Abstract
The forests of Southeast Asia harbour high levels of species diversity, providing a plethora of ecosystem services. However, this biodiversity is threatened by both climate and land-use change, the impacts of which are poorly understood. We modelled 1,498 tree species distributions under four shared socioeconomic pathways (SSPs) with varying global change intensities, and classified species into 11 spatially associated groups. We found both global changes to cause severe losses in tree distributions. Only under SSP1–2.6, the sustainable pathway with low intensities in both global changes, were distribution losses mitigated. Unexpectedly, losses were overall greatest under intermediate climate change pathways SSP2–4.5 and SSP3–7.0 rather than under the most extreme pathway, SSP5–8.5. This was because, although climate-driven losses were greatest under SSP5–8.5, land-use-driven losses were much more extensive under SSP2–4.5 and SSP3–7.0 than under SSP5–8.5. Crucially, other than SSP1–2.6, each SSP led to worst-case scenarios for several species groups. Our findings highlight that to most effectively conserve Southeast Asian tree distributions, policymakers need to prioritize a sustainable pathway that mitigates both global changes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data used in this study are publicly available, except for occurrence data from SING Herbarium, Singapore Botanical Gardens. Occurrence data were obtained from the Global Biodiversity Information Facility (https://www.gbif.org) and the SING Herbarium. Past and future climate variables were obtained from the WorldClim database (https://www.worldclim.org/). Past land-use variables were obtained from the HILDA+ Global Land Use Change database (https://doi.org/10.1594/PANGAEA.921846) and future land-use variables from the Land Use Harmonised dataset (https://luh.umd.edu/) and the GCAM–Demeter land-use dataset by Chen et al.23 (https://data.pnnl.gov/group/nodes/dataset/13192). Static soil variables were obtained from the SoilGrids database (https://soilgrids.org/). The results data used to make Figs. 1–4 are available via Figshare (https://doi.org/10.6084/m9.figshare.22547140) (ref. 51).
Code availability
The computer code used in the analyses is available on request from the corresponding author. The code used to make Figs. 1–4 is available via Figshare (https://doi.org/10.6084/m9.figshare.22547140) (ref. 51).
References
Joyce, E., Thiele, K., Slik, F. & Crayn, D. Checklist of the vascular flora of the Sunda–Sahul Convergence Zone. Biodivers. Data J. 8, e51094 (2020).
Pillay, R. et al. Tropical forests are home to over half of the world’s vertebrate species. Front. Ecol. Environ. 20, 10–15 (2022).
Sodhi, N. S., Koh, L. P., Brook, B. W. & Ng, P. K. Southeast Asian biodiversity: an impending disaster. Trends Ecol. Evol. 19, 654–660 (2004).
Woodruff, D. S. Biogeography and conservation in Southeast Asia: how 2.7 million years of repeated environmental fluctuations affect today’s patterns and the future of the remaining refugial-phase biodiversity. Biodivers. Conserv. 19, 919–941 (2010).
Urban, M. C. Accelerating extinction risk from climate change. Science 348, 571–573 (2015).
Newbold, T., Oppenheimer, P., Etard, A. & Williams, J. J. Tropical and Mediterranean biodiversity is disproportionately sensitive to land-use and climate change. Nat. Ecol. Evol. 4, 1630–1638 (2020).
Colwell, R. K., Brehm, G., Cardelús, C. L., Gilman, A. C. & Longino, J. T. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322, 258–261 (2008).
Trisos, C. H., Merow, C. & Pigot, A. L. The projected timing of abrupt ecological disruption from climate change. Nature 580, 496–501 (2020).
Achard, F. et al. Determination of tropical deforestation rates and related carbon losses from 1990 to 2010. Glob. Change Biol. 20, 2540–2554 (2014).
Jamaludin, J., De Alban, J. D. T., Carrasco, L. R. & Webb, E. L. Spatiotemporal analysis of deforestation patterns and drivers reveals emergent threats to tropical forest landscapes. Environ. Res. Lett. 17, 054046 (2022).
Karger, D. N., Kessler, M., Lehnert, M. & Jetz, W. Limited protection and ongoing loss of tropical cloud forest biodiversity and ecosystems worldwide. Nat. Ecol. Evol. 5, 854–862 (2021).
Pang, S. E. H., Slik, J. W. F., Zurell, D. & Webb, E. L. The clustering of spatially associated species unravels patterns in tropical tree species distributions. Ecosphere 14, e4589 (2023).
Powers, R. P. & Jetz, W. Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Change 9, 323–329 (2019).
Guisan, A. et al. Predicting species distributions for conservation decisions. Ecol. Lett. 16, 1424–1435 (2013).
Struebig, M. J. et al. Targeted conservation to safeguard a biodiversity hotspot from climate and land-cover change. Curr. Biol. 25, 372–378 (2015).
Pang, S. E. H., De Alban, J. D. T. & Webb, E. L. Effects of climate change and land cover on the distributions of a critical tree family in the Philippines. Sci. Rep. 11, 276 (2021).
IPCC Climate Change 2021: The Physical Science Basis (eds Masson-Delmotte, V. et al.) (Cambridge Univ. Press, 2021).
Riahi, K. et al. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: an overview. Glob. Environ. Change 42, 153–168 (2017).
Warren, R. et al. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Change 3, 678–682 (2013).
IUCN Red List Categories and Criteria: Version 3.1 (IUCN SSC, 2001).
Beech, E., Rivers, M., Oldfield, S. & Smith, P. P. GlobalTreeSearch: the first complete global database of tree species and country distributions. J. Sustain. For. 36, 454–489 (2017).
Hurtt, G. C. et al. Harmonization of global land-use change and management for the period 850-2100 (LUH2) for CMIP6. Geosci. Model Dev. 13, 5425–5464 (2020).
Chen, M. et al. Global land use for 2015–2100 at 0.05° resolution under diverse socioeconomic and climate scenarios. Sci. Data 7, 320 (2020).
Hannah, L. et al. 30% land conservation and climate action reduces tropical extinction risk by more than 50%. Ecography 43, 943–953 (2020).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Zeng, Y., Friess, D. A., Sarira, T. V., Siman, K. & Koh, L. P. Global potential and limits of mangrove blue carbon for climate change mitigation. Curr. Biol. 31, 1737–1743.e3 (2021).
Sarira, T. V., Zeng, Y., Neugarten, R., Chaplin-Kramer, R. & Koh, L. P. Co-benefits of forest carbon projects in Southeast Asia. Nat. Sustain. 5, 393–396 (2022).
Wyse, S. V., Dickie, J. B. & Willis, K. J. Seed banking not an option for many threatened plants. Nat. Plants 4, 848–850 (2018).
Meyfroidt, P. & Lambin, E. F. The causes of the reforestation in Vietnam. Land Use Policy 25, 182–197 (2008).
Meyfroidt, P. & Lambin, E. F. Forest transition in Vietnam and displacement of deforestation abroad. Proc. Natl Acad. Sci. USA 106, 16139–16144 (2009).
Xian, J., Xia, C. & Cao, S. Cost–benefit analysis for China’s Grain for Green Program. Ecol. Eng. 151, 105850 (2020).
Yue, Y. et al. Large scale reforestation of farmlands on sloping hills in South China karst. Landsc. Ecol. 35, 1445–1458 (2020).
Esquivel‐Muelbert, A. et al. Compositional response of Amazon forests to climate change. Glob. Change Biol. 25, 39–56 (2019).
Peterson, A. T., Papeş, M. & Soberón, J. Mechanistic and correlative models of ecological niches. Eur. J. Ecol. 1, 28–38 (2015).
Pang, S. E. H., Zeng, Y., De Alban, J. D. T. & Webb, E. L. Occurrence–habitat mismatching and niche truncation when modelling distributions affected by anthropogenic range contractions. Divers. Distrib. 28, 1327–1343 (2022).
Song, X.-P. et al. Massive soybean expansion in South America since 2000 and implications for conservation. Nat. Sustain. 4, 784–792 (2021).
Hausfather, Z. & Peters, G. P. Emissions—the ‘business as usual’ story is misleading. Nature 577, 618–620 (2020).
Cassman, K. G. & Grassini, P. A global perspective on sustainable intensification research. Nat. Sustain. 3, 262–268 (2020).
Derived Dataset (GBIF, 2023); https://doi.org/10.15468/dd.htbsz8
Govaerts, R., Lughadha, E. N., Black, N., Turner, R. & Paton, A. The World Checklist of Vascular Plants, a continuously updated resource for exploring global plany diversity. Sci. Data 8, 215 (2021).
Zizka, A. et al. CoordinateCleaner: standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 10, 744–751 (2019).
Hengl, T. et al. SoilGrids250m: global gridded soil information based on machine learning. PLoS ONE 12, e0169748 (2017).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1 km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Kamworapan, S. & Surussavadee, C. Evaluation of CMIP5 global climate models for simulating climatological temperature and precipitation for Southeast Asia. Adv. Meteorol. 2019, 1067365 (2019).
Knutti, R., Masson, D. & Gettelman, A. Climate model genealogy: generation CMIP5 and how we got there. Geophys. Res. Lett. 40, 1194–1199 (2013).
Winkler, K., Fuchs, R., Rounsevell, M. & Herold, M. Global land use changes are four times greater than previously estimated. Nat. Commun. 12, 2501 (2021).
Olson, D. M. et al. Terrestrial Ecoregions of the World: a new map of life on Earth. Bioscience 51, 933–938 (2001).
Chamberlain, S. A. & Szöcs, E. taxize: taxonomic search and retrieval in R. F1000Research 2, 191 (2013).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013).
Hijmans, R. J. terra: Spatial Data Analysis. R package version 1.7-65 https://rspatial.github.io/terra/ (2022).
Pang, S. E. H., Slik, J. W. F., Chisholm, R. A. & Webb, E. L. Data and code for figures for 'Conserving Southeast Asian trees requires mitigating both climate and land-use change'. Figshare https://doi.org/10.6084/m9.figshare.22547140 (2023).
Acknowledgements
We thank J. M. T. Miyajima for her feedback on the writing and figures, and Y. Zeng for his comments on the manuscript. We also thank L. R. Carrasco and J. Y. Lim for their comments on an earlier draft of this manuscript. We thank SING Herbarium, Singapore Botanical Gardens, for providing access to their plant occurrence records.
Author information
Authors and Affiliations
Contributions
S.E.H.P. and E.L.W. conceived the study. S.E.H.P. designed the methodology and carried out the data preparation, modelling of tree distributions and analyses of distribution outcomes. S.E.H.P. visualized the results and prepared all figures and tables with inputs from J.W.F.S, R.A.C. and E.L.W. All authors contributed to the interpretation of the results and writing of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Peer review
Peer review information
Nature Sustainability thanks Jiban Deb, Zhenzhong Zeng, Pieter Zuidema and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Distribution of tree species richness across Southeast Asia for all 1498 species, 11 species groups, and for all species across our study period.
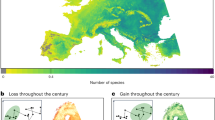
(a) The projected distribution of weighted tree species richness across SEA based on a dataset of 1498 species. Weighted richness was calculated by weighting the presence of each species based on the size of their respective species group—each species group contributed equally—before min-max scaling the values (1 represents the presence of all 1498 species). Lower data availability in Sundaland meant a smaller fraction of known tree species from that region could be modelled, as compared to Indo–Burma and subtropical East Asia. (b) The projected distribution of tree species richness for each group, which was scaled to 0-1 for consistency in values across panels. A total of 11 spatially distinct species groups were identified. Species groups were labelled based on expert knowledge and quantitative examinations of underlying environmental conditions. (a, b) Projections were under climate conditions for the year 1960 and an undisturbed land use scenario. (c) The projected distribution of weighted tree species richness in 1960 and 2019 and the loss of, and gain in, weighted richness from 1960 to 2019, due to past climate and land use changes. Projected gains followed a realistic migration rate. Within richness maps, blacked out areas indicated non-forested areas that persisted from 1960 to 2090, across SSPs. Colour gradients for weighted richness, and the loss of, and gain in, weighted richness, were scaled to 0–0.34 for clarity, with 0.34 representing the highest weighted richness value observed across grid cells, scenarios, and time periods. Climate-related changes in weighted richness was less distinct because climate change impacts among species (groups) were more spatially variable; for more detailed spatial trends see group-specific maps (Supplementary Figs. 7–17). Maps follow a transverse cylindrical equal-area projection with grid cells of 5 km by 5 km.
Extended Data Fig. 2 The mean net changes in tree species distributions due to past global changes from 1960 to 2019 for all 1498 tree species and among the identified 11 species groups.
The mean net changes in tree species distributions (bars and points) due to past global changes from 1960 to 2019 for (a) all 1498 tree species (weighted mean) and (b) among the identified 11 species groups: SEA tropical deciduous lowland (n = 51), Mainland SEA deciduous lowland (n = 210), Central Bornean tropical submontane (n = 56), Sundaic tropical coastal & lowland (n = 185), SEA coastal & mangrove (n = 183), Sundaic tropical lowland (n = 160), Indo–Burmese deciduous lowland & submontane (n = 177), East Asian subtropical submontane (n = 85), SEA temperate & tropical montane (n = 112), Southern Tibetan plateau (n = 150), and Sundaic tropical montane (n = 129). Error bars indicate the 95% confidence intervals.
Extended Data Fig. 3 The gross changes in tree species distributions due to combined global changes, climate change only, or land-use change only from 1960 to 2019 for all 1498 tree species and among the identified 11 species groups.
The gross changes—as gains (blue) and losses (yellow)—in tree species distributions due to combined global changes (bar), climate change only (circle), or land-use change only (asterisk) from 1960 to 2019 for (a) all 1498 tree species (weighted mean) and (b) among the identified 11 species groups (mean).
Extended Data Fig. 4 The mean gross changes in tree species distributions among the identified 11 species groups due to future global changes from 2019 to 2090 under four separate SSPs.
The mean gross changes—as gain (blue) or loss (yellow)—in tree species distributions among the identified 11 species groups due to future global changes from 2019 to 2090 under four separate SSPs: combined (bar), climate change only (circle), or land-use change only (asterisk).
Extended Data Fig. 5 The environmental conditions that characterized the representative distribution of each species group.
The environmental conditions that characterised the representative distribution (as seen in Extended Data Fig. 1b) of each species group. For each representative distribution, environmental conditions from the 95th percentile of pixels were extracted and further weighted based on the pixel’s value (that is, proportion of member species present) to derive the boxplots shown. Hence, the environmental characteristics described here represent the conditions of only the most suitable of sites (or where more of each cluster’s member species is present). The box plots show the median (central line) and interquartile range (IQR) (box), with whiskers extending to ±1.5 × IQR. Values here were used to inform the labels of each species group. Species groups were ordered based on their distribution changes across pathways (as is in the main text): C10 = SEA tropical & deciduous lowland (pixels = 10648); C11 = Mainland SEA deciduous lowland (pixels = 10695); C1 = Central Bornean tropical submontane (pixels = 10342); C4 = Sundaic tropical coastal & lowland (pixels = 10176); C3 = SEA coastal & mangrove (pixels = 10420); C5 = Sundaic tropical lowland (pixels = 10223); C9 = Indo–Burmese deciduous lowland & submontane (pixels = 11511); C7 = East Asian subtropical submontane (pixels = 10943); C8 = SEA temperate & tropical montane (pixels = 10470); C6 = Southern Tibetan Plateau (pixels = 10432); and C2 = Sundaic tropical montane (pixels = 3537). The number of pixels used to quantify the environmental conditions characterising representative distributions differed among clusters because of differences in the number of pixels with tied valued and/or a narrower representative distribution (that is, C2).
Supplementary information
Supplementary Information
Supplementary Figs. 1–41, Tables 1 and 2, Sections 1–7 and references.
Supplementary Data 1
Species list of the final 1,498 tree species that were used in this study. The list includes information on their taxonomy, vernacular name, IUCN status, sample sizes, specific model performances and whether selected for the reanalysis using a subset of species with more sampling data and better model performing.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pang, S.E.H., Slik, J.W.F., Chisholm, R.A. et al. Conserving Southeast Asian trees requires mitigating both climate and land-use change. Nat Sustain 7, 1313–1323 (2024). https://doi.org/10.1038/s41893-024-01417-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41893-024-01417-4