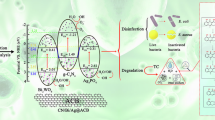
Abstract
Biological wastewater treatment (BWT) is critical to safeguard the aqueous environment. However, enzyme activities in microbial metabolism are inhibited by low temperatures, making BWT implementation in cold environments challenging. Here we propose a strategy to endow BWT facilities with low-temperature operation resilience by integrating photothermal technology with BWT using photothermal carriers (PTCs). Specifically, α-Fe2O3@polyaniline was coated onto a high-conductivity SiC ceramic matrix on a PTC, forming functional partitions for bacterial colonization. The upper layers of the PTCs have an interlaced porous structure and photothermal functions, which provided stable energy conversion and light shielding. The heat conducted downward formed a mesophilic, lightless zone in the lower PTC layers, resulting in high thermal conduction and bioaffinity. Consequently, anammox bacteria, a key biome for sustainable BWT, can be enriched in these PTCs, as evidenced by a 21.4% increase in abundance and a 2.2-fold increase in biomass. With the use of PTCs in a BWT facility under 0.6 kW m−2 illumination, the nitrogen removal performance at low temperature (15 °C) was 5.8 times higher than the case without the use of PTCs. Overall, this work shows how solar energy can be used to enhance the resilience of BWT to low temperatures, improving the applicability of BWT in cold regions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw meta-omics datasets have been deposited in the National Center for Biotechnology Information database under BioProject PRJNA1093580 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1093580). Source data are provided with this paper.
Code availability
All computer codes generated during this study are available from the corresponding author.
References
Wang, Z. et al. A loading rate switch strategy for stable nitritation in mainstream municipal wastewater. Nat. Sustain. 7, 305–314 (2024).
Zhang, M., Ji, B. & Liu, Y. Microalgal-bacterial granular sludge process: a game changer of future municipal wastewater treatment? Sci. Total Environ. 752, 141957 (2021).
Lu, S., Liu, X., Liu, C., Cheng, G. & Shen, H. Influence of photoinhibition on nitrification by ammonia-oxidizing microorganisms in aquatic ecosystems. Rev. Environ. Sci. Biotechnol. 19, 531–542 (2020).
Huo, T. et al. Metabolic acclimation of anammox consortia to decreased temperature. Environ. Int. 143, 105915 (2020).
Gilbert, E. M. et al. Low temperature partial nitritation/anammox in a moving bed biofilm reactor treating low strength wastewater. Environ. Sci. Technol. 48, 8784–8792 (2014).
Petropoulos, E., Dolfing, J., Davenport, R. J., Bowen, E. J. & Curtis, T. P. Developing cold-adapted biomass for the anaerobic treatment of domestic wastewater at low temperatures (4, 8 and 15 °C) with inocula from cold environments. Water Res. 112, 100–109 (2017).
Smith, A. L., Skerlos, S. J. & Raskin, L. Psychrophilic anaerobic membrane bioreactor treatment of domestic wastewater. Water Res. 47, 1655–1665 (2013).
Kouba, V. et al. High-rate partial nitritation of municipal wastewater after psychrophilic anaerobic pretreatment. Environ. Sci. Technol. 51, 11029–11038 (2017).
Xu, J. et al. Start-up of aerobic granular biofilm at low temperature: performance and microbial community dynamics. Sci. Total Environ. 698, 134311 (2020).
Lv, Y., Pan, J., Huo, T., Zhao, Y. & Liu, S. Enhanced microbial metabolism in one stage partial nitritation-anammox system treating low strength wastewater by novel composite carrier. Water Res. 163, 114872 (2019).
Xu, Z. et al. Photosynthetic hydrogen production by droplet-based microbial micro-reactors under aerobic conditions. Nat. Commun. 11, 5985 (2020).
Tetteh, E. K., Amankwa, M. O. & Yeboah, C. Emerging carbon abatement technologies to mitigate energy-carbon footprint: a review. Clean. Mater. 2, 100020 (2021).
Zhang, J. et al. Photothermal Janus anode with photosynthesis-shielding effect for activating low-temperature biological wastewater treatment. Adv. Funct. Mater. 30, 2070045 (2020).
Ding, C. et al. Photothermal enhanced enzymatic activity of lipase covalently immobilized on functionalized Ti3C2TX nanosheets. Chem. Eng. J. 378, 122205 (2019).
Wang, Y. et al. Solar photothermal electrodes for highly efficient microbial energy harvesting at low ambient temperatures. ChemSusChem 11, 4071–4076 (2018).
Wen, G., Deng, X., Wan, Q., Xu, X. & Huang, T. Photoreactivation of fungal spores in water following UV disinfection and their control using UV-based advanced oxidation processes. Water Res. 148, 1–9 (2019).
Gomelsky, M. & Hoff, W. D. Light helps bacteria make important lifestyle decisions. Trends Microbiol. 19, 441–448 (2011).
Li, X. et al. Composite carrier enhanced bacterial adhesion and nitrogen removal in partial nitrification/anammox process. Sci. Total Environ. 868, 161659 (2023).
Liu, D. et al. Ti3C2 MXene as an excellent anode material for high-performance microbial fuel cells. J. Mater. Chem. A 6, 20887–20895 (2018).
Scheidweiler, D. et al. Spatial structure, chemotaxis and quorum sensing shape bacterial biomass accumulation in complex porous media. Nat. Commun. 15, 191 (2024).
Okshevsky, M. & Meyer, R. L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 41, 341–352 (2015).
Hurme, R., Berndt, K. D., Normark, S. J. & Rhen, M. A proteinaceous gene regulatory thermometer in salmonella. Cell 90, 55–64 (1997).
Almblad, H. et al. Bacterial cyclic diguanylate signaling networks sense temperature. Nat. Commun. 12, 1986 (2021).
Tang, X., Guo, Y., Jiang, B. & Liu, S. Metagenomic approaches to understanding bacterial communication during the anammox reactor start-up. Water Res. 136, 95–103 (2018).
Pan, J. et al. Bacterial communication coordinated behaviors of whole communities to cope with environmental changes. Environ. Sci. Technol. 57, 4253–4265 (2023).
Cheng, S. & Logan, B. E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 9, 492–496 (2007).
Yuan, Y., Zhou, S., Liu, Y. & Tang, J. Nanostructured macroporous bioanode based on polyaniline-modified natural loofah sponge for high-performance microbial fuel cells. Environ. Sci. Technol. 47, 14525–14532 (2013).
Tang, W., Yang, K., Qin, J., Li, X. & Niu, X. A 16-year dataset (2000–2015) of high-resolution (3 h, 10 km) global surface solar radiation. Earth Syst. Sci. Data 11, 1905–1915 (2019).
Zhao, Y. et al. Insight into the aggregation capacity of anammox consortia during reactor start-up. Environ. Sci. Technol. 52, 3685–3695 (2018).
Guo, Q. et al. Anaerobic ammonium oxidation (anammox) under realistic seasonal temperature variations: characteristics of biogranules and process performance. Bioresour. Technol. 192, 765–773 (2015).
da Silva, R. J. et al. Extraction of plasmid DNA by use of a magnetic maghemite-polyaniline nanocomposite. Anal. Biochem. 575, 27–35 (2019).
Ray, A. et al. Polyaniline: doping, structure and derivatives. Synth. Met. 29, 41–50 (1989).
Yu, H. Q. Molecular Insights into extracellular polymeric substances in activated sludge. Environ. Sci. Technol. 54, 7742–7750 (2020).
Solano, C., Echeverz, M. & Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 18, 96–104 (2014).
Hira, D. et al. Anammox organism KSU-1 expresses a novel His/DOPA ligated cytochrome c. J. Mol. Biol. 430, 1189–1200 (2018).
Ferousi, C. et al. Discovery of a functional, contracted heme-binding motif within a multiheme cytochrome. J. Biol. Chem. 294, 16953–16965 (2019).
Ling, Y. et al. The influence of oxygen content on the thermal activation of hematite nanowires. Angew. Chem. 124, 4150–4155 (2012).
Hou, X., Liu, S. & Zhang, Z. Role of extracellular polymeric substance in determining the high aggregation ability ofanammox sludge. Water Res. 75, 51–62 (2015).
Grasso, D., Subramaniam, K., Butkus, M., Strevett, K. & Bergendahl, J. A review of non-DLVO interactions in environmental colloidal systems. Rev. Environ. Sci. Biotechnol. 1, 17–38 (2002).
Xu, Y. & Cui, G. Influence of spectral characteristics of the Earth’s surface radiation on the greenhouse effect: principles and mechanisms. Atmos. Environ. 244, 117908 (2021).
Goody, R. M. & Yung, Y. L. Atmospheric Radiation: Theoretical Basis (Oxford Academic, 1989); https://doi.org/10.1093/oso/9780195051346.001.0001
Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface (ASTM International, 2012).
Xu, Y. et al. Origami system for efficient solar driven distillation in emergency water supply. Chem. Eng. J. 356, 869–876 (2019).
Fonseca, D. L. & Bassin, J. P. Investigating the most appropriate methods for attached solids determination in moving-bed biofilm reactors. Bioprocess. Biosyst. Eng. 42, 1867–1878 (2019).
Henning, N. et al. Biological transformation of fexofenadine and sitagliptin by carrier-attached biomass and suspended sludge from a hybrid moving bed biofilm reactor. Water Res. 167, 115034 (2019).
Li, X. Y. & Yang, S. F. Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Res. 41, 1022–1030 (2007).
Osnes, T., Sandstad, O., Skar, V., Osnes, M. & Kierulf, P. Total protein in common duct bile measured by acetonitrile precipitation and a micro bicinchoninic acid (BCA) method. Scand. J. Clin. Lab. Invest. 53, 757–763 (1993).
Loewus, F. A. Improvement in anthrone method for determination of carbohydrates. Anal. Chem. 24, 219 (1952).
Sinclair, P. R., Gorman, N. & Jacobs, J. M. Measurement of heme concentration. Curr. Protoc. Toxicol. https://doi.org/10.1002/0471140856.tx0803s00 (1999).
Huang, D. Q. et al. Removal of extracellular deoxyribonucleic acid increases the permeability and mass transfer of anammox granular sludge with different sizes. Chemosphere 302, 134898 (2022).
Corinaldesi, C., Danovaro, R. & Dell’Anno, A. Simultaneous recovery of extracellular and intracellular DNA suitable for molecular studies from marine sediments. Appl. Environ. Microbiol. 71, 46–50 (2005).
Darling, A. E. et al. PhyloSift: phylogenetic analysis of genomes and metagenomes. PeerJ 9, e243 (2014).
Yang, Y. et al. Discovery of a new genus of anaerobic ammonium oxidizing bacteria with a mechanism for oxygen tolerance. Water Res. 226, 119165 (2022).
Tong, F. et al. The microbiome of the buffalo digestive tract. Nat. Commun. 13, 823 (2022).
Speth, D. R. et al. Genome-based microbial ecology of anammox granules in a full-scale wastewater treatment system. Nat. Commun. 7, 11172 (2016).
Acknowledgements
This work was supported by the National Natural Science Foundations of China (grant no. 52270016) (S.L.) and National Key Research and Development Program of China (grant no. 2022YFC3203003) (S.L.).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the intellectual development of this study, discussed the results and commented on the paper. J.S. and S.L. conceived and designed the study. J.S., Y.F. and Y.M. acquired the data. X.W., L.K., Q.Z. and K.Z. analysed and interpreted the data. J.S., R.Z. and S.L. drafted and revised the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Texts 1–23, Figs. 1–20, Tables 1–4 and References.
Source data
Source Data Figs. 1–5
Source data for the main figures in the paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, J., Feng, Y., Zheng, R. et al. Solar-enhanced low-temperature biological wastewater treatment. Nat Sustain 8, 1048–1057 (2025). https://doi.org/10.1038/s41893-025-01591-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41893-025-01591-z
This article is cited by
-
From mineralization to low-carbon conversion: emerging paradigms in water decontamination
Science China Chemistry (2026)
-
Solar-enhanced biological wastewater treatment
Nature Sustainability (2025)
-
Synergistic catalysis: sodium perborate-mediated advanced oxidation processes coupled with MIL-88B(Fe) for photocatalytic degradation of methylene blue
Research on Chemical Intermediates (2025)