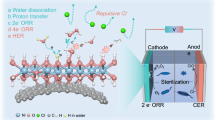
Abstract
Electrocatalytic two-electron oxygen reduction reaction (2e− ORR) in seawater offers a sustainable route for hydrogen peroxide (H2O2) production. However, due to the high concentration of Cl− ions and competitive 4e− ORR, there is a lack of efficient and long-term stable seawater electrocatalysts. Here we report a high-performance electrocatalyst design based on NiPS3 nanosheets enabling efficient H2O2 production from seawater. Specifically, the NiPS3 nanosheets deliver a 2e− ORR selectivity of ∼98%, a H2O2 yield of 6.0 mol gcat−1 h−1 and robust stability for over 1,000 h in simulated seawater. Underlying the exciting performance is the synergy of the S2−, Ni2+ and P4+ sites where the octahedral S2− skeleton repels Cl− ions, the Ni2+ sites enable the modest binding strength of *OOH intermediate, and the P4+ sites interact with H2O to trigger the protonation of proximal O atom of *OOH. The seawater electrocatalysis system also allows for scalable synthesis of solid H2O2, tandem oxidation reaction of biomass to organic acid and direct use of the produced H2O2 as a sterilizing agent. Once integrated with photovoltaics, the solar-powered electrolysis device can operate in real seawater. Our findings pave the way for sustainable conversion of seawater into value-added products.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available within the paper and Supplementary Information. Source data are provided with this paper.
References
Xia, C. et al. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 366, 226–231 (2019).
Sun, Y., Han, L. & Strasser, P. A comparative perspective of electrochemical and photochemical approaches for catalytic H2O2 production. Chem. Soc. Rev. 49, 6605–6631 (2020).
Hou, H. L., Zeng, X. K. & Zhang, X. W. Production of hydrogen peroxide through photocatalytic processes: a critical review of recent advances. Angew. Chem. Int. Ed. 59, 17356 (2020).
Edwards, J. K. & Hutchings, G. J. Palladium and gold–palladium catalysts for the direct synthesis of hydrogen peroxide. Angew. Chem. Int. Ed. 47, 9192–9198 (2008).
Zhang, C. et al. Crystal engineering enables cobalt-based metal–organic frameworks as high-performance electrocatalysts for H2O2 production. J. Am. Chem. Soc. 14, 7797–7799 (2023).
Fan, R. et al. Ultrastable electrocatalytic seawater splitting at ampere-level current density. Nat. Sustain. 7, 158–167 (2024).
Jin, H. et al. Emerging materials and technologies for electrocatalytic seawater splitting. Sci. Adv. 9, eadi7755 (2023).
Chang, J. et al. Dual-doping and synergism toward high-performance seawater electrolysis. Adv. Mater. 33, 2101425 (2021).
Hu, H., Wang, X., Attfield, J. P. & Yang, M. Metal nitrides for seawater electrolysis. Chem. Soc. Rev. 53, 163–203 (2024).
Tan, X. et al. Toward an understanding of the enhanced CO2 electroreduction in NaCl electrolyte over CoPc molecule-implanted graphitic carbon nitride catalyst. Adv. Energy Mater. 11, 2100075 (2021).
Bai, S. et al. On factors of ions in seawater for CO2 reduction. Appl. Catal. B 323, 122166 (2023).
Zhao, Q. et al. Approaching a high-rate and sustainable production of hydrogen peroxide: oxygen reduction on Co–N–C single-atom electrocatalysts in simulated seawater. Energy Environ. Sci. 14, 5444–5456 (2021).
Zhang, E. et al. Engineering the local atomic environments of indium single-atom catalysts for efficient electrochemical production of hydrogen peroxide. Angew. Chem. Int. Ed. 61, e202117347 (2022).
Li, J. et al. Interaction of metal ions in high efficiency seawater hydrogen peroxide production by a carbon-based photocatalyst. Appl. Catal. B 343, 123541 (2024).
Mase, K. et al. Seawater usable for production and consumption of hydrogen peroxide as a solar fuel. Nat. Commun. 7, 11470 (2016).
Chen, L. et al. Seawater electrolysis for fuels and chemicals production: fundamentals, achievements, and perspectives. Chem. Soc. Rev. 53, 7455–7488 (2024).
Wang, N. et al. Regulating N species in N-doped carbon electro-catalysts for high-efficiency synthesis of hydrogen peroxide in simulated seawater. Adv. Sci. 10, 2302446 (2023).
Rao, T. et al. Phase transitions and water splitting applications of 2D transition metal dichalcogenides and metal phosphorous trichalcogenides. Adv. Sci. 8, 2002284 (2021).
Wang, F. et al. New frontiers on van der Waals layered metal phosphorous trichalcogenides. Adv. Funct. Mater. 28, 1802151 (2018).
Li, W. et al. Expediting oxygen evolution by optimizing cation and anion complexity in electrocatalysts based on metal phosphorous trichalcogenides. Angew. Chem. Int. Ed. 62, e202214570 (2023).
Liu, Y. et al. Synergizing hydrogen spillover and deprotonation by the internal polarization field in a MoS2/NiPS3 vertical heterostructure for boosted water electrolysis. Adv. Mater. 34, 2203615 (2022).
Wang, H. et al. Exfoliated 2D layered and nonlayered metal phosphorous trichalcogenides nanosheets as promising electrocatalysts for CO2 reduction. Angew. Chem. Int. Ed. 62, e202217253 (2023).
Yang, P. et al. Highly enhanced chloride adsorption mediates efficient neutral CO2 electroreduction over a dual-phase copper catalyst. J. Am. Chem. Soc. 145, 8714–8725 (2023).
Zhang, S. et al. Boosted photoreforming of plastic waste via defect-rich NiPS3 nanosheets. J. Am. Chem. Soc. 145, 6410–6419 (2023).
Mi, M. et al. Variation between antiferromagnetism and ferrimagnetism in NiPS3 by electron doping. Adv. Funct. Mater. 32, 2112750 (2022).
Wu, Z. et al. Novel photodynamic therapy using two-dimensional NiPS3 nanosheets that target hypoxic microenvironments for precise cancer treatment. Nanophotonics 12, 81–98 (2023).
Budniak, A. K. et al. Spectroscopy and structural investigation of iron phosphorus trisulfide-FePS3. Adv. Optical Mater. 10, 2102489 (2022).
Perry, S. C. et al. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 3, 442–458 (2019).
Cheng, Z., Abernathy, H. & Liu, M. Raman spectroscopy of nickel sulfide Ni3S2. Phys. Chem. C 111, 17997–18000 (2007).
Lliev, M. N. & Schmid, H. Raman spectroscopy of M3B7O13X boracites (M = Cr,Co,Ni,Cu,Zn,Cd; X = Cl,Br,I). J. Raman Spectrosc. 45, 267–273 (2014).
Du, K. et al. Weak van der Waals stacking, wide-range band gap, and Raman study on ultrathin layers of metal phosphorus trichalcogenides. ACS Nano 10, 1738–1743 (2016).
Zhang, C. et al. Trimetallic sulfide hollow superstructures with engineered d-band center for oxygen reduction to hydrogen peroxide in alkaline solution. Adv. Sci. 9, 2104768 (2022).
Sun, Y. et al. Activity-selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal–nitrogen–carbon catalysts. J. Am. Chem. Soc. 141, 12372–12381 (2019).
Wang, Z. et al. Hydrogen peroxide generation with 100% Faradaic efficiency on metal-free carbon black. ACS Catal. 11, 2454–2459 (2021).
Chen, S. et al. Chemical identification of catalytically active sites on oxygen-doped carbon nanosheet to decipher the high activity for electro-synthesis hydrogen peroxide. Angew. Chem. Int. Ed. 60, 16607–16614 (2021).
Fan, M. et al. N–B–OH site-activated graphene quantum dots for boosting electrochemical hydrogen peroxide production. Adv. Mater. 35, 2209086 (2023).
Jung, E. et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020).
Hayyan, M., Hashim, M. A. & AlNashef, I. M. Superoxide ion: generation and chemical implications. Chem. Rev. 116, 3029–3085 (2016).
Woo, J. et al. Heteroatom-doped carbon-based oxygen reduction electrocatalysts with tailored four-electron and two-electron selectivity. Chem. Commun. 57, 7350–7361 (2021).
Li, Z. et al. High-efficiency electroreduction of O2 into H2O2 over ZnCo bimetallic triazole frameworks promoted by ligand activation. Angew. Chem. Int. Ed. 63, e202314266 (2024).
Roth, D., Stirn, J., Stephan, D. W. & Greb, L. Lewis superacidic catecholato phosphonium ions: phosphorus-ligand cooperative C–H bond activation. J. Am. Chem. Soc. 143, 15845–15851 (2021).
Kang, X. et al. A corrosion-resistant RuMoNi catalyst for efficient and long-lasting seawater oxidation and anion exchange membrane electrolyzer. Nat. Commun. 14, 3617 (2023).
Xue, S. et al. NiPS3 nanosheet–graphene composites as highly efficient electrocatalysts for oxygen evolution reaction. ACS Nano 12, 5297–5305 (2018).
Chi, W. S. et al. Employing electrostatic self-assembly of tailored nickel sulfide nanoparticles for quasi-solid-state dye-sensitized solar cells with Pt-free counter electrodes. Chem. Commun. 48, 9501–9503 (2012).
Xia, C. et al. Confined local oxygen gas promotes electrochemical water oxidation to hydrogen peroxide. Nat. Catal. 3, 125–134 (2020).
Li, X. et al. Hydrogen peroxide-mediated tandem catalysis for electrifying chemical synthesis. Chem Catal. 4, 100997 (2024).
Bagno, A. et al. Prediction of the 1H and 13C NMR spectra of r-D-glucose in water by DFT methods and MD simulations. J. Org. Chem. 72, 7373–7381 (2007).
Kumar, S., Sharma, S., Kansai, S. K. & Elumalai, S. Efficient conversion of glucose into fructose via extraction-assisted isomerization catalyzed by endogenous polyamine spermine in the aqueous phase. ACS Omega 5, 2406–2418 (2020).
Ajmi, K. et al. Polyvinyl acetate processing wastewater treatment using combined Fenton’s reagent and fungal consortium: application of central composite design for conditions optimization. J. Hazard. Mater. 358, 243–255 (2018).
Kerley, M. S. et al. Alkaline hydrogen peroxide treatment unlocks energy in agricultural by-products. Science 230, 820–822 (1985).
Jia, J. et al. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 7, 13237 (2016).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P. & Burke, K. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Wang, J. et al. Cu-doped iron oxide for the efficient electrocatalytic nitrate reduction reaction. Nano Lett. 23, 1897–1903 (2023).
Wang, H.-F. & Liu, Z.-P. Comprehensive mechanism and structure-sensitivity of ethanol oxidation on platinum: new transition-state searching method for resolving the complex reaction network. J. Am. Chem. Soc. 130, 10996–11004 (2008).
Shang, C. & Liu, Z.-P. Constrained Broyden minimization combined with the dimer method for locating transition state of complex reactions. J. Chem. Theory Comput. 6, 1136–1144 (2010).
Zhang, X.-J., Shang, C. & Liu, Z.-P. Double-ended surface walking method for pathway building and transition state location of complex reactions. J. Chem. Theory Comput. 9, 5745–5753 (2013).
Acknowledgements
This work received financial support from the National Natural Science Foundation of China NSFC 22075085 (C.Y.), 22475072 (C.Y.) and 22305084 (C.Z.) and East China Normal University Multifunctional Platform for Innovation (004).
Author information
Authors and Affiliations
Contributions
C.Y., C.L. and C.Z. conceived the idea, supervised the work and revised the paper. C.Z. performed most of the experiment. P.S. and G.W. carried out the DFT calculation. Y.Z. and T.B. took part in the material characterizations and electrochemical measurements. X.Z., Z.L. and Y.W. provided suggestions on experimental results. All authors contributed to the discussion and revision of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Mingyang Deng, Juncai Dong, Young Jin Sa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–54 and Tables 1–4.
Source data
Source Data Fig. 1
Characterization data.
Source Data Fig. 2
Electrochemical results.
Source Data Fig. 3
Electrochemical results and calculation data.
Source Data Fig. 4
Application results.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, C., Shan, P., Zou, Y. et al. Stable and high-yield hydrogen peroxide electrosynthesis from seawater. Nat Sustain 8, 542–552 (2025). https://doi.org/10.1038/s41893-025-01538-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41893-025-01538-4
This article is cited by
-
Sustainable chlorine cycle enabled by single atom catalysis
Nature Communications (2025)
-
Hydrogen-bonded organic framework@conductive metal-organic framework heterostructures for ampere-level hydrogen peroxide production
Nature Communications (2025)