Abstract
Under a warming climate, wildfires are becoming more frequent and severe. Multicountry studies evaluating associations between wildfire fine particulate matter (PM2.5) and respiratory hospitalizations are lacking. Here we evaluate the short-term effects of wildfire-specific PM2.5 on respiratory hospitalizations from 1,052 communities across Australia, Brazil, Canada, Chile, New Zealand, Vietnam, Thailand and Taiwan, during 2000–2019. A 1 µg m−3 increase in wildfire-specific PM2.5 was associated with increased hospitalization risks for all-cause respiratory, asthma, chronic obstructive pulmonary disease, acute upper respiratory infection, influenza and pneumonia by 0.36%, 0.48%, 0.38%, 0.42%, 0.79% and 0.36%, respectively. Higher risks were observed among populations ≤19 or ≥60 years old, from low-income or high non-wildfire PM2.5 communities, and residing in Brazil, Thailand, Taiwan and Vietnam. Australia and New Zealand exhibited a greater hospitalization risk for asthma associated with wildfire-specific PM2.5. Compared with non-wildfire PM2.5, wildfire-specific PM2.5 posed greater hospitalization risks for all respiratory diseases and a greater burden of asthma. Wildfire-specific PM2.5 contributed to 42.4% of PM2.5-linked respiratory hospitalizations, dominating in Thailand. Overall, the substantial contribution of wildfire-specific PM2.5 to respiratory hospitalizations demands continued mitigation and adaptation efforts across most countries. Intervention should be prioritized for influenza, children, adolescents, the elderly and populations in low-income or high-polluted communities.
Similar content being viewed by others
Main
In recent decades, wildfires, encompassing all types of fire in vegetated landscapes, have been increasingly reported worldwide. This trend is particularly evident in Australia, the Brazilian Amazon, Europe, Russia, Canada and the western United States1. Notably, Canada experienced a record-breaking series of wildfires in 2023, which burned an estimated 185,000 km2, impacting all provinces and territories2. Severe wildfires were also experienced in the United States and Europe during the 2023 fire season3. Climate change is expected to increase the frequency, duration and intensity of extreme wildfire events4. The global fire-prone area is projected to increase by 29%, by the end of the twenty-first century5. This may result in amplified socioeconomic and health burdens6. Wildfires harm human health primarily through wildfire smoke (for example, toxic gases and particles), of which ~90% of the total particle mass is comprised of fine particulate matter with diameters ≤2.5 µm (PM2.5)7.
Compared to urban-sourced PM2.5, the higher submicronic particle content8 and distinct chemical composition of wildfire-specific PM2.5 probably contribute to its increased toxicity at equivalent doses1, potentially posing a greater threat to human health, with respiratory complications as a particular concern6,9. Despite the substantial burden of wildfire-related health impacts on low- and middle-income countries (LMICs)10, research investigating the health consequences of wildfire-specific PM2.5 in these regions remains surprisingly limited. Previous investigations have largely been conducted in high-income countries, such as the United States and Canada, focusing on a specific local area or fire event9,11,12,13,14,15,16,17. This focus potentially hinders the generalizability of findings to other countries disproportionately burdened by wildfires, such as Chile and Brazil. Furthermore, studies in LMICs are imperative to inform targeted intervention and resource allocation directed towards areas with the greatest need, ultimately contributing to global health equity. To date, only two multicountry studies have investigated the impacts of wildfire-specific PM2.5 on respiratory health. One investigated a single outcome (acute respiratory infection) within a specific population (children)18 and the other one focused solely on all-cause respiratory mortality19, without covering other major types of respiratory diseases.
Additionally, few multicountry studies have quantified the spatiotemporal burden of respiratory diseases attributable to wildfire-specific PM2.5, which limits their ability to account for the dynamic vulnerability of populations over time. Further, previous investigations used heterogeneous modelling approaches. This poses methodological challenges in establishing a more representative association between short-term wildfire-specific PM2.5 exposure and respiratory diseases. Finally, few existing literature compared the association of respiratory hospitalizations with wildfire and non-wildfire PM2.5 exposure. Therefore, a systematic analysis of the risks and burden of cause-specific respiratory diseases associated with wildfire-specific PM2.5 is warranted, to provide more comparable evidence, by applying a unified analytic framework to different communities.
Here, by leveraging hospitalization data from eight countries and territories, over 20 years, we aimed to: (1) characterize the concentration–response (C–R) relationship between wildfire-specific PM2.5 and hospitalization risks for all-cause respiratory, asthma, chronic obstructive pulmonary disease (COPD), acute upper respiratory infection (AURI), influenza and pneumonia; (2) identify vulnerable populations; (3) quantify the spatiotemporal trends of respiratory hospitalizations attributable to wildfire-specific PM2.5; and (4) compare the health impacts of wildfire and non-wildfire PM2.5 on respiratory hospitalizations.
Results
Summary statistics
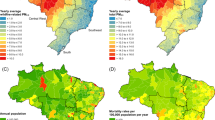
Annually, 1,777,941 all-cause respiratory, 130,618 asthma, 223,661 COPD, 97,275 AURI, 38,502 influenza and 692,691 pneumonia hospitalizations were included in our analyses. These data covered 1,052 communities, in eight countries and territories, during different periods of 2000–2019. On average, there were approximately five all-cause respiratory hospitalizations, two pneumonia hospitalizations and less than one hospitalization from asthma, COPD, AURI and influenza per day (Supplementary Table 1). Countries and territories contributed to a mean of 16.5 years (s.d. = 5.2) of the data. Brazil contributed the highest number of respiratory hospitalizations, followed by Thailand (Supplementary Table 2). Overall, males, children and adolescents, the elderly ≥60 years and individuals from low-income communities or areas with higher non-wildfire PM2.5 levels bore a slightly larger proportion of respiratory hospitalizations relative to their counterparts (Supplementary Table 2). The median daily concentration of wildfire-specific PM2.5 and non-wildfire PM2.5 across study communities was 1.4 (interquartile range (IQR) 3.6, range 0.0–169.2) µg m−3 and 13.4 (IQR 11.0, range 0.0–198.4) µg m−3, respectively. The median daily concentration of non-wildfire PM2.5 was highest in Chile, followed by Thailand and Taiwan (Supplementary Table 1). The average daily concentration of wildfire-specific PM2.5 was pronounced in communities of Brazil, Thailand, Chile, New South Wales Australia, Vietnam and Northwest Canada (Fig. 1 and Supplementary Table 1).
The spatial distribution of average daily wildfire-specific PM2.5 (µg m−3) across 1,052 study communities in Canada, Brazil, New Zealand, Thailand, Australia, Chile, Vietnam and Taiwan during 2000–2019.
Respiratory hospitalization risk and wildfire-specific PM2.5
Wildfire-specific PM2.5 generated persistently elevated risks in respiratory hospitalizations on the current day and the previous day of wildfire-specific PM2.5 exposure (lag 0 and lag 1) (Extended Data Fig. 1). The hospitalization risk of respiratory diseases peaked on the day of exposure (relative risk (RR) = 1.0030, 95% confidence interval (CI) 1.0027–1.0033), followed by a lag of 1 day (RR = 1.0006, 1.0005–1.0008), per 1 µg m−3 increase in wildfire-specific PM2.5. The risks were markedly lower and the CIs contained 1, when longer lag day periods were investigated (Extended Data Fig. 1). Therefore, in the final analyses, we focused on cumulative RR over the lag of 0–1 days, expressed as RR.
When considering the pooled cumulative C–R relationship between wildfire-specific PM2.5 and respiratory hospitalizations during the lag 0–1 days, we observed a monotonically increasing linear or supralinear curve for all categories of respiratory hospitalizations examined. Overall, the RR of respiratory hospitalization from different causes responded linearly to the elevating wildfire-specific PM2.5. This finding was corroborated by community-specific quasi-Bayesian information criterion (qBIC) values. In >92% of the study communities, linear models demonstrated a better fit than nonlinear models, as indicated by lower qBIC values (Supplementary Table 3). The strongest relationship was observed for influenza, where the RR exhibited a notable initial increase before 20 µg m−3, followed by a gentler incline up to 50 µg m−3. Similar C–R patterns were observed for AURI, asthma and pneumonia. The C–R curve of COPD exhibited the most pronounced degree of linearity (Fig. 2).
a–f, C–R curves for all-cause respiratory disease (a), asthma (b), COPD (c), AURI (d), influenza (e) and pneumonia (f). The centre of the error bands represents the mean effect estimates comparing days with lower versus higher wildfire-specific PM2.5 levels across 1,052 study communities, with error bands indicating uncertainty ranges derived from the 95% CIs of 1,000 Monte Carlo samples. All the models were controlled for temperature, relative humidity, non-wildfire PM2.5, day of week, seasonality and long-term trend.
Overall, wildfire-specific PM2.5 presented consistent positive associations, for each of the respiratory hospitalizations examined. A 1 µg m−3 increase in wildfire-specific PM2.5, during the lag 0–1 days, was significantly associated with elevated risks in all-cause respiratory (RR = 1.0036, 1.0032–1.0038), asthma (RR = 1.0048, 1.0040–1.0057), COPD (RR = 1.0038, 1.0032–1.0042), AURI (RR = 1.0042, 1.0032–1.0050), influenza (RR = 1.0079, 1.0059–1.0096) and pneumonia (RR = 1.0036, 1.0032–1.0040) (Table 1). Sensitivity analyses confirmed the robustness of our results (Supplementary Figs. 1 and 2 and Supplementary Table 8).
We found that age, community income level, community non-wildfire PM2.5 level and country or territory significantly modified the association between wildfire-specific PM2.5 and respiratory hospitalization risks (Fig. 3 and Supplementary Table 5). Specifically, individuals ≤19 or ≥60 years old presented a greater hospitalization risk in all-cause respiratory disease and pneumonia, compared with those aged 20–59 years old. Greater risks were observed in communities with high non-wildfire PM2.5 levels for all-cause respiratory disease, COPD and pneumonia. In contrast, low non-wildfire PM2.5 communities presented a higher hospitalization risk for asthma. Furthermore, individuals in low-income communities experienced higher hospitalization risks from all-cause respiratory diseases, COPD and pneumonia, compared to higher-income communities. Conversely, greater hospitalization risks for asthma were observed in high-income communities. At the country and territory level, residents of Taiwan generally experienced higher cause-specific risks than other countries and territories. Whereas greater risks were observed, for all-cause respiratory disease in Brazil, Thailand and Vietnam; asthma in New Zealand and Australia; COPD, influenza and pneumonia in Brazil; and AURI in Canada (Supplementary Table 5).
a–d, Relative risk modified by sex (a), age (b), community non-wildfire PM2.5 level (c) and community income level (d). The centre of the error bars represents the mean effect estimates comparing days with lower versus higher wildfire-specific PM2.5 levels across 1,052 study communities, with error bars indicating uncertainty ranges derived from the 95% CIs of 1,000 Monte Carlo samples. All the models were controlled for temperature, relative humidity, non-wildfire PM2.5, day of week, seasonality and long-term trend. Income level was categorized according to tertiles of the community-level GDP per capita: GDP per capita ≤ tertile 1 as low income; tertile 1 < GDP per capita ≤ tertile 2 as middle income; and GDP per capita > tertile 2 as high income. Non-wildfire PM2.5 level was classified according to the 50th percentile of non-wildfire PM2.5 concentration distributions (17.25 µg m−3): average daily non-wildfire PM2.5 during the study period ≤17.25 µg m−3 as low non-wildfire PM2.5 level; and >17.25 µg m−3 as high non-wildfire PM2.5 level. A Wald-type test was used to test the significance of the effect modifications. *A two-sided P for the difference is statistically significant (<0.05).
Annual attributable burden
Overall, 25,321 (20,478–30,114) all-cause respiratory hospitalizations were attributed to wildfire-specific PM2.5 annually, equating to an attributable fraction (AF) of 1.42% (1.15–1.69%). For each year, on average, up to 1.49% (0.86–2.10%) of asthma, 1.30% (0.89–1.70%) of COPD, 1.77% (0.88–2.64%) of AURI, 2.84% (0.90–4.41%) of influenza and 1.57% (1.24–1.89%) of pneumonia hospitalizations, were attributed to wildfire-specific PM2.5. When community income levels were examined, a larger attributable burden was observed in low-income communities for most respiratory diseases and in middle-income communities for asthma. When examined at the country and territory level, the highest cause-specific AFs were found in Taiwan, ranging from 2.29% (1.82–2.76%) for all-cause respiratory diseases to 3.78% (1.65–5.86%) for AURI. In contrast, a relatively lower AF was observed in Canada for asthma (0.45% (0.12–0.77%)), COPD (0.19% (0.04–0.33%)) and AURI (0.62% (0.18–1.05%)) (Table 2 and Supplementary Table 7).
Comparisons between wildfire-specific and non-wildfire PM2.5
Wildfires only constituted a small portion (17.1%) of ambient PM2.5, compared to non-wildfire sources, with a median concentration of 1.4 (IQR 3.6) µg m−3 and 13.4 (IQR 11.0) µg m−3, respectively. However, wildfire-specific PM2.5 was associated with a significantly greater risk and contributed to a substantial proportion of hospitalizations for all the major types of respiratory diseases examined, compared with non-wildfire PM2.5 (Fig. 4 and Supplementary Tables 10 and 11).
a, The relative risks of hospitalizations for cause-specific respiratory disease per 1 µg m−3 increase in wildfire-specific and non-wildfire PM2.5. b, The AFs of hospitalizations for cause-specific respiratory diseases associated with wildfire-specific and non-wildfire PM2.5. The centre of the error bars represents the mean effect estimates comparing days with lower versus higher wildfire-specific PM2.5 levels across 1,052 study communities, with error bars indicating uncertainty ranges derived from the 95% CIs of 1,000 Monte Carlo samples. Models for wildfire-specific PM2.5 were controlled for temperature, relative humidity, non-wildfire PM2.5, day of week, seasonality and long-term trend. Models for non-wildfire PM2.5 were controlled for temperature, relative humidity, wildfire-specific PM2.5, day of week, seasonality and long-term trend. A Wald-type test was used to test the significance of the difference between effect estimates of wildfire-specific and non-wildfire PM2.5. *A two-sided P for difference <0.05.
The spatiotemporal pattern of annual AF of all-cause respiratory hospitalization due to wildfire-specific PM2.5 varied. We found an overall increasing trend in annual AF. This pattern extended to Australia, Canada, Chile, New Zealand and Taiwan during 2000–2019. Taiwan experienced an exceptional rise during the most recent 5 years. In contrast, the annual AF in Brazil and Vietnam decreased over 2005–2019 and 2000–2019, respectively (Extended Data Fig. 2).
For most study periods, wildfire-specific PM2.5 contributed to a substantial proportion of PM2.5-related respiratory hospitalizations, especially in Brazil, Chile, New Zealand, Thailand and Taiwan. In Australia, the annual AF difference (wildfire AF minus non-wildfire AF) exhibited a continuous upward trend from 2005 to 2019, cumulating in wildfire very close to non-wildfire for the first time in 2015–2019. In Brazil, wildfire-specific PM2.5 carried a greater annual AF than non-wildfire PM2.5 in 2010–2014, with the annual AF difference declining over time. A notable reduction was exhibited in the contribution of wildfire-specific PM2.5 to the PM2.5-related AF from 2010–2014 to 2015–2019. Declining annual AF of wildfire PM2.5 in Canada and New Zealand (2005–2019) was accompanied by a growing annual AF difference, especially in New Zealand, where wildfire smoke emerged as the dominant source of PM2.5-linked respiratory hospitalizations. From 2005 to 2014, Chile experienced a rising trend in annual AF of wildfire PM2.5. In Taiwan, both annual AF of wildfire-specific PM2.5 and AF differences increased from 2000 to 2019, respectively. In Vietnam, annual AF of wildfire-specific and non-wildfire PM2.5 increased from 2000 to 2019, with their differences decreasing (Extended Data Fig. 2).
Discussion
To the best of our knowledge, this is the largest study investigating the association between acute exposure to wildfire-specific PM2.5 and cause-specific respiratory hospitalizations. Overall, short-term wildfire-specific PM2.5 exposure was linearly associated with elevated risks in various respiratory hospitalizations, especially for influenza, individuals ≤19 or ≥60 years old, populations in low-income or high-polluted communities and residents of Brazil, Vietnam, Thailand and Taiwan. An estimated 1.42% (1.15–1.69%) of all-cause respiratory hospitalizations were attributable to wildfire-specific PM2.5, increasing overall, in Australia, Taiwan and Vietnam during various periods between 2010 and 2019. Compared with non-wildfire PM2.5, wildfire-specific PM2.5 posed a greater hospitalization risk for all the major type of respiratory diseases. Wildfire emerged as a notable source of PM2.5-linked respiratory hospitalizations overall, in Brazil, Chile, New Zealand, Thailand and Taiwan.
Our findings of the linear C–R relationship20,21 and the elevated risks of respiratory hospitalizations with short-term exposure to wildfire-specific PM2.5 were broadly in line with previous studies11,12,13,14,15,16,17,18,19. However, they mostly focused on several cities or territories within a single country and reported highly heterogeneous risk estimates. For example, a study in Darwin, Australia, found a 9.1% (RR = 1.091, 1.023–1.163) increase in all-cause respiratory hospitalizations per 10 µg m−3 increase in PM2.5 during bushfire events, one day after exposure22. Another study of the 2003 southern California wildfires found a 2.8% (RR = 1.028, 1.014–1.041) increase in respiratory hospitalizations for 10 µg m−3 increase in moving average PM2.5 exposure during the wildfire period23. Others found that a 10 μg m−3 increase in wildfire-related PM2.5 was associated with a 5.09% (RR = 1.0509, 1.0473–1.0544) increase in respiratory hospital admissions over 0–1 days after the exposure in Brazil during 2000–201514. The effect varied across studies possibly due to factors including study population and period, wildfire severity, exposure assessment and time window and modelling strategy. Our unified assessment approach across countries and territories ensured robust comparability and avoids potential publication bias.
The underlying mechanism for the health effects of wildfire-specific PM2.5 remains unclear. Nonetheless, the potential pathways by which PM2.5 affects the respiratory system may also apply to wildfire-specific PM2.5, including injury from free radical peroxidation, unbalanced intracellular calcium homeostasis and inflammation24. Other mechanisms for influenza and asthma are provided in Supplementary Discussion 1. In particular, the greater susceptibility of influenza could be due to greater transmission and gene modification of the influenza virus. Specifically, ambient PM2.5 is suggested as a direct transmission mode for influenza virus infection to the human alveolar epithelium, with nearly half (47%) of inhaled PM2.5 reaching the alveolar epithelium, the primary target site for influenza infection25. Furthermore, PM2.5 may not only act as a carrier but also influence the virus itself. PM2.5 components potentially modify the influenza virus genome25 and pre-exposure to PM2.5 may alter the antiviral response of bronchial epithelial cells, increasing their susceptibility to infection26. The unique characteristics of wildfire-specific PM2.5, including its chemical composition and long-range transport capability, might further facilitate this process, potentially increasing human susceptibility to influenza infection.
Aligned with a previous study in Southern California, 200323, we found that individuals ≤19 or ≥60 years old demonstrated a greater vulnerability to respiratory hospitalization associated with wildfire-specific PM2.5. Such stronger associations among children and adolescents might be explained by environmental, behavioural (for example, prolonged outdoor activities) and physiological factors (for example, underdeveloped detoxification systems)27. However, previous studies have yielded conflicting results. A meta-analysis suggested that young people might be less vulnerable to adverse respiratory effects from wildfire smoke exposure than are adults28. This discrepancy warrants further investigation.
Socioeconomic status played a complex role in the association between wildfire-specific PM2.5 and respiratory diseases. Individuals from disadvantaged communities experienced substantially amplified risks in respiratory hospitalization associated with wildfire-specific PM2.5, supported by a previous study in Northern California29. This greater vulnerability may be multifactorial, possibly including more childhood respiratory infections, lower housing conditions and indoor air quality, deficient nutrition and occupational exposures30. Further unravelling of how socioeconomic status modulates wildfire-specific PM2.5-related health effects demands use of multidimensional measures of socioeconomic status.
Non-wildfire PM2.5 significantly modified the association between wildfire-specific PM2.5 and respiratory hospitalizations. Consistent findings were observed across most respiratory diseases, indicating that individuals residing in areas with higher non-wildfire PM2.5 levels were more susceptible to wildfire smoke. This was probably due to impaired lung function20 affected by chronic exposure to PM2.5 from other sources. This finding is in line with a recent study from North Carolina, United States, reporting that individuals residing in areas with higher chronic PM2.5 exposure may exhibit heightened susceptibility to hospitalization during acute PM2.5 spikes31. Additionally, chronic inflammation resulting from regular exposures to higher levels of air pollution31 may further exacerbate the population susceptibility, during short-term increases in wildfire-specific PM2.5.
We observed significant spatiotemporal variations in hospitalization risks from respiratory diseases, related to wildfire-specific PM2.5. This may be attributed to differences in exposure levels of wildfire-specific and non-wildfire PM2.5, climate conditions, population susceptibility, basic health status and socioeconomic status (Supplementary Discussion 2). Specifically, as we discussed before, areas with higher levels of air pollution may bear greater health risks from wildfire smoke due to impaired lung function and chronic inflammation. This could contribute to the higher respiratory hospitalization rates in Taiwan, Brazil, Thailand and Vietnam, regions with historically higher median concentrations of non-wildfire PM2.5. Additionally, the pronounced annual concentrations of wildfire-specific PM2.5 may also play a role, a finding recently reported31. It is impractical for residents to stay indoors or seek shelter for extended periods, during prolonged wildfire smoke pollution events, as would be feasible for shorter periods, such as in Brazil, Thailand and Vietnam. What is more, housing design also varies across countries and territories. Hotter climates (tropical and subtropical) tend to have more open housing, which offers less protection from outdoor pollution. It is noteworthy that stronger associations of wildfire-specific PM2.5 with asthma hospitalization were found in Australia and New Zealand. This may be explained by poorer resident adaptability, substantial asthma burdens32,33 and high concentrations of various allergens (for example, pollen, dust mites and fungal spores)34. Furthermore, wildfire-specific PM2.5 toxicity can vary by biomass type and fire intensity across communities35. This could also contribute to the geographical disparities in the observed effect estimates.
During the study period, the proportions of respiratory hospitalizations attributable to wildfire-specific PM2.5 increased overall, in Australia, Taiwan and Vietnam. This rising burden could be attributable to increased population vulnerability and exposure to wildfire-specific PM2.5 (refs. 36,37). However, a decreasing trend was exhibited in countries commonly considered wildfire-prone areas (for example, Brazil, Canada and Chile). This may be due to improved resident adaptation ability, increased protective measures taken by residents and a long history of wildfire risk management38. Nonetheless, this does not diminish the severity or the need for addressing the wildfire-related health burden in these regions. While both Brazil and Chile have witnessed declines in the proportion of respiratory hospitalizations attributed to wildfire-specific PM2.5 exposure, wildfires remained a important contributor to PM2.5-linked respiratory hospitalizations in Brazil and the dominant source in Chile. This highlights a greater threat of PM2.5 from wildfire than non-wildfire sources.
The public health significance of PM2.5 from wildfire and non-wildfire sources could be different. A previous study reported that wildfire-specific PM2.5 may pose greater health risks than PM2.5 from other sources39. This may be due to the potential heightened toxicity of wildfire-specific PM2.5, accounting for an increased presence of smaller particles (for example, submicrometre particles and ultrafine particles) and a higher concentration of oxidative and pro-inflammatory components (for example, polycyclic aromatic hydrocarbons and aldehydes)1. This coincided with our findings that wildfire-specific PM2.5 posed a greater hospitalization risk for various respiratory diseases than did non-wildfire PM2.5. Therefore, continued mitigation efforts are warranted to attenuate and reverse the rising difference in the proportion of respiratory hospitalizations due to wildfire and non-wildfire particles, especially in Chile, New Zealand, Thailand, Taiwan and Vietnam.
As by far the largest study on the association between wildfire-specific PM2.5 and respiratory hospitalizations, this study has several strengths. First, we used an extensive multicountry dataset with high statistical power and a unified well-established two-stage analytical framework, including both the exposure assessment method and risk assessment model. This ensured the robustness, generalizability and comparison of the results across countries and territories. No previous study has done this comprehensive consistent assessment, for cause-specific respiratory hospitalization with wildfire-specific PM2.5. Second, the communities included in this study were predominantly regions with a history of wildfires and thereby serve as areas suitable for robust investigations on wildfire PM2.5 and respiratory health. Third, we identified vulnerable populations from different causes, which can inform the development of targeted interventions and aid in addressing environmental injustice. Fourth, the spatiotemporal assessment of the respiratory health burden attributable to wildfire-specific PM2.5 allows for a better understanding of the extent to which wildfire air pollution has affected different populations, in multiple countries and territories over varying periods. This may aid in resource allocation and more cost-effective policy-making. Finally, we compared the effect estimates, AF and spatiotemporal burden of respiratory hospitalizations attributable to wildfire and non-wildfire PM2.5 as few previous studies have reported such findings40. This provided supportive quantitative evidence on the stronger detrimental health effects of PM2.5 from wildfire relative to other sources.
Some limitations should be acknowledged. Despite extensive spatiotemporal coverage, our dataset was still incomplete. For example, some country- and territory-specific effect estimates may not be fully nationally representative as a result of the inclusion of a part of the communities. Specifically, Taiwan only had data from six municipalities; Australia only had data from New South Wales; and data from some communities did not cover the full study period. Moreover, although PM2.5 is the key component of wildfire smoke mixtures, other air pollutants such as ozone were not considered in this analysis. However, our results changed minimally when we controlled for wildfire-specific ozone in the model across all investigated causes. This suggests that the presence of wildfire-specific ozone has a negligible influence on the estimated effects of wildfire-specific PM2.5 on respiratory hospitalizations. Additionally, community-level wildfire-specific PM2.5 exposures were used in the analysis, which may not fully reflect personal exposures because of variations in housing quality and personal protective behaviours. The grid resolution used may not fully capture spatial variability, potentially leading to exposure measurement errors. To address this, we used population-weighted exposure. Overall, our results should be conservative given that a coarser exposure assignment may bias the effect estimates towards the null41. Meanwhile, the potential measurement error introduced by the uncertainties of the fire emission inventory, the GEOS-Chem simulations and machine learning models may also underestimate the effect estimates. Future studies with expanded health data, coupled with broader spatiotemporal coverage and more effect modifiers and air pollutants, could further advance the assessment of the health impacts of wildfire-specific air pollution.
Public health implications
Wildfire impacts are still likely to intensify as the global climate continues to warm1. In the context of the notable contribution of wildfire-specific PM2.5 exposure to respiratory burdens, prioritizing robust mitigation and adaptation strategies across diverse countries and territories emerges as a critical public health imperative, especially for diseases, populations and areas experiencing a greater susceptibility. Some solutions consist of raising awareness of wildfire-related health risks targeted at policy-makers, clinicians and the public, improving emission control, closely monitoring wildfire air pollution levels and strengthening community preparedness through resource planning and allocation.
Methods
Hospitalization and socioeconomic data
On the basis of the international classification of diseases nineth (ICD-9) revision or ICD-10 codes, we obtained daily hospitalization data for all-cause respiratory diseases, asthma, COPD, AURI, influenza and pneumonia across 1,052 communities in eight countries and territories, with a median area of 5,185.3 km2 (IQR 11,664.2 km2) and a median population of 139,256 (IQR 271,027). The hospitalization data for all countries and territories fell within the period 2000 to 2019, with differing start and/or end dates: for Australia, Brazil and New Zealand, hospitalization data were from 2000 to 2019; for Canada, data were from 2005 to 2019; for Chile, data were from 2001 to 2019; for Thailand, data were from 2015 to 2019; for Taiwan, data were from 2000 to 2018; and for Vietnam, data were from 2002 to 2015. Additional details on data collection, ICD codes and data coverage are provided in Supplementary Methods 1.
We estimated the community-level gross domestic product (GDP) per capita, by dividing the total GDP by the population count in each community, using data from the global gridded GDP (10 × 10 km2) (ref. 42) and the gridded population of the world (GPW, v.4, 1 × 1 km2) (ref. 43). Community-specific population and total GDP were calculated by aggregating the corresponding values of grid cells within each community. We then categorized community-specific socioeconomic status, based on tertiles of GDP per capita, across study communities: GDP per capita ≤ tertile 1 as low income; tertile 1 < GDP per capita ≤ tertile 2 as middle income; and GDP per capita > tertile 2 as high income. We also assigned the community non-wildfire PM2.5 level according to the 50th percentile of non-wildfire PM2.5 concentration distribution across study communities (≤17.25 µg m−3 as low level and >17.25 µg m−3 as high level)44.
Finally, we analysed a multicountry daily hospitalization dataset of cause-specific respiratory diseases, across 1,052 communities, in eight countries and territories, during different periods of 2000–2019, characterized by age, sex, community income level, community non-wildfire PM2.5 level and country and territory. More information on data collection and processing is in Supplementary Methods 1.
Wildfire-specific PM2.5 and meteorological data
As detailed in our previous work37, daily concentrations of wildfire-specific PM2.5 at a 0.25° × 0.25° (~28 × 28 km2) spatial resolution during 2000–2019, were estimated using a chemical transport model (GEOS-Chem) and calibrated through a machine learning approach. Briefly, wildfire-specific PM2.5 was estimated as differences in GEOS-Chem-derived simulations for total PM2.5, under two scenarios with and without wildfire emissions. The GEOS-Chem-derived estimates of total PM2.5 from the original 2.0° × 2.5° spatial resolution (~220 × 280 km2) were interpolated to 0.25° × 0.25° grid-cell resolution to match other auxiliary variables used in machine learning calibration, using inverse distance weighted spatial interpolation10,37,45. Then we trained a random forest machine learning model to calibrate the GEOS-Chem-derived estimates of total PM2.5 against ground-level daily total PM2.5 measurements, with daily gridded (0.25° × 0.25° resolution) meteorological parameters (mean/minimum/maximum temperature, temperature variations, relative humidity, wind speed, precipitation, air pressure and ultraviolet radiation) and calendar year, calendar month, day of week, day of year, longitude and latitude as auxiliary variables37. According to tenfold cross-validation, the random forest-calibrated total PM2.5 concentrations accounted for 91.0% (root mean squared error = 8.5 µg m−3) of the variability observed in ground-level measurements37. Finally, the calibrated wildfire-specific PM2.5 concentrations were estimated by multiplying the calibrated total PM2.5 concentrations with the GEOS-Chem-derived wildfire-to-all ratio in each 0.25° × 0.25° grid cell.
We obtained the daily ambient temperature and dewpoint temperature from the European Centre for medium-range weather forecasts reanalysis v.5 (ERA5)46, at the 0.25° × 0.25° grid-cell resolution. These data were used to calculate the daily relative humidity for each community. Details on the collection and calculation of meteorological data are shown in Supplementary Methods 2. Subsequently, three community-specific, daily data values were assigned to each study community: the population-weighted wildfire-specific PM2.5 concentrations, temperature and relative humidity. For communities smaller than the 0.25° × 0.25° grid, the value assigned to such a community is the value of the grid cell it falls entirely within. The value assigned to a community exceeding the grid size is the average value across all grid cells covered at least partly by that community.
Statistical analysis
We implemented a standard two-stage analytic framework, to estimate the association between short-term exposure to daily wildfire-specific PM2.5 and cause-specific respiratory hospitalization. The two-stage design is a flexible and computationally efficient analytical framework commonly used in environmental epidemiology to model large, heterogeneous data from multiple communities19,47,48,49. This approach allows for separate modelling of community-specific characteristics, preserving crucial local nuances and mitigating potential biases that could arise from pooling diverse data into a single model (Supplementary Methods 3).
In the first stage, we used a quasi-Poisson regression with a distributed lag nonlinear model in each community, with the following equation:
Where Yit denotes the hospitalization counts in community i on day t; cb is a distributed lag model incorporated with a crossbasis47 function of a linear term over the current and previous seven (lag 0–7) days and two internal knots placed at equally spaced values in the log scale of lag days; ns is the natural cubic spline function; and d.f. is the degree of freedom. To account for the confounding effects of temperature and relative humidity (RH), we included moving averaged daily ambient temperature (Temp) and RH during lag 0–7 days in the model. Daily non-wildfire PM2.5 concentrations were adjusted to control for the potential confounding effects of PM2.5 from other sources; time variable was fitted in an ns term of seven degrees of freedom per year to account for long-term trends and seasonality; DOW is the categorical variable for day of the week to control for weekly pattern and β is its coefficient; and α is the intercept.
In the second stage, we pooled the community-specific cumulative effect estimates over the lag period within each country and territory, using a random-effect meta-analytic model. This allowed us to generate the country- and territory-specific estimates, as per a 1 µg m−3 increment in wildfire-specific PM2.5. Our preliminary analyses revealed moderate heterogeneity in effect estimates across communities for cause-specific respiratory hospitalization (I2 34–58%) (Supplementary Table 4). Consequently, we pooled the community-specific estimates to derive overall estimates.
To characterize the C–R relationship between wildfire-specific PM2.5 and respiratory hospitalization, we fitted a B-spline function with two knots at the 25th and 75th percentiles of mean wildfire-specific PM2.5 concentration distributions50,51,52. For each country and territory, individual community percentiles were first averaged to obtain country- and territory-level values, which were then used to compute an overall average across all countries and territories50,51,52. To further test the linearity of the C–R relationships, we compared community-specific qBIC values of linear models with nonlinear models (Supplementary Methods 4). We conducted various sensitivity analyses to assess the robustness of our results (Supplementary Methods 5).
To explore potential effect modifiers, we performed subgroup analyses by age, sex, community income level and community non-wildfire PM2.5 level. We tested the significance of the effect modifications using the Wald-type test53.
Under a causal assumption, we estimated attributable cases and AF of respiratory hospitalizations attributable to short-term exposure to wildfire-specific PM2.5, using the pooled country- and territory-specific effect estimates, as detailed elsewhere54. Briefly, we calculated the respiratory hospitalizations attributable to wildfire-specific PM2.5 in each community, using pooled country- and territory-specific effect estimates. Subsequently, we divided the total hospitalizations attributable to wildfire-specific PM2.5 by the total hospitalizations across all the study communities or communities within a specific country and territory, to obtain the overall and country- and territory-specific AF. We quantified the uncertainty in attributable cases and AF using 95% empirical CI, calculated with 1,000 Monte Carlo simulations54. We further explored the temporal trends of attributable burden, which were first calculated on the basis of year-specific effect estimates and then aggregated by four different periods: 2000–2004, 2005–2009, 2010–2014 and 2015–2019.
To compare the health impacts of wildfire-specific PM2.5 and non-wildfire PM2.5, we estimated the hospitalization risk for all respiratory diseases included, as per 1 µg m−3 increase in non-wildfire PM2.5. We quantified the proportion of respiratory hospitalizations attributable to non-wildfire PM2.5 during the study period. We measured the difference in the proportion of respiratory hospitalizations attributable to wildfire-specific and non-wildfire PM2.5, as AF of wildfire-specific PM2.5 minus AF of non-wildfire PM2.5 (termed AF difference), across different periods of countries and territories.
All analyses were implemented by R software (v.4.0.5 for Windows) using mixmeta53 and dlnm55 R packages. A two-sided P < 0.05 was determined as statistically significant.
Inclusion and ethics
This study has been approved by the Monash University Human Research Ethics Committee.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Publicly available data are found here: the global gridded GDP (https://www.nature.com/articles/sdata20184); the GPW (https://cmr.earthdata.nasa.gov/search/concepts/C1597158029-SEDAC.html); surface temperature and ambient dewpoint temperature from ERA5 (https://www.ecmwf.int/en/forecasts/datasets/reanalysis-datasets/era5); modern-era retrospective analysis for research and applications v.2 (MERRA-2) data, biomass burning emissions inventory of global fire emissions database v.4.1 (GFED v.4.1) data and anthropogenic emissions inventory of EDGAR v.4.2 data that support the GEOS-Chem model development and wildfire-specific PM2.5 simulation in this study are available from https://gmao.gsfc.nasa.gov/reanalysis/MERRA-2/, https://daac.ornl.gov/VEGETATION/guides/fire_emissions_v4_R1.html and http://edgar.jrc.ec.europa.eu/, respectively. For respiratory hospitalization data, the authors are not permitted to share the data. Interested researchers should contact the corresponding author S.L. (shanshan.li@monash.edu) on a case-by-case basis. Source data are provided with this paper.
Code availability
Analysis codes are available from the corresponding authors on request and will be shared on https://github.com/Yiwen-Zhang-259/Multicountry_Wildfire_Respiratory_Hospitalization.git.
Change history
18 April 2025
In the version of this article initially published, sections of the map for Canada in Fig. 1 were missing detail and have now been updated in the HTML and PDF versions of the article.
References
Xu, R. et al. Wildfires, global climate change, and human health. New Engl. J. Med. 383, 2173–2181 (2020).
Fire Statistics (Canadian Interagency Forest Fire Centre, 2023); https://ciffc.net/statistics
Zhou, S., Xu, R., Chen, G., Yu, P. & Guo, Y. Where is the boundary of wildfire smoke? Innov. Med. 1, 100024 (2023).
Morawska, L. et al. The state of science on severe air pollution episodes: quantitative and qualitative analysis. Environ. Int. 156, 106732 (2021).
Senande-Rivera, M., Insua-Costa, D. & Miguez-Macho, G. Spatial and temporal expansion of global wildland fire activity in response to climate change. Nat. Commun. 13, 1208 (2022).
Reid, C. E. et al. Critical review of health impacts of wildfire smoke exposure. Environ. Health Perspect. 124, 1334–1343 (2016).
Vicente, A. et al. Emission factors and detailed chemical composition of smoke particles from the 2010 wildfire season. Atmos. Environ. 71, 295–303 (2013).
Makkonen, U., Hellén, H., Anttila, P. & Ferm, M. Size distribution and chemical composition of airborne particles in south-eastern Finland during different seasons and wildfire episodes in 2006. Sci. Total Environ. 408, 644–651 (2010).
Zhang, Y. et al. Health impacts of wildfire smoke on children and adolescents: a systematic review and meta-analysis. Curr. Environ. Health Rep. 11, 46–60 (2024).
Xue, T. et al. Associations between exposure to landscape fire smoke and child mortality in low-income and middle-income countries: a matched case-control study. Lancet Planet. Health 5, e588–e598 (2021).
Reid, C. E. et al. Differential respiratory health effects from the 2008 northern California wildfires: a spatiotemporal approach. Environ. Res. 150, 227–235 (2016).
Matz, C. J. et al. Health impact analysis of PM2.5 from wildfire smoke in Canada (2013–2015, 2017–2018). Sci. Total Environ. 725, 138506 (2020).
Liu, J. C. et al. Wildfire-specific fine particulate matter and risk of hospital admissions in urban and rural counties. Epidemiology 28, 77 (2017).
Ye, T. et al. Risk and burden of hospital admissions associated with wildfire-related PM2.5 in Brazil, 2000–15: a nationwide time-series study. Lancet Planet. Health 5, e599–e607 (2021).
Morgan, G. et al. Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology 21, 47–55 (2010).
Johnston, F. H. et al. Air pollution events from forest fires and emergency department attendances in Sydney, Australia 1996–2007: a case-crossover analysis. Environ. Health 13, 105 (2014).
Martin, K. L., Hanigan, I. C., Morgan, G. G., Henderson, S. B. & Johnston, F. H. Air pollution from bushfires and their association with hospital admissions in Sydney, Newcastle and Wollongong, Australia 1994–2007. Aust. NZ J. Public Health 37, 238–243 (2013).
Li, J. et al. Landscape fire smoke enhances the association between fine particulate matter exposure and acute respiratory infection among children under 5 years of age: findings of a case-crossover study for 48 low-and middle-income countries. Environ. Int. 171, 107665 (2023).
Chen, G. et al. Mortality risk attributable to wildfire-related PM2.5 pollution: a global time series study in 749 locations. Lancet Planet. Health 5, e579–e587 (2021).
Kelly, F. J. & Fussell, J. C. Air pollution and public health: emerging hazards and improved understanding of risk. Environ. Geochem. Health 37, 631–649 (2015).
Wen, J., Heft-Neal, S., Baylis, P., Boomhower, J. & Burke, M. Quantifying fire-specific smoke exposure and health impacts. Proc. Natl Acad. Sci. USA 120, e2309325120 (2023).
Crabbe, H. Risk of respiratory and cardiovascular hospitalisation with exposure to bushfire particulates: new evidence from Darwin, Australia. Environ. Geochem. Health 34, 697–709 (2012).
Delfino, R. J. et al. The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occup. Environ. Med. 66, 189–197 (2009).
Xing, Y.-F., Xu, Y.-H., Shi, M.-H. & Lian, Y.-X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 8, E69 (2016).
Hsiao, T.-C. et al. Interactions of chemical components in ambient PM2.5 with influenza viruses. J. Hazard. Mater. 423, 127243 (2022).
Chivé, C. et al. Exposure to PM2.5 modulate the pro-inflammatory and interferon responses against influenza virus infection in a human 3D bronchial epithelium model. Environ. Pollut. 348, 123781 (2024).
Goldizen, F. C., Sly, P. D. & Knibbs, L. D. Respiratory effects of air pollution on children. Pediatr. Pulmonol. 51, 94–108 (2016).
Kondo, M. C. et al. Meta-analysis of heterogeneity in the effects of wildfire smoke exposure on respiratory health in North America. Int. J. Environ. Res. Public Health 16, 960 (2019).
Reid, C. et al. Effect modification of the association between fine particulate air pollution during a wildfire event and respiratory health by area-level measures of socio-economic status, race/ethnicity, and smoking prevalence. Environ. Res. Health 1, 025005 (2023).
Doiron, D. et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur. Resp. J. 54, 1802140 (2019).
Cowan, K. N. et al. Effect measure modification of the association between short-term exposures to PM2.5 and hospitalizations by long-term PM2.5 exposure among a cohort of people with chronic obstructive pulmonary disease (COPD) in North Carolina, 2002–2015. Environ. Health 22, 49 (2023).
Chronic Respiratory Conditions (Australian Institute of Health and Welfare, 2023); www.aihw.gov.au/reports/chronic-respiratory-conditions/chronic-respiratory-conditions/contents/summary
Asthma (Asthma and Respiratory Foundation of New Zealand, 2023); www.asthmafoundation.org.nz/your-health/living-with-asthma
Gautier, C. & Charpin, D. Environmental triggers and avoidance in the management of asthma. J. Asthma Allergy 10, 47–56 (2017).
Kim, Y. H. et al. Mutagenicity and lung toxicity of smoldering vs. flaming emissions from various biomass fuels: implications for health effects from wildland fires. Environ. Health Perspect. 126, 017011 (2018).
Xu, R. et al. Climate change, environmental extremes, and human health in Australia: challenges, adaptation strategies, and policy gaps. Lancet Reg. Health Western Pacific 40, 100936 (2023).
Xu, R. et al. Global population exposure to landscape fire air pollution from 2000 to 2019. Nature 621, 521–529 (2023).
Tymstra, C., Stocks, B. J., Cai, X. & Flannigan, M. D. Wildfire management in Canada: review, challenges and opportunities. Prog. Disaster Sci. 5, 100045 (2020).
Aguilera, R., Corringham, T., Gershunov, A. & Benmarhnia, T. Wildfire smoke impacts respiratory health more than fine particles from other sources: observational evidence from southern California. Nat. Commun. 12, 1493 (2021).
Jiao, A., Headon, K., Han, T., Umer, W. & Wu, J. Associations between short-term exposure to wildfire particulate matter and respiratory outcomes: a systematic review. Sci. Total Environ. 907, 168134 (2024).
Smith, G. S. et al. Air pollution and pulmonary tuberculosis: a nested case–control study among members of a northern California health plan. Environ. Health Perspect. 124, 761–768 (2016).
Kummu, M., Taka, M. & Guillaume, J. H. A. Gridded global datasets for Gross Domestic Product and Human Development Index over 1990–2015. Sci. Data 5, 180004 (2018).
Center For International Earth Science Information Network-CIESIN-Columbia University. Gridded Population of the World, Version 4 (GPWv4): Population Density, Revision 11 (Version 4.11) [Data set]. Earth Data https://doi.org/10.7927/H49C6VHW (2017).
Wang, Q. et al. Independent and combined effects of heatwaves and PM 2.5 on preterm birth in Guangzhou, China: a survival analysis. Environ. Health Perspect. 128, 017006 (2020).
Ye, T. et al. Short-term exposure to wildfire-related PM2.5 increases mortality risks and burdens in Brazil. Nat. Commun. 13, 7651 (2022).
Hersbach, H. et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 146, 1999–2049 (2020).
Gasparrini, A. et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 386, 369–375 (2015).
Liu, C. et al. Ambient particulate air pollution and daily mortality in 652 cities. New Engl. J. Med. 381, 705–715 (2019).
Vicedo-Cabrera, A. M. et al. Short term association between ozone and mortality: global two stage time series study in 406 locations in 20 countries. Br. Med. J. 368, m108 (2020).
Huang, W.-Z. et al. Improved morbidity-based air quality health index development using Bayesian multi-pollutant weighted model. Environ. Res. 204, 112397 (2022).
Chen, R. et al. Fine particulate air pollution and daily mortality. A nationwide analysis in 272 Chinese cities. Am. J. Resp. Crit. Care Med. 196, 73–81 (2017).
Zhang, Y. et al. Short-term exposure to wildfire-specific PM2.5 and diabetes hospitalization: a study in multiple countries and territories. Diabetes Care 47, 1664–1672 (2024).
Sera, F., Armstrong, B., Blangiardo, M. & Gasparrini, A. An extended mixed-effects framework for meta-analysis. Stat. Med. 38, 5429–5444 (2019).
Gasparrini, A. & Leone, M. Attributable risk from distributed lag models. BMC Med. Res. Method. 14, 55 (2014).
Gasparrini, A. Distributed lag linear and non-linear models in R: the package dlnm. J. Stat. Softw. 43, 1–20 (2011).
Acknowledgements
This study is supported by Australian Research Council (DP210102076) and Australian National Health & Medical Research Council (APP2000581). Yiwen Zhang is supported by the NHMRC e-Asia Joint Research Program Grant (GNT2000581). R.X. is supported by Monash Faculty of Medicine Nursing and Health Science (FMNHS) Bridging Postdoctoral Fellowships 2022 and VicHealth Postdoctoral Research Fellowships 2022. W. Huang and T.Y. are supported by China Scholarship Council funds (W. Huang, 202006380055; T.Y., 201906320051). S.L. was supported by an Emerging Leader Fellowship (GNT2009866) of the Australian National Health and Medical Research Council. Y.G. was supported by Career Development Fellowship (GNT1163693) and Leader Fellowship (GNT2008813) of the Australian National Health and Medical Research Council.
Funding
Open access funding provided by Monash University.
Author information
Authors and Affiliations
Contributions
S.L. and Y.G. designed the study and are co-senior authors. Yiwen Zhang conducted the statistical analysis and took lead in drafting the manuscript and interpreting the results. R.X. estimated the wildfire-specific PM2.5 exposure data. R.X. and W. Huang provided substantial scientific input in interpreting the results and editing of the manuscript. R.X., W. Huang, T.Y., P.Y., W.Y., Y.W., Y.L., Z.Y., B.W., K.J., J.S., M.J.A., A.J., Y.G. and S.L. revised and edited the manuscript. Yiwen Zhang, R.X., W. Huang, T.Y., P.Y., W.Y., Y.W., Y.L., Z.Y. and B.W. cleaned the data. A.C., B.J., D.G., E.L., F.H.J., G.M., L.D.K., Ying Zhang, G.G.M., J.H., J.A., Y.L.G., L.M., M.S.Z.S.C., P.H.N.S., P.M., P.B., S.H., W. Hu and D.P. provided the data and contributed to the submitted version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Santu Ghosh, Colleen Reid and Jennifer Stowell for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The respiratory hospitalization risks associated with a 1 µg/m3 increase in wildfire-specific PM2.5 over the lag 0–7 days.
The center of the error bars represents the mean effect estimates comparing days with lower versus higher wildfire-specific PM2.5 levels across 1052 study communities, with error bars indicating uncertainty ranges derived from the 95% CIs of 1,000 Monte Carlo samples. The models controlled for temperature, relative humidity, non-wildfire PM2.5, day of week, seasonality and long-term trend. Abbreviations: PM2.5, particulate matter with diameter ≤ 2.5 µm; CI, confidence interval.
Extended Data Fig. 2 Spatiotemporal pattern of average annual attributable fraction (%) of all-cause respiratory hospitalizations, attributed to wildfire-specific and non-wildfire PM2.5, during 2000–2019.
2000–04, years from 2000 to 2004; 2005–09, years from 2005 to 2009; 2010–14, years from 2010 to 2014; 2015–19, years from 2015 to 2019. The black points and line curves represent the temporal trend of difference between the proportion of hospitalizations attributable to wildfire-specific PM2.5 and non-wildfire PM2.5, measured as AF of wildfire-specific PM2.5 minus AF of non-wildfire PM2.5. Models for wildfire-specific PM2.5 were controlled for temperature, relative humidity, non-wildfire PM2.5, day of week, seasonality and long-term trend. Models for non-wildfire PM2.5 were controlled for temperature, relative humidity, wildfire-specific PM2.5, day of week, seasonality and long-term trend. Abbreviations: AF, attributable fraction; AUS, Australia; BRA, Brazil; CAN Canada; CHL, Chile; NZL, New Zealand; THA, Thailand; TWN, Taiwan; and VNM, Vietnam.
Supplementary information
Supplementary Information
Supplementary methods, discussion, Figs. 1 and 2 and Tables 1–11.
Source data
Source Data Fig. 1
Source data.
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Source Data Fig. 4
Source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Xu, R., Huang, W. et al. Respiratory risks from wildfire-specific PM2.5 across multiple countries and territories. Nat Sustain 8, 474–484 (2025). https://doi.org/10.1038/s41893-025-01533-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41893-025-01533-9