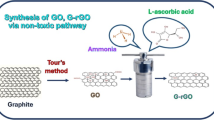
Abstract
Although graphene and graphene-related two-dimensional materials (GR2Ms) hold much potential for various applications, the current methods for their large-scale production rely heavily on graphite minerals and energy-intensive techniques. Here we report a one-step dehydration–condensation method for the economical and green preparation of GR2Ms on a gram scale from biomass at room temperature under atmospheric pressure using only concentrated sulfuric acid. This protocol has been applied successfully to various types of biomass and carbohydrates, delivering a 33% mass yield of GR2M product. The properties of the product are consistent with those of classical reduced graphene oxide (RGO), with the twist that it does not need to be produced from graphite minerals. The mild reaction conditions substantially reduce the energy input, while providing a facile platform for monitoring the kinetics of RGO nucleation and growth. Compared with conventional methods, a 98% reduction in energy consumption is achieved. Overall, the results of this research pave a new avenue to scalable and sustainable GR2M production.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All of the data needed to evaluate the conclusions in the paper are presented in the paper and/or its Supplementary Information. Source data are provided with this paper.
References
Geim, A. K. Graphene: status and prospects. Science 324, 1530–1534 (2009).
Li, D. & Kaner, R. B. Graphene-based materials. Science 320, 1170–1171 (2008).
Ni, G. X. et al. Fundamental limits to graphene plasmonics. Nature 557, 530–533 (2018).
Park, J. M. et al. Robust superconductivity in magic-angle multilayer graphene family. Nat. Mater. 21, 877–883 (2022).
Guo, S., Garaj, S., Bianco, A. & Ménard-Moyon, C. Controlling covalent chemistry on graphene oxide. Nat. Rev. Phys. 4, 247–262 (2022).
Chang, D. et al. Reversible fusion and fission of graphene oxide-based fibers. Science 372, 614–617 (2021).
Stoller, M. D., Park, S., Zhu, Y., An, J. & Ruoff, R. S. Graphene-based ultracapacitors. Nano Lett. 8, 3498–3502 (2008).
Chen, X. et al. Recent advances in fluorinated graphene from synthesis to applications: critical review on functional chemistry and structure engineering. Adv. Mater. 34, 2101665 (2022).
Ming, X. et al. 2D-topology-seeded graphitization for highly thermally conductive carbon fibers. Adv. Mater. 34, 2201867 (2022).
Yang, Z. et al. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: a review. Carbon 141, 467–480 (2019).
Yousefi, N., Lu, X., Elimelech, M. & Tufenkji, N. Environmental performance of graphene-based 3D macrostructures. Nat. Nanotechnol. 14, 107–119 (2019).
Wang, K., Nam Hui, K., Hui, K. S., Peng, S. & Xu, Y. Recent progress in metal–organic framework/graphene-derived materials for energy storage and conversion: design, preparation, and application. Chem. Sci. 12, 5737–5766 (2021).
Jiang, S., Neuman, T., Boeglin, A., Scheurer, F. & Schull, G. Topologically localized excitons in single graphene nanoribbons. Science 379, 1049–1054 (2023).
Kireev, D. et al. Fabrication, characterization and applications of graphene electronic tattoos. Nat. Protoc. 16, 2395–2417 (2021).
Chen, X. et al. Laser-triggered bottom-up transcription of chemical information: toward patterned graphene/MoS2 heterostructures. J. Am. Chem. Soc. 144, 9645–9650 (2022).
Zhuo, H.-Y. et al. Theoretical understandings of graphene-based metal single-atom catalysts: stability and catalytic performance. Chem. Rev. 120, 12315–12341 (2020).
Lin, L., Peng, H. & Liu, Z. Synthesis challenges for graphene industry. Nat. Mater. 18, 520–524 (2019).
Choi, S. H. et al. Large-scale synthesis of graphene and other 2D materials towards industrialization. Nat. Commun. 13, 1484 (2022).
Wyss, K. M., Luong, D. X. & Tour, J. M. Large-scale syntheses of 2D materials: flash Joule heating and other methods. Adv. Mater. 34, 2106970 (2022).
Luong, D. X. et al. Gram-scale bottom-up flash graphene synthesis. Nature 577, 647–651 (2020).
Mayyas, M. et al. Liquid-metal-templated synthesis of 2D graphitic materials at room temperature. Adv. Mater. 32, 2001997 (2020).
Shan, J. et al. Copper acetate-facilitated transfer-free growth of high-quality graphene for hydrovoltaic generators. Natl Sci. Rev. 9, nwab169 (2022).
Chen, C. et al. Sub-10-nm graphene nanoribbons with atomically smooth edges from squashed carbon nanotubes. Nat. Electron. 4, 653–663 (2021).
Kolmer, M. et al. Rational synthesis of atomically precise graphene nanoribbons directly on metal oxide surfaces. Science 369, 571–575 (2020).
Bar-On, Y. M., Phillips, R. & Milo, R. The biomass distribution on Earth. Proc. Natl Acad. Sci. USA 115, 6506–6511 (2018).
Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 41, 1538–1558 (2012).
Dodds, D. R. & Gross, R. A. Chemicals from biomass. Science 318, 1250–1251 (2007).
Sherwood, J. The significance of biomass in a circular economy. Bioresour. Technol. 300, 122755 (2020).
Raiho, A. M. et al. 8000-year doubling of Midwestern forest biomass driven by population- and biome-scale processes. Science 376, 1491–1495 (2022).
Olah, G. A., Prakash, G. K. S. & Goeppert, A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 133, 12881–12898 (2011).
Luo, X. et al. Protection strategies enable selective conversion of biomass. Angew. Chem. Int. Ed. 59, 11704–11716 (2020).
Wang, J. et al. Benefit analysis of multi-approach biomass energy utilization toward carbon neutrality. Innovation 4, 100423 (2023).
Wang, G. et al. A review of recent advances in biomass pyrolysis. Energy Fuels 34, 15557–15578 (2020).
Wang, C. et al. Improvement of the carbon yield from biomass carbonization through sulfuric acid pre-dehydration at room temperature. Bioresour. Technol. 355, 127251 (2022).
Hoekman, S. K., Broch, A. & Robbins, C. Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 25, 1802–1810 (2011).
Xie, L. et al. Sequential superassembly of nanofiber arrays to carbonaceous ordered mesoporous nanowires and their heterostructure membranes for osmotic energy conversion. J. Am. Chem. Soc. 143, 6922–6932 (2021).
Thompson, E., Danks, A. E., Bourgeois, L. & Schnepp, Z. Iron-catalyzed graphitization of biomass. Green Chem. 17, 551–556 (2015).
Xia, S. et al. Fe–Co based synergistic catalytic graphitization of biomass: influence of the catalyst type and the pyrolytic temperature. Energy 239, 122262 (2022).
Sun, L., Gong, Y., Li, D. & Pan, C. Biomass-derived porous carbon materials: synthesis, designing, and applications for supercapacitors. Green Chem. 24, 3864–3894 (2022).
Song, W.-L. et al. Cellulose-derived flake graphite as positive electrodes for Al-ion batteries. Sustain. Energy Fuels 3, 3561–3568 (2019).
Liu, W.-J., Li, W.-W., Jiang, H. & Yu, H.-Q. Fates of chemical elements in biomass during its pyrolysis. Chem. Rev. 117, 6367–6398 (2017).
Wang, D.-C. et al. Confined chemical transitions for direct extraction of conductive cellulose nanofibers with graphitized carbon shell at low temperature and pressure. J. Am. Chem. Soc. 143, 11620–11630 (2021).
Ferrari, A. C. et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 97, 187401 (2006).
Suganuma, S. et al. Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J. Am. Chem. Soc. 130, 12787–12793 (2008).
Liu, W. et al. Supramolecular gold stripping from activated carbon using α-cyclodextrin. J. Am. Chem. Soc. 143, 1984–1992 (2021).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Ahmad Farid, M. A. & Andou, Y. A route towards graphene from lignocellulosic biomass: technicality, challenges, and their prospective applications. J. Clean. Prod. 380, 135090 (2022).
Liu, F. et al. Achievements and challenges of graphene chemical vapor deposition growth. Adv. Funct. Mater. 32, 2203191 (2022).
Hummers, W. S. & Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339–1339 (1958).
Marcano, D. C. et al. Improved synthesis of graphene oxide. ACS Nano 4, 4806–4814 (2010).
Chen, J., Yao, B., Li, C. & Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 64, 225–229 (2013).
Nam, S., French, A. D., Condon, B. D. & Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 135, 1–9 (2016).
Liu, T. et al. One-step room-temperature preparation of expanded graphite. Carbon 119, 544–547 (2017).
Li, H. et al. Layer-by-layer assembly and UV photoreduction of graphene–polyoxometalate composite films for electronics. J. Am. Chem. Soc. 133, 9423–9429 (2011).
Wang, B., Likodimos, V., Fielding, A. J. & Dryfe, R. A. W. In situ electron paramagnetic resonance spectroelectrochemical study of graphene-based supercapacitors: comparison between chemically reduced graphene oxide and nitrogen-doped reduced graphene oxide. Carbon 160, 236–246 (2020).
Acknowledgements
We thank M.-X. Wang at Tsinghua University most sincerely for fruitful discussions. This work was supported by the National Natural Science Foundation of China (22193020, 22193022 and 22371251, Q.-H.G.), the China Postdoctoral Science Foundation (2022M722734, D.-C.W.), the Tsinghua University Initiative Scientific Research Program (Q.-H.G.), the Postdoctoral Science Preferential Funding of Zhejiang Province (ZJ2022100, D.-C.W.), the Intergovernmental Scientific and Technological Innovation Cooperation Project of Zhejiang Province (2023C04031, D.-C.W.) and the Starry Night Science Fund of the ZJU Shanghai Institute for Advanced Study (SN-ZJU-SIAS-006, Q.-H.G.). H.-Y.Y. acknowledges support from the National Natural Science Foundation of China (42125105). We thank Zhejiang University (ZJU) and the ZJU-Hangzhou Global Scientific and Technological Innovation Center (ZJU-HIC) for their continued support. J.F.S. acknowledges support from Northwestern University. We thank G.-Q. Zhu, L.-N. Wang, X.-F. Yu, Y.-X. Qian, X.-C. Bo, Y. Wu, X. Xiao and S.-T. Han for their helpful discussions on characterization protocols.
Author information
Authors and Affiliations
Contributions
D.-C.W., J.F.S. and Q.-H.G. conceived the study; D.-C.W., J.F.S. and Q.-H.G. designed the experiments; D.-C.W. conducted the experiments and characterizations, analysed the data; D.-C.W., J.-Z.L., Y.L., S.-N.L. and Q.-H.G. conducted the NMR and ESI-MS analyses and proposed the mechanism; D.-C.W. and S.Z. contributed to the XPS studies; J.-Z.L., Y.W. and Q.-H.G. independently performed the replications and validated the experiments; all of the authors contributed to the data analysis; D.-C.W., H.-Y.Y., L.Q., J.F.S. and Q.-H.G. wrote the draft paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
D.-C.W. and Q.-H.G. have filed two patent applications (Chinese Patent application number 202210494990.3 and US Patent application number US18138020) based on this work, which have been lodged with the ZJU-Hangzhou Global Scientific and Technological Innovation Center. The other authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Hailin Peng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Several features in D-C RGO growth.
a, AFM Images of zoomed-in view of nuclei 1 and 5 in Fig. 5a in the main text. The white arrows point to wrinkles, which were formed as a result of internal stress. b, The RGO microcrystals observed in the AFM images polymerize towards the center of the nuclei and grow into a new layer. c, Irregular edges. d, HRTEM Images and SAED pattern of grain boundary after the combination of the D-C RGO. The similar results were obtained in three independent experiments.
Extended Data Fig. 2 A set of evidence for layer-by-layer growth of D-C RGO.
An AFM image of a growing D-C RGO nucleus. Statistics on the mean length of each layer in the layer-by-layer, the bottom-up growth rate fitting R2 is 0.989. The error bars are presented as the mean ± s.d. (n = 5). The similar results were obtained in three independent experiments.
Extended Data Fig. 3 AFM Images of nucleation and growth for D-C-ginger-RGO.
a, The nucleation process. b, Ribbon-like growth mode. c, Large area of D-C RGO and exfoliated few-layer RGO. The similar results were obtained in three independent experiments.
Extended Data Fig. 4 AFM Images of nucleation and growth for D-C-straw-RGO.
a, The nucleation process. b, Ribbon-like growth mode. c, Large area of D-C RGO and exfoliated few-layer RGO. The similar results were obtained in three independent experiments.
Extended Data Fig. 5 AFM Images of nucleation and growth for D-C-cellulose-RGO.
a, The nucleation process. b, Ribbon-like growth mode. c, Large area of D-C RGO and exfoliated few-layer RGO. The similar results were obtained in three independent experiments.
Extended Data Fig. 6 AFM Images of nucleation and growth for D-C-glucose-RGO.
a, The nucleation process. b, Ribbon-like growth mode. c, Large area of D-C RGO and exfoliated few-layer RGO. The similar results were obtained in three independent experiments.
Supplementary information
Supplementary Information
Supplementary text, characterizations, Figs. 1–72 and Tables 1–5.
Supplementary Video 1
Experimental procedure for the one-step preparation of D–C RGO at room temperature.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Extended Data Fig. 2
Statistical source data for Extended Data Fig. 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, DC., Lv, JZ., Zhong, S. et al. One-step conversion of biomass to reduced graphene oxide at room temperature. Nat Sustain 7, 1699–1708 (2024). https://doi.org/10.1038/s41893-024-01480-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41893-024-01480-x