Abstract
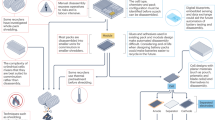
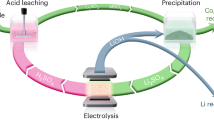
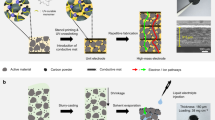
Recycling large quantities of lithium-ion batteries facing retirement is pivotal for resource conservation and environmental sustainability. Direct recycling, while offering a promising avenue with reduced waste compared with pyrometallurgy and hydrometallurgy, often involves intricate and long processes. Here we introduce a water electrolysis-induced separation approach, using H2 or O2 gas bubbling to efficiently separate electrode materials from current collectors. The process achieves 99.5% materials recovery with metal impurities <40 ppm within 34 s for LiFePO4 and 3 s for graphite at 10 mA cm−2, with minimal energy consumption of 11 and 1.1 kJ kgcell−1. Moreover, this approach accommodates various electrode types, encompassing cathodes and anodes from spent batteries or manufacturing scraps. The subsequent dry electrode manufacturing process with lithium replenishment substantially enhances environmental sustainability by eliminating the use of N-methyl pyrrolidone, while maintaining performance through the effective mixing of active materials and conductive agents. An EverBatt analysis underscores a remarkable reduction in energy consumption and waste generation compared with industrially adopted recycling methods. This finding provides an efficient and sustainable solution for battery recycling while ensuring high-quality materials production.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting this research are available in the main text and the Supplementary Information and original data can be obtained from the corresponding authors upon reasonable request. The EverBatt database can be downloaded via https://www.anl.gov/amd/reference/everbatt-model-download-form. Source data are provided with this paper.
Change history
15 May 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41893-025-01583-z
References
Blomgren, G. E. The development and future of lithium ion batteries. J. Electrochem. Soc. 164, A5019–A5025 (2016).
Bauer, C. et al. Charging sustainable batteries. Nat. Sustain. 5, 176–178 (2022).
Mayyas, A., Moawad, K., Chadly, A. & Alhseinat, E. Can circular economy and cathode chemistry evolution stabilize the supply chain of Li-ion batteries? Extr. Ind. Soc. 14, 101253 (2023).
Dunn, J., Kendall, A. & Slattery, M. Electric vehicle lithium-ion battery recycled content standards for the US—targets, costs, and environmental impacts. Resour. Conserv. Recycl. 185, 106488 (2022).
Tao, Y., Rahn, C. D., Archer, L. A. & You, F. Second life and recycling: Energy and environmental sustainability perspectives for high-performance lithium-ion batteries. Sci. Adv. 7, eabi7633 (2021).
Grosjean, C., Miranda, P. H., Perrin, M. & Poggi, P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sustain. Energ. Rev. 16, 1735–1744 (2012).
Holzer, A., Windisch-Kern, S., Ponak, C. & Raupenstrauch, H. A novel pyrometallurgical recycling process for lithium-ion batteries and its application to the recycling of LCO and LFP. Metals 11, 149 (2021).
Pan, C. & Shen, Y. F. Pyrometallurgical recycling of spent lithium-ion batteries from conventional roasting to synergistic pyrolysis with organic wastes. J. Energy Chem. 85, 547–561 (2023).
Sattar, R., Ilyas, S., Bhatti, H. N. & Ghaffar, A. Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep. Purif. Technol. 209, 725–733 (2019).
Vieceli, N. et al. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manag. 71, 350–361 (2018).
Xu, P. P. et al. Efficient direct recycling of lithium-ion battery cathodes by targeted healing. Joule 4, 2609–2626 (2020).
Fan, M. et al. In situ electrochemical regeneration of degraded LiFePO4 electrode with functionalized prelithiation separator. Adv. Energy Mater. 12, 2103630 (2022).
Wang, J. X. et al. Direct conversion of degraded LiCoO2 cathode materials into high-performance LiCoO2: a closed-loop green recycling strategy for spent lithium-ion batteries. Energy Storage Mater. 45, 768–776 (2022).
Yang, J., Wang, W., Yang, H. & Wang, D. One-pot compositional and structural regeneration of degraded LiCoO2 for directly reusing it as a high-performance lithium-ion battery cathode. Green Chem. 22, 6489–6496 (2020).
Yu, X. L. et al. Achieving low-temperature hydrothermal relithiation by redox mediation for direct recycling of spent lithium-ion battery cathodes. Energy Storage Mater. 51, 54–62 (2022).
Yang, Y., Huang, G. Y., Xu, S. M., He, Y. H. & Liu, X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy 165, 390–396 (2016).
Fan, E. S. et al. Selective recovery of Li and Fe from spent lithium-ion batteries by an environmentally friendly mechanochemical approach. ACS Sustain. Chem. Eng. 6, 11029–11035 (2018).
Chen, X. P., Li, S. Z., Wu, X., Zhou, T. & Ma, H. R. In-situ recycling of coating materials and Al foils from spent lithium ion batteries by ultrasonic-assisted acid scrubbing. J. Clean. Prod. 258, 120943 (2020).
Zhao, Y. et al. A novel three-step approach to separate cathode components for lithium-ion battery recycling. Rare Met. 40, 1431–1436 (2021).
Jena, K. K. & Choi, D. S. Recycling of cathode active materials from spent lithium-ion batteries (LIBs): effective methodology for environmental remediation. Mater. Chem. Phys. 311, 128532 (2024).
Liu, K. & Zhang, F. S. Innovative leaching of cobalt and lithium from spent lithium-ion batteries and simultaneous dechlorination of polyvinyl chloride in subcritical water. J. Hazard. Mater. 316, 19–25 (2016).
Yao, H. et al. Recycling of spent lithium iron phosphate cathodes: challenges and progress. ACS Appl. Mater. Interfaces 16, 67087–67105 (2024).
Natarajan, S. & Aravindan, V. An urgent call to spent LIB recycling: whys and wherefores for graphite recovery. Adv. Energy Mater. 10, 2002238 (2020).
Cheng, Q., Marchetti, B., Chen, X. Y., Xu, S. & Zhou, X. D. Separation, purification, regeneration and utilization of graphite recovered from spent lithium-ion batteries—a review. J. Environ. Chem. Eng. 10, 107312 (2022).
Song, D. W. et al. Heat treatment of LiCoO2 recovered from cathode scraps with solvent method. J. Power Sources 249, 137–141 (2014).
Zhang, X. X. et al. Sustainable recycling and regeneration of cathode scraps from industrial production of lithium-ion batteries. ACS Sustain. Chem. Eng. 4, 7041–7049 (2016).
Zhang, H. et al. Transient and dry recycling of battery materials with negligible carbon footprint and roll-to-roll scalability. Energy Environ. Sci. 16, 2561–2571 (2023).
Chen, Z. et al. Reaction-passivation mechanism driven materials separation for recycling of spent lithium-ion batteries. Nat. Commun. 14, 4648 (2023).
He, L. P., Sun, S. Y., Song, X. F. & Yu, J. G. Recovery of cathode materials and Al from spent lithium-ion batteries by ultrasonic cleaning. Waste Manag. 46, 523–528 (2015).
Gastol, D. et al. Reclaimed and up-cycled cathodes for lithium-ion batteries. Glob. Chall. 6, 2200046 (2022).
Lei, C. H. et al. Lithium ion battery recycling using high-intensity ultrasonication. Green Chem. 23, 4710–4715 (2021).
Zhang, B. et al. Self-powered recycling of spent lithium iron phosphate batteries via triboelectric nanogenerator. Energy Environ. Sci. 16, 3873–3884 (2023).
Ji, G. et al. Direct regeneration of degraded lithium-ion battery cathodes with a multifunctional organic lithium salt. Nat. Commun. 14, 584 (2023).
Yang, Y. et al. Simultaneous anodic de-lithiation/cathodic lithium-embedded regeneration method for recycling of spent LiFePO4 battery. Energy Storage Mater. 65, 103081 (2024).
Han, F. et al. Alkali-enhanced polyvinylidene fluoride cracking to deeply remove aluminum impurities for regeneration of battery-grade lithium iron phosphate. Chem. Eng. J. 483, 148973 (2024).
Chen, W. et al. Flash recycling of graphite anodes. Adv. Mater. 35, e2207303 (2023).
Yuwen, C. et al. Efficient recovery and regeneration of waste graphite through microwave stripping from spent batteries anode for high-performance lithium-ion batteries. J. Clean. Prod. 333, 130197 (2022).
Yuan, C., Deng, Y. L., Li, T. H. & Yang, F. Manufacturing energy analysis of lithium ion battery pack for electric vehicles. CIRP Ann-Manuf. Techn. 66, 53–56 (2017).
Dai, Q. et al. EverBatt: A Closed-Loop Battery Recycling Cost and Environmental Impacts Model (Argonne National Lab, 2019).
Acknowledgements
C.W. thanks the support of the National Key Research and Development Program of China (2022YFB3803400), the Shanghai Pujiang Program (22PJ1413400) and the Fundamental Research Funds for Central Universities.
Author information
Authors and Affiliations
Contributions
F.Y. and C.W. conceived the research idea. F.Y., X.C., W.W. and G.L. designed the experiments. F.Y., X.C. and G.Q. carried out electrode fabrication and electrochemical characterization. Q.N., G.Q. and W.W. assisted with material characterization. T.W. and C.W. assisted in revising the paper. All authors discussed the results and contributed to the data analysis. F.Y. and X.C. wrote the paper with contributions from all authors. S.L., Y.H, J.L. and C.W. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Youngsik Kim, Xiangyang Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–55 and Table 1.
Supplementary Video 1
The separation of LFP electrode via the WES method.
Supplementary Video 2
The separation of LFP electrodes from a spent pouch cell via the WES method.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, F., Chen, X., Qu, G. et al. Electrode separation via water electrolysis for sustainable battery recycling. Nat Sustain 8, 520–529 (2025). https://doi.org/10.1038/s41893-025-01539-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41893-025-01539-3
This article is cited by
-
Flash upcycling of spent LiCoO2 into oxygen-suppressed lithium-replenishing agent for high-performance batteries
Nature Communications (2026)
-
Bioinspired Injection Therapy for Spent LiFePO4 Batteries: A Non-Invasive Strategy for Capacity Regeneration and Longevity Enhancement
Nano-Micro Letters (2026)
-
Interfacial Evolution and Accelerated Aging Mechanism for LiFePO4/Graphite Pouch Batteries Under Multi-Step Indirect Activation
Nano-Micro Letters (2026)
-
Elimination of lattice strain to reconstruct ion transport channels facilitates direct regeneration of spent LiFePO4 cathode materials
Science China Materials (2026)
-
Advanced direct recycling enables upcycling of spent lithium-ion batteries
Science China Chemistry (2026)