (2017).
Long, T. M. et al. Exercise training improves vascular function and secondary health measures in survivors of pediatric oncology related cerebral insult. PLoS ONE 13, e0201449 (2018).
Marmol-Perez, A. et al. Every move counts to improve bone health at clinical sites in young pediatric cancer survivors: The iBoneFIT Project. Med. Sci. Sports Exerc. 56, 1085−1093 (2024).
Matthews, E. E., Neu, M., Cook, P. F. & King, N. Sleep in mother and child dyads during treatment for pediatric acute lymphoblastic leukemia. Oncol. Nurs. Forum. 41, 599−610 (2014).
Mendoza, J. A. et al. A fitbit and facebook mhealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: a pilot study. Pediatr. Blood Cancer https://doi.org/10.1002/pbc.26660 (2017).
Merz, E. L. et al. Bedtime digital media use, sleep and fatigue among survivors of childhood cancer, their siblings and healthy control sibling pairs. Psychol. Health Med. 28, 2137−2146 (2023).
Miller, J. M. et al. Cancer survivors exercise at higher intensity in outdoor settings: the GECCOS trial. Pediatr. Blood Cancer. 68, e28850 (2021).
Misawa, M. et al. A telerehabilitation program to improve visual perception in children and adolescents with hemianopia consecutive to a brain tumor: a single-arm feasibility and proof-of-concept trial. eClinicalMedicine 78, 102955 (2024).
Müller, C., Krauth, K. A., Gerß, J. & Rosenbaum, D. Physical activity and health-related quality of life in pediatric cancer patients following a 4 week inpatient rehabilitation program. Support Care Cancer 24, 3793–3802 (2016).
Muller, C. et al. Effects of an exercise intervention on bone mass in pediatric bone tumor patients. Int. J. Sports Med. 35, 696−703 (2014).
Nessle, C. N., Flora, C., Sandford, E., Choi, S. W. & Tewari, M. High-frequency temperature monitoring at home using a wearable device: a case series of early fever detection and antibiotic administration for febrile neutropenia with bacteremia. Pediatr. Blood Cancer. 64, e29835 (2022).
Nunes, M. D. R. et al. Pain, sleep patterns and health-related quality of life in paediatric patients with cancer. Eur. J. Cancer Care 28, e13029 (2019).
Orsey, A. D. & Wakefield, D. B. Does socioeconomic status impact physical activity and sleep among children with cancer? Pediatr. Blood Cancer. 63, 2004−10 (2016).
Ovans, J. A., Hooke, M. C., Bendel, A. E. & Tanner, L. R. Physical therapist coaching to improve physical activity in children with brain tumors: a pilot study. Pediatr. Phys. Ther. 30, 310–317 (2018).
Pickering, L. et al. Brain tumours in children and adolescents may affect the circadian rhythm and quality of life. Acta paediatr. 110, 3376−3386 (2021).
Pitt, E., Cashion, C., Rumble, S. & Bradford, N. Associations between health behaviors, health self-efficacy, and long-term outcomes in survivors of childhood cancer: a cross-sectional study. Semin. Oncol. Nursing. 39, 151434 (2023).
Rehorst-Kleinlugtenbelt, L. B., Bekkering, W. P., van der Torre, P., van der Net, J. & Takken, T. Physical activity level objectively measured by accelerometery in children undergoing cancer treatment at home and in a hospital setting: a pilot study. Pediatr. Hematol. Oncol. J. 4, 82–88 (2019).
Rogers, V. E. et al. Circadian activity rhythms and fatigue of adolescent cancer survivors and healthy controls: a pilot study. J. Clin. Sleep. Med. 16, 1141–1147 (2020).
Rogers, V. E., Zhu, S., Ancoli-Israel, S. & Hinds, P. S. Impairment in circadian activity rhythms occurs during dexamethasone therapy in children with leukemia. Pediatr. Blood Cancer 61, 1986–1991 (2014).
Rogers, V. E. et al. Relationship between circadian activity rhythms and fatigue in hospitalized children with CNS cancers receiving high-dose chemotherapy. Support Care Cancer. 28, 1459−1467 (2020).
Rogers, V. E. et al. A pilot randomized controlled trial to improve sleep and fatigue in children with central nervous system tumors hospitalized for high-dose chemotherapy. Pediatr. Blood Cancer. 66, e27814 (2019).
Rosen, G. et al. The effects of dexamethasone on sleep in young children with acute lymphoblastic leukemia. Sleep. Med. 16, 503–509 (2015).
Sabel, M. et al. Active video gaming improves body coordination in survivors of childhood brain tumours. Disabil. Rehabil. 38, 2073−84 (2016).
Savaş, E. H., Semerci, R. & Bayram, C. The effect of a biofeedback-based virtual reality game on pain, fear and anxiety levels during port catheter needle insertion in pediatric oncology patients: a randomized controlled study. Eur. J. Oncol. Nurs. 70, 102621 (2024).
Savaş, E. H. et al. A biofeedback based virtual reality game for pediatric population (BioVirtualPed): a feasibility trial. Semin Oncol. Nurs. 40, 151615 (2024).
Setoyama, A., Ikeda, M. & Kamibeppu, K. Objective assessment of sleep status and its correlates in hospitalized children with cancer: exploratory study. Pediatr. Int. official J. Japan Pediatr. Soc. 58, 842−9 (2016).
Steur, L. M. H. et al. High prevalence of parent-reported sleep problems in pediatric patients with acute lymphoblastic leukemia after induction therapy. Pediatr. Blood Cancer 67, e28165 (2020).
Steur, L. M. H. et al. Sleep-wake rhythm disruption is associated with cancer-related fatigue in pediatric acute lymphoblastic leukemia. Sleep 43, zsz320 (2020).
Steur, L. M. H. et al. The impact of maintenance therapy on sleep-wake rhythms and cancer-related fatigue in pediatric acute lymphoblastic leukemia. Support Care Cancer. 28, 5983−5993 (2020).
Swartz, M. C. et al. Feasibility and acceptability findings of an energy balance data repository of children, adolescents, and young adults with cancer. J. Clin. Med. 9, 2879 (2020).
Traube, C. et al. Sleep in hospitalized children with cancer: a cross-sectional study. Hosp Pediatr. 10, 969−976 (2020).
van Deuren, S. et al. Fatigue-related cognitive-behavioral factors in survivors of childhood cancer: comparison with chronic fatigue syndrome and survivors of adult-onset cancer. J. Adolesc. Young Adult Oncol. 10, 92−99 (2020).
Van Dijk-Lokkart, E. M. et al. Longitudinal development of cancer-related fatigue and physical activity in childhood cancer patients. Pediatr. Blood Cancer. 66, e27949 (2019).
van Hulst, A. M. et al. Hydrocortisone to reduce dexamethasone-induced neurobehavioral side-effects in children with acute lymphoblastic leukaemia-results of a double-blind, randomised controlled trial with cross-over design. Eur. J. Cancer 187, 124–133 (2023).
Vyhlídal, T., Dygrýn, J. & Chmelík, F. Actigraphy-based characteristics of sleep in paediatric cancer patients in remission and a comparison with their healthy peers in the recovery stay. Nat. Sci. Sleep. 14, 1449−1456 (2022).
Vyhlidal, T., Dygryn, J., Hruba, J. & Chmelik, F. Accelerometry-based assessment of physical activity and sedentary behavior in adult survivors of childhood acute lymphoblastic leukemia and their healthy peers. Sci. Rep. 13, 7439 (2023).
Vyhlídal, T., Dygrýn, J., Pelclová, J. & Chmelík, F. Movement behaviours in paediatric cancer survivors during recovery and school weeks. Front. Oncol. 12, 971805 (2022).
Wang, Y. M. et al. Trajectory of sleep, depression, and quality of life in pediatric HSCT recipients. Transplant. Cell. Ther. 30, 632.e1−632.e5 (2024.
Williamson Lewis, R. et al. Feasibility of fitbit use in adolescent survivors of pediatric cancer: who consistently uses it and for how long?. J. Adolesc. Young. Adult Oncol. 12, 529–536 (2023).
Withycombe, J. S. et al. Can steps per day reflect symptoms in children and adolescents undergoing cancer treatment?. Cancer Nurs. 45, 345–353 (2022).
Wu, W. W., Yu, T. H., Jou, S. T., Hung, G. Y. & Tang, C. C. Factors associated with walking performance among adolescents undergoing cancer treatment: a correlational study. J. Child Health Care 27, 574–586 (2023).
Yurkiewicz, I. R., Simon, P., Liedtke, M., Dahl, G. & Dunn, T. Effect of fitbit and ipad wearable technology in health-related quality of life in adolescent and young adult cancer patients. J. Adolesc. Young. Adult Oncol. 7, 579–583 (2018).
Zupanec, S. et al. A sleep hygiene and relaxation intervention for children with acute lymphoblastic leukemia: a pilot randomized controlled trial. Cancer Nurs. 40, 488–496 (2017).
Perez, A. J. & Zeadally, S. Recent advances in wearable sensing technologies. Sens. (Basel) 21, 6828 (2021).
Liang, X. et al. Age-related differences in accelerometer-assessed physical activity and sleep parameters among children and adolescents with and without autism spectrum disorder: a meta-analysis. JAMA Netw. Open 6, e2336129–e2336129 (2023).
Herzig, D. et al. Relation of heart rate and its variability during sleep with age, physical activity, and body composition in young children. Front. Physiol. 8, 109 (2017).
Fleming, S. et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 377, 1011–1018 (2011).
Whitehead, L., Robinson, S., Arabiat, D., Jenkins, M. & Morelius, E. The report of access and engagement with digital health interventions among children and young people: systematic review. JMIR Pediatr. Parent 7, e44199 (2024).
Zahedivash, A. et al. Utility of smart watches for identifying arrhythmias in children. Commun. Med. 3, 167 (2023).
Dinh-Le, C., Chuang, R., Chokshi, S. & Mann, D. Wearable health technology and electronic health record integration: scoping review and future directions. JMIR Mhealth Uhealth 7, e12861 (2019).
Rogers, V. E. et al. Circadian activity rhythms and fatigue of adolescent cancer survivors and healthy controls: a pilot study. J. Clin. Sleep Med. 16, 1141−1147 (2020).
Canali, S., Schiaffonati, V. & Aliverti, A. Challenges and recommendations for wearable devices in digital health: data quality, interoperability, health equity, fairness. PLOS Digit. Health 1, e0000104 (2022).
Smuck, M., Odonkor, C. A., Wilt, J. K., Schmidt, N. & Swiernik, M. A. The emerging clinical role of wearables: factors for successful implementation in healthcare. npj Digit. Med. 4, 45 (2021).
Huhn, S. et al. The impact of wearable technologies in health research: scoping review. JMIR Mhealth Uhealth 10, e34384 (2022).
Roos, L. G. & Slavich, G. M. Wearable technologies for health research: opportunities, limitations, and practical and conceptual considerations. Brain, Behav., Immun. 113, 444–452 (2023).
Kucukkaya, A., Goktas, P. & Semerci Şahin, R. Exploring barriers and inequalities in access to comprehensive care for pediatric oncology patients: a systematic review. Semin. Oncol. Nurs. 14, 151852 (2025).
Jongebloed, H. et al. The digital divide in rural and regional communities: a survey on the use of digital health technology and implications for supporting technology use. BMC Res. Notes 17, 90 (2024).
Badr, J., Motulsky, A. & Denis, J.-L. Digital health technologies and inequalities: a scoping review of potential impacts and policy recommendations. Health Policy 146, 105122 (2024).
Ahmed, M. M. et al. Integrating digital health innovations to achieve universal health coverage: promoting health outcomes and quality through global public health equity. Healthcare 13, 1060 (2025).
Gong, E. et al. Bridging the digital divide to promote prevention and control of non-communicable diseases for all in China and beyond. BMJ 387, e076768 (2024).
Pan, C.-C. et al. Sociodemographics and digital health literacy in using wearables for health promotion and disease prevention: cross-sectional nationwide survey in Germany. J. Prev. 46, 371−391 (2024).
Halman, A. & Oshlack, A. Catchii: Empowering literature review screening in healthcare. Res Synth. Methods 15, 157–165 (2024).
Acknowledgements
The first author (L.C.) was awarded an Australian Government Research Training Program Scholarship (RTP) place with offset of PhD tuition fees. She is funded by Cancer Therapies, Stem Cell Medicine, Murdoch Children’s Research Institute. EP was supported by the Murdoch Children’s Research Institute by a Clinician Scientist Fellowship. G.B. is supported by an NHMRC Investigator Grant (1194497) and the Royal Children’s Hospital Foundation. R.C. is supported by the Kids Cancer Project, The Royal Children’s Hospital Foundation, Cancer Co Lab (previously Victorian Paediatric Cancer Consortium), Medical Research Future Fund grant number GHMG: 2024900 and holds a Murdoch Children’s Research Institute (MCRI) Clinician Scientist Tier 2 Fellowship and a VESKI FAIR Fellowship. R.C., J.S. and D.A.E. are supported by Novo Nordisk Foundation grant number NNF21CC0073729. JS is funded by Cancer Co Lab (previously the Victorian Paediatric Cancer Consortium). S.G. holds an honorary appointment with The University of Melbourne and holds a Cancer Council Fellowship. The Murdoch Children’s Research Institute is supported by the Victorian Government’s Operational Infrastructure Support Program and Australian Government NHMRC Independent Research Institute Infrastructure Support Scheme.
Author information
Authors and Affiliations
Contributions
L.C., S.G., R.C. and D.E. were involved in the conceptualization, study design and methodology of this scoping review. L.C. was responsible for completing the literature searches on all databases. L.C. and S.G. were responsible for title/abstract screening, full text screening and data extraction. L.C. was responsible for data analysis and wrote the original draft manuscript. S.G., R.C., D.E., G.B., E.P. and J.S. revised and provided edits for the manuscript. R.C., D.E., G.B. and E.P. provided supervision for the project. All Authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Collier, L., Grimshaw, S.L., Stolper, J. et al. Scoping review of the utilization of wearable devices in pediatric and young adult oncology. npj Digit. Med. 8, 488 (2025). https://doi.org/10.1038/s41746-025-01842-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-025-01842-5