Abstract
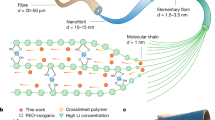
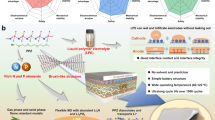
As the most abundant and renewable biopolymer, cellulose has found applications in a range of fields such as healthcare, packaging, electronics and environmental remediation, contributing to the transition towards sustainability. Here we apply a green and scalable process transforming cellulose to a robust electrolyte exhibiting lithium (Li) ion conductivity of 1.09 × 10−3 S cm−1 with a transference number of 0.81 and mechanical strength of 12 MPa. Our process takes advantage of the rich hydroxyl groups in the cellulose which are replaced by phthalic anhydride through an esterification reaction to form cellulose phthalate (CP). Combined experimental and theoretical analyses reveal that the introduction of phthalate groups is essential to not only ensure effective multi-oxygen interaction with Li ions to create fast ion transportation channels, but also facilitates the intermolecular hydrogen bond responsible for the impressive mechanical properties. The CP biopolymer film is even compatible with most commercial cathode materials, and our solid-state Li/CP/LiFePO4 cells show better performance and notably good stability over 1,000 cycles than that of a baseline Li-ion cell with a flammable organic liquid electrolyte. Our study unlocks the enormous potential of cellulose utilization in batteries and opens an avenue for the development of abundant and sustainable solid-state electrolytes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the Article and its Supplementary Information file. Should any raw data files be needed in another format, they are available from the corresponding authors upon reasonable request.
Code availability
The input files for ORCA, VASP, Chargemol, LAMMPS, CHGNet and ASE used in this study are available from the corresponding authors by request. All the codes used are either commercial or open source and can be accessed through their homepages.
References
Li, T. et al. Developing fibrillated cellulose as a sustainable technological material. Nature 590, 47–56 (2021).
Klemm, D., Heublein, B., Fink, H.-P. & Bohn, A. Cellulose: fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 44, 3358–3393 (2005).
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Prakash, P. et al. A soft co-crystalline solid electrolyte for lithium-ion batteries. Nat. Mater. 22, 627–635 (2023).
Zahiri, B. et al. Revealing the role of the cathode–electrolyte interface on solid-state batteries. Nat. Mater. 20, 1392–1400 (2021).
Christie, A. M., Lilley, S. J., Staunton, E., Andreev, Y. G. & Bruce, P. G. Increasing the conductivity of crystalline polymer electrolytes. Nature 433, 50–53 (2005).
Khurana, R., Schaefer, J. L., Archer, L. A. & Coates, G. W. Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: a new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 136, 7395–7402 (2014).
Dixit, M. B. et al. Polymorphism of garnet solid electrolytes and its implications for grain-level chemo-mechanics. Nat. Mater. 21, 1298–1305 (2022).
Zhang, Q. et al. Sulfide-based solid-state electrolytes: synthesis, stability, and potential for all-solid-state batteries. Adv. Mater. 31, 1901131 (2019).
Wang, Z. et al. Why cellulose-based electrochemical energy storage devices? Adv. Mater. 33, 2000892 (2021).
Himmel, M. E. et al. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315, 804–807 (2007).
Kubát, J. & Pattyranie, C. Transition in cellulose in the vicinity of −30 °C. Nature 215, 390–391 (1967).
Yang, C. et al. Copper-coordinated cellulose ion conductors for solid-state batteries. Nature 598, 590–596 (2021).
Cao, Y. et al. Room temperature ionic liquids (RTILs): a new and versatile platform for cellulose processing and derivatization. Chem. Eng. J. 147, 13–21 (2009).
Marson, G. A. & El Seoud, O. A. Cellulose dissolution in lithium chloride/N, N-dimethylacetamide solvent system: relevance of kinetics of decrystallization to cellulose derivatization under homogeneous solution conditions. J. Polym. Sci. Pol. Chem. 37, 3738–3744 (1999).
Wu, J. et al. Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 5, 266–268 (2004).
Zhang, J. et al. Homogeneous esterification of cellulose in room temperature ionic liquids. Polym. Int. 64, 963–970 (2015).
Moon, R. J., Martini, A., Nairn, J., Simonsen, J. & Youngblood, J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 40, 3941–3994 (2011).
Chen, F., Wang, X., Armand, M. & Forsyth, M. Cationic polymer-in-salt electrolytes for fast metal ion conduction and solid-state battery aplications. Nat. Mater. 21, 1175–1182 (2022).
Zhao, Y. et al. Design strategies for polymer electrolytes with ether and carbonate groups for solid-state lithium metal batteries. Chem. Mater. 32, 6811–6830 (2020).
Liang, Z., Cabarcos, O. M., Allara, D. L. & Wang, Q. Hydrogen-bonding-directed layer-by-layer assembly of conjugated polymers. Adv. Mater. 16, 823–827 (2004).
Wu, Y., Wang, S., Li, H., Chen, L. & Wu, F. Progress in thermal stability of all-solid-state-Li-ion-batteries. InfoMat 3, 827–853 (2021).
Yang, X. et al. Determining the limiting factor of the electrochemical stability window for PEO-based solid polymer electrolytes: main chain or terminal –OH group? Energ. Environ. Sci. 13, 1318–1325 (2020).
Wu, N. et al. Fast Li+ conduction mechanism and interfacial chemistry of a NASICON/polymer composite electrolyte. J. Am. Chem. Soc. 142, 2497–2505 (2020).
Xu, B. et al. Interfacial chemistry enables stable cycling of all-solid-state li metal batteries at high current densities. J. Am. Chem. Soc. 143, 6542–6550 (2021).
Xiao, P. et al. Synthesis, characterization and properties of novel cellulose derivatives containing phosphorus: cellulose diphenyl phosphate and its mixed esters. Cellulose 21, 2369–2378 (2014).
Lin, Z. et al. A wide-temperature superior ionic conductive polymer electrolyte for lithium metal battery. Nano Energy 73, 104786 (2020).
Liu, Y. A.-O. et al. Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries. Science 375, 739–745 (2022).
Mong, A. L. et al. Tough and flexible, super ion-conductive electrolyte membranes for lithium-based secondary battery applications. Adv. Funct. Mater. 31, 2008586 (2021).
Su, Y. et al. Rational design of a topological polymeric solid electrolyte for high-performance all-solid-state alkali metal batteries. Nat. Commun. 13, 4181 (2022).
He, X., Larson, J. M., Bechtel, H. A. & Kostecki, R. In situ infrared nanospectroscopy of the local processes at the Li/polymer electrolyte interface. Nat. Commun. 13, 1398 (2022).
Zhou, Q., Ma, J., Dong, S., Li, X. & Cui, G. Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries. Adv. Mater. 31, 1902029 (2019).
Peng, Z. et al. High-power lithium metal batteries enabled by high-concentration acetonitrile-based electrolytes with vinylene carbonate additive. Adv. Funct. Mater. 30, 2001285 (2020).
Zhang, J. et al. Synthesis of cellulose benzoates under homogeneous conditions in an ionic liquid. Cellulose 16, 299–308 (2009).
Goodlett, V. W., Dougherty, J. T. & Patton, H. W. Characterization of cellulose acetates by nuclear magnetic resonance. J. Polym. Sci. A 9, 155–161 (1971).
Vijayakumar, M., Emery, J., Bohnke, O., Vold, R. L. & Hoatson, G. L. 7Li NMR analysis on perovskite structured Li0.15La0.28TaO3. Solid State Ion. 177, 1673–1676 (2006).
Evans, J., Vincent, C. A. & Bruce, P. G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28, 2324–2328 (1987).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 136, B864–B871 (1964).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Manz, T. A. & Limas, N. G. Introducing DDEC6 atomic population analysis: part 1. Charge partitioning theory and methodology. RSC Adv. 6, 47771–47801 (2016).
Neese, F., Wennmohs, F., Becker, U. & Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 152, 224108 (2020).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Martínez, L., Andrade, R., Birgin, E. G. & Martínez, J. M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 30, 2157–2164 (2009).
Thompson, A. P. et al. LAMMPS—a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 271, 108171 (2022).
Deng, B. et al. CHGNet as a pretrained universal neural network potential for charge-informed atomistic modelling. Nat. Mach. Intell. 5, 1031–1041 (2023).
Larsen, A. H. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Mat. 29, 273002 (2017).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos 22025507 and 21931012), the Key Research Program of Frontier Sciences, CAS (ZDBS-LYSLH020), the Beijing National Laboratory for Molecular Sciences (BNLMS-CXXM-202010), the RGC General Research Fund (grant no. 17309620), Seed Fund of the University of Hong Kong (project code: 2201101550) and Hong Kong Quantum AI Lab Limited, Air @ InnoHK of Hong Kong Government. We thank Q. Li, A. Guan, N. Wu and J. Xiang from the Center for Physiochemical Analysis and Measurement in the Institute of Chemistry (CAS) for the NMR test. The authors also thank N. Grundish for polishing the manuscript to improve its readability.
Author information
Authors and Affiliations
Contributions
J.L. synthesized and characterized the cellulose-based electrolytes; Z.H., S.C. and G.H.C. carried out the theoretical analysis and the related discussions; S.Z., H.Z. and S.G. participated in the test and discussion of the electrochemical characterizations; G.Z and Z.P. performed the NMR characterization; Y.Q. and Y.L. contributed to the design of cellulose-based electrolytes; A.-M.C. supervised the project; and Y.L., Y.Q., J.L. and A.-M.C. wrote the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Seung Woo Lee, Chunpeng Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25, discussion and Tables 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Hu, Z., Zhang, S. et al. Molecular engineering of renewable cellulose biopolymers for solid-state battery electrolytes. Nat Sustain 7, 1481–1491 (2024). https://doi.org/10.1038/s41893-024-01414-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41893-024-01414-7