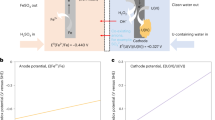
Abstract
Efficient uranium extraction from seawater has the potential to secure an abundant and reliable supply of nuclear fuel, providing affordable energy with minimized carbon emissions. Among the many extraction methods, the electrochemical route has emerged as a promising choice owing to its fast kinetics and materials regeneration. The major challenges facing this technology, however, lie in its high energy consumption, low extraction efficiency and poor selectivity. Here we show a bipolar electrochemical uranium extraction (EUE) system that combines cathodic direct electroreduction of uranium species and electrochemistry-assisted indirect uranium reduction at the anode. The EUE device operates at an ultra-low voltage of merely 0.6 V, exhibiting an efficiency of ~100% for sources with a wide range of uranium concentrations (1–100 ppm). This bipolar EUE system in natural seawater displays excellent uranium selectivity (above 85.3%), long-term stability (45 cycles), low energy consumption (1,944 kWh kg−1 U) and cost advantage (US$83.2 kg−1 U). This work opens an avenue to the electrochemical system design for sustainable recycling of different waste resources.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available within the Article and its Supplementary Information. Source data are provided with this paper.
References
Yang, L. et al. Bioinspired hierarchical porous membrane for efficient uranium extraction from seawater. Nat. Sustain. 5, 71–80 (2021).
Xie, Y. et al. Uranium extraction from seawater: material design, emerging technologies and marine engineering. Chem. Soc. Rev. 52, 97–162 (2023).
Ye, Y. et al. Spontaneous electrochemical uranium extraction from wastewater with net electrical energy production. Nat. Water 1, 887–898 (2023).
Gao, P. et al. Ultra-highly efficient enrichment of uranium from seawater via studtite nanodots growth–elution cycle. Nat. Commun. 15, 6700 (2024).
Yuan, Y. et al. Selective extraction of uranium from seawater with biofouling-resistant polymeric peptide. Nat. Sustain. 4, 708–714 (2021).
Zhu, Z. et al. Versatile carbon-based materials from biomass for advanced electrochemical energy storage systems. eScience 4, 100249 (2024).
Meng, Z. et al. Micro/nano metal–organic frameworks meet energy chemistry: a review of materials synthesis and applications. eScience 3, 100092 (2023).
Yuan, Y. et al. DNA nano-pocket for ultra-selective uranyl extraction from seawater. Nat. Commun. 11, 5708 (2020).
Kaushik, A. et al. Large-area self-standing thin film of porous hydrogen-bonded organic framework for efficient uranium extraction from seawater. Chem 8, 2749–2765 (2022).
Wang, Y. et al. Electrochemical‐mediated regenerable FeII active sites for efficient uranium extraction at ultra‐low cell voltage. Angew. Chem. Int. Ed. 62, e202217601 (2023).
Abney, C. W., Mayes, R. T., Saito, T. & Dai, S. Materials for the recovery of uranium from seawater. Chem. Rev. 117, 13935–14013 (2017).
Chi, F., Zhang, S., Wen, J., Xiong, J. & Hu, S. Highly efficient recovery of uranium from seawater using an electrochemical approach. Ind. Eng. Chem. Res. 57, 8078–8084 (2018).
Zhang, S. et al. Confining Ti-oxo clusters in covalent organic framework micropores for photocatalytic reduction of the dominant uranium species in seawater. Chem 9, 3172–3184 (2023).
Zhang, Y. et al. Boosting uranium extraction from seawater by micro-redox reactors anchored in a seaweed-like adsorbent. Nat. Commun. 15, 9124 (2024).
Qi, J.-X. et al. Ocean wave-driven covalent organic framework/ZnO heterostructure composites for piezocatalytic uranium extraction from seawater. Nat. Commun. 16, 1078 (2025).
Tsarev, S., Collins, R. N., Fahy, A. & Waite, T. D. Reduced uranium phases produced from anaerobic reaction with nanoscale zerovalent iron. Environ. Sci. Technol. 50, 2595–2601 (2016).
Wang, H. et al. Yeast‐raised polyamidoxime hydrogel prepared by ice crystal dispersion for efficient uranium extraction from seawater. Adv. Sci. 11, 2306534 (2024).
Yuan, Y. et al. High-capacity uranium extraction from seawater through constructing synergistic multiple dynamic bonds. Nat. Water 3, 89–98 (2025).
Wu, W. et al. Uranium extraction from seawater via hydrogen bond porous organic cages. J. Am. Chem. Soc. 147, 2228–2236 (2025).
Li, R. et al. A cascade electro-dehydration process for simultaneous extraction and enrichment of uranium from simulated seawater. Water Res. 240, 120079 (2023).
Ye, Y. et al. Electrochemical removal and recovery of uranium: effects of operation conditions, mechanisms, and implications. J. Hazard. Mater. 432, 128723 (2022).
Zhang, C. et al. Overcoming chemical dissociation processes: electrochemical modulation of high‐affinity binding sites for rapid uranium extraction from seawater. Adv. Funct. Mater. 35, 2412712 (2024).
Oladeji, A. V., Courtney, J. M., Fernandez-Villamarin, M. & Rees, N. V. Electrochemical metal recycling: recovery of palladium from solution and in situ fabrication of palladium-carbon catalysts via impact electrochemistry. J. Am. Chem. Soc. 144, 18562–18574 (2022).
Li, M. et al. An electrochemical strategy for simultaneous heavy metal complexes wastewater treatment and resource recovery. Environ. Sci. Technol. 56, 10945–10953 (2022).
Deng, H. et al. Potential‐mediated recycling of copper from brackish water by an electrochemical copper pump. Adv. Sci. 9, 2203189 (2022).
Wang, Z. et al. Constructing an ion pathway for uranium extraction from seawater. Chem 6, 1683–1691 (2020).
Li, T. et al. Heart trabeculae‐inspired superhydrophilic electrode for electric‐assisted uranium extraction from seawater. Adv. Funct. Mater. 35, 2412349 (2024).
Zhang, S., Li, H. & Wang, S. Porous aromatic framework electrodes boost electrochemical uranium extraction. ACS Cent. Sci. 10, 7–9 (2024).
Li, J. et al. Layered charge separation in surface boron doped copper with phosphate groups boosts the electrochemical uranium extraction from seawater. Appl. Catal. B 347, 123770 (2024).
Liu, C. et al. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat. Energy 2, 17007 (2017).
Tsouris, C. Uranium extraction: fuel from seawater. Nat. Energy 2, 17022 (2017).
Yang, H. et al. Functionalized iron–nitrogen–carbon electrocatalyst provides a reversible electron transfer platform for efficient uranium extraction from seawater. Adv. Mater. 33, 2106621 (2021).
Jin, B., Gao, J., Zhang, Y. & Shao, M. Deprotonated of layered double hydroxides during electrocatalytic water oxidation for multi‐cations intercalation. Smart Mol. 2, e20230026 (2024).
Liu, X. et al. Highly efficient electrocatalytic uranium extraction from seawater over an amidoxime‐functionalized In–N–C catalyst. Adv. Sci. 9, 2201735 (2022).
Li, J. et al. Regulation of perovskite oxides composition for the efficient electrocatalytic reactions. Smart Mol. 1, e20220005 (2023).
Rashid, J. et al. A facile synthesis of bismuth oxychloride–graphene oxide composite for visible light photocatalysis of aqueous diclofenac sodium. Sci. Rep. 10, 14191 (2020).
Deng, Y., Handoko, A. D., Du, Y., Xi, S. & Yeo, B. S. In situ raman spectroscopy of copper and copper oxide surfaces during electrochemical oxygen evolution reaction: identification of CuIII oxides as catalytically active species. ACS Catal. 6, 2473–2481 (2016).
Poliakova, T. et al. Uranium oxides structural transformation in human body liquids. Sci. Rep. 13, 4088 (2023).
Zhao, Y. et al. Pressure-induced phase transformation of botallackite α-Cu2(OH)3Cl with a two-dimensional layered structure synthesized via a hydrothermal strategy. J. Phys. Chem. C 124, 9581–9590 (2020).
Yuan, K. et al. Electrochemical and spectroscopic evidence on the one-electron reduction of U(VI) to U(V) on magnetite. Environ. Sci. Technol. 49, 6206–6213 (2015).
Qin, Y. et al. CO intermediate‐assisted dynamic Cu sintering during electrocatalytic CO2 reduction on Cu–N–C catalysts. Angew. Chem. Int. Ed. 63, e202404763 (2024).
Prestipino, C. et al. Structural determination of copper species on the alumina-supported copper chloride catalyst: a detailed EXAFS study. J. Phys. Chem. B 107, 5022–5030 (2003).
Lin, T. et al. Ion pair sites for efficient electrochemical extraction of uranium in real nuclear wastewater. Nat. Commun. 15, 4149 (2024).
Tang, X. et al. Sulfur edge in molybdenum disulfide nanosheets achieves efficient uranium binding and electrocatalytic extraction in seawater. Nanoscale 14, 6285–6290 (2022).
Zhang, Y., Zhou, J., Wang, D., Cao, R. & Li, J. Performance of MXene incorporated MOF-derived carbon electrode on deionization of uranium(VI). Chem. Eng. J. 430, 132702 (2022).
Zhou, J. et al. Pseudocapacitive deionization of uranium(VI) with WO3/C electrode. Chem. Eng. J. 398, 125460 (2020).
Huang, J., Liu, Z., Huang, D., Jin, T. & Qian, Y. Efficient removal of uranium(VI) with a phytic acid-doped polypyrrole/carbon felt electrode using double potential step technique. J. Hazard. Mater. 433, 128775 (2022).
Kim, J. et al. Uptake of uranium from seawater by amidoxime-based polymeric adsorbent: field experiments, modeling, and updated economic assessment. Ind. Eng. Chem. Res. 53, 6076–6083 (2014).
Lindner, H. & Schneider, E. Review of cost estimates for uranium recovery from seawater. Energy Econ. 49, 9–22 (2015).
Chen, D. et al. Enhanced and selective uranium extraction onto electrospun nanofibers by regulating the functional groups and photothermal conversion performance. Chem. Eng. J. 480, 148108 (2024).
Hao, M. et al. Modulating uranium extraction performance of multivariate covalent organic frameworks through donor–acceptor linkers and amidoxime nanotraps. JACS Au 3, 239–251 (2023).
Acknowledgements
We acknowledge support from the National Key R&D Program of China (grant no. 2021YFA1500900 to S.W.), the National Natural Science Foundation of China (grant nos. 22425021 to S.W. and 22272047 and 21905088 to Yanyong W.), the Provincial Natural Science Foundation of Hunan (grant no. 2022JJ10006 to Yanyong W.) and Hunan Provincial Innovation Foundation for Postgraduate (grant no. CX20240455 to Yanjing W.).
Author information
Authors and Affiliations
Contributions
S.W. and Yanyong W. proposed the research direction and guided the project. Yanyong W. proposed the design concept of the system. Yanjing W. conceived the research and performed experiments. S.W., Yanyong W. and Yanjing W. co-wrote the paper with input from all authors. G.W. helped with the cell device design. C.-L.D. and T.T.T.N. carried out the in situ XAFS test. Z.L., J.Y., C.X., S.D., F.Z., Y.C., J.W., Q.L., X.P. and Y.Y. provided suggestions on the experimental results and discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Hui Wu, Wenkun Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–45, Tables 1–23 and Movie 1.
Supplementary Video 1
Video of a small-scale seawater trial in 100 l natural seawater using the bipolar EUE electrolyser stack.
Source data
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Wen, G., Liu, Z. et al. Bipolar electrochemical uranium extraction from seawater with ultra-low cell voltage. Nat Sustain 8, 682–691 (2025). https://doi.org/10.1038/s41893-025-01567-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41893-025-01567-z
This article is cited by
-
Ultra-low voltage bipolar electrochemistry: a game-changer for seawater uranium extraction
Science China Materials (2025)