Abstract
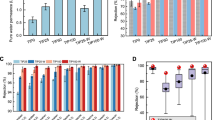
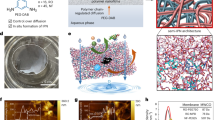
Global water scarcity motivates sustainable clean water production from non-traditional water sources. While existing reverse osmosis (RO) membranes dominate seawater desalination, they are far from ideal for purifying diverse water sources due to inadequate removal of various low-molecular-weight contaminants. Here we overcome this limitation by developing ultraselective polyamide RO membranes via in situ interfacial plasmonic nanoheating integrated interfacial polymerization (IP). The rapid localized heating at the nano-interface of IP boosts the reactivity of monomers, improves the local mass transfer of amine monomers, and facilitates interfacial degassing/vaporization. Consequently, the resultant RO membrane, featuring highly crosslinked polyamide with extensive internal nanovoids, exhibits superior removal for a wide spectrum of toxic and hard-to-remove contaminants found in different water sources, revealing transformative potential for various water treatment scenarios. It also shows a transcendent desalination performance (water permeance of 3.4 l m−2 h−1 bar−1 and NaCl rejection of 99.7%), which further enables efficient desalination of natural seawater for high-quality freshwater, indicating great promise for practical applications. Our study opens a route to develop high-performance RO membranes for effectively purifying diverse water sources towards sustainable clean water production.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in this Article and its Supplementary Information. Source data are provided with this paper.
References
Eliasson, J. The rising pressure of global water shortages. Nature 517, 6 (2015).
Mekonnen, M. M. & Hoekstra, A. Y. Four billion people facing severe water scarcity. Sci. Adv. 2, e1500323 (2016).
Caldera, U. & Breyer, C. Learning curve for seawater reverse osmosis desalination plants: capital cost trend of the past, present, and future. Water Resour. Res. 53, 10523–10538 (2017).
Tang, C. Y. et al. Potable water reuse through advanced membrane technology. Environ. Sci. Technol. 52, 10215–10223 (2018).
Finnerty, C. T. K. et al. The future of municipal wastewater reuse concentrate management: drivers, challenges, and opportunities. Environ. Sci. Technol. 58, 3–16 (2024).
Sima, L. C. & Elimelech, M. More than a drop in the bucket: decentralized membrane-based drinking water refill stations in southeast Asia. Environ. Sci. Technol. 47, 7580–7588 (2013).
Werber, J. R., Deshmukh, A. & Elimelech, M. The critical need for increased selectivity, not increased water permeability, for desalination membranes. Environ. Sci. Technol. Lett. 3, 112–120 (2016).
Jones, E., Qadir, M., van Vliet, M. T. H., Smakhtin, V. & Kang, S. M. The state of desalination and brine production: a global outlook. Sci. Total Environ. 657, 1343–1356 (2019).
Park, H. B., Kamcev, J., Robeson, L. M., Elimelech, M. & Freeman, B. D. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356, eaab0530 (2017).
Yang, Z., Guo, H. & Tang, C. Y. Y. The upper bound of thin-film composite (TFC) polyamide membranes for desalination. J. Membr. Sci. 590, 117297 (2019).
Bernstein, R., Belfer, S. & Freger, V. Toward improved boron removal in RO by membrane modification: feasibility and challenges. Environ. Sci. Technol. 45, 3613–3620 (2011).
Lim, Y. J., Goh, K., Kurihara, M. & Wang, R. Seawater desalination by reverse osmosis: current development and future challenges in membrane fabrication – a review. J. Membr. Sci. 629, 119292 (2021).
Akin, I., Arslan, G., Tor, A., Cengeloglu, Y. & Ersoz, M. Removal of arsenate [As(V)] and arsenite [As(III)] from water by SWHR and BW-30 reverse osmosis. Desalination 281, 88–92 (2011).
Chen, A. S. C., Wang, L., Sorg, T. J. & Lytle, D. A. Removing arsenic and co-occurring contaminants from drinking water by full-scale ion exchange and point-of-use/point-of-entry reverse osmosis systems. Water Res. 172, 115455 (2020).
Kimura, K., Toshima, S., Amy, G. & Watanabe, Y. Rejection of neutral endocrine disrupting compounds (EDCs) and pharmaceutical active compounds (PhACs) by RO membranes. J. Membr. Sci. 245, 71–78 (2004).
Guo, H. et al. Tweak in puzzle: tailoring membrane chemistry and structure toward targeted removal of organic micropollutants for water reuse. Environ. Sci. Technol. Lett. 9, 247–257 (2022).
Freger, V. & Ramon, G. Z. Polyamide desalination membranes: formation, structure, and properties. Prog. Polym. Sci. 122, 101451 (2021).
Culp, T. E. et al. Nanoscale control of internal inhomogeneity enhances water transport in desalination membranes. Science 371, 72–75 (2021).
Ma, X.-H. et al. Nanofoaming of polyamide desalination membranes to tune permeability and selectivity. Environ. Sci. Technol. Lett. 5, 123–130 (2018).
Ukrainsky, B. & Ramon, G. Z. Temperature measurement of the reaction zone during polyamide film formation by interfacial polymerization. J. Membr. Sci. 566, 329–335 (2018).
Peng, L. E. et al. Does interfacial vaporization of organic solvent affect the structure and separation properties of polyamide RO membranes? J. Membr. Sci. 625, 119173 (2021).
Gan, Q., Peng, L. E., Guo, H., Yang, Z. & Tang, C. Y. Cosolvent-assisted interfacial polymerization toward regulating the morphology and performance of polyamide reverse osmosis membranes: increased m-phenylenediamine solubility or enhanced interfacial vaporization? Environ. Sci. Technol. 56, 10308–10316 (2022).
Peng, L. E. et al. Tailoring polyamide rejection layer with aqueous carbonate chemistry for enhanced membrane separation: mechanistic insights, chemistry–structure–property relationship, and environmental implications. Environ. Sci. Technol. 53, 9764–9770 (2019).
Song, X. et al. Intrinsic nanoscale structure of thin film composite polyamide membranes: connectivity, defects, and structure–property correlation. Environ. Sci. Technol. 54, 3559–3569 (2020).
Song, X., Gan, B., Yang, Z., Tang, C. Y. & Gao, C. Confined nanobubbles shape the surface roughness structures of thin film composite polyamide desalination membranes. J. Membr. Sci. 582, 342–349 (2019).
Gan, Q. et al. Nanofoamed polyamide membranes: mechanisms, developments, and environmental implications. Environ. Sci. Technol. 58, 20812–20829 (2024).
Di Vincenzo, M. et al. Biomimetic artificial water channel membranes for enhanced desalination. Nat. Nanotechnol. 16, 190–196 (2021).
Lin, L., Lopez, R., Ramon, G. Z. & Coronell, O. Investigating the void structure of the polyamide active layers of thin-film composite membranes. J. Membr. Sci. 497, 365–376 (2016).
Wong, M. C. Y., Lin, L., Coronell, O., Hoek, E. M. V. & Ramon, G. Z. Impact of liquid-filled voids within the active layer on transport through thin-film composite membranes. J. Membr. Sci. 500, 124–135 (2016).
Hu, Y. W., Wang, F., Yang, Z. & Tang, C. Y. Modeling nanovoid-enhanced water permeance of thin film composite membranes. J. Membr. Sci. 675, 121555 (2023).
Wen, Y. et al. Metal-organic framework enables ultraselective polyamide membrane for desalination and water reuse. Sci. Adv. 8, eabm4149 (2022).
Deshmukh, A., Lienhard, J. H. & Elimelech, M. Heat diffusion during thin-film composite membrane formation. J. Membr. Sci. 696, 122493 (2024).
Jauffred, L., Samadi, A., Klingberg, H., Bendix, P. M. & Oddershede, L. B. Plasmonic heating of nanostructures. Chem. Rev. 119, 8087–8130 (2019).
Naldoni, A., Shalaev, V. M. & Brongersma, M. L. Applying plasmonics to a sustainable future. Science 356, 908–909 (2017).
Zhou, L. et al. Self-assembly of highly efficient, broadband plasmonic absorbers for solar steam generation. Sci. Adv. 2, e1501227 (2016).
Zhan, C. et al. Plasmonic nanoreactors regulating selective oxidation by energetic electrons and nanoconfined thermal fields. Sci. Adv. 7, eabf0962 (2021).
Hirsch, L. R. et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl Acad. Sci. USA 100, 13549–13554 (2003).
Huschka, R. et al. Light-induced release of DNA from gold nanoparticles: nanoshells and nanorods. J. Am. Chem. Soc. 133, 12247–12255 (2011).
Liu, Y. et al. Silver nanoparticle enhanced metal-organic matrix with interface-engineering for efficient photocatalytic hydrogen evolution. Nat. Commun. 14, 541 (2023).
Shao, P. et al. Mixed-valence molybdenum oxide as a recyclable sorbent for silver removal and recovery from wastewater. Nat. Commun. 14, 1365 (2023).
Bastus, N. G., Piella, J. & Puntes, V. Quantifying the sensitivity of multipolar (dipolar, quadrupolar, and octapolar) surface plasmon resonances in silver nanoparticles: the effect of size, composition, and surface coating. Langmuir 32, 290–300 (2016).
Christopher, P., Xin, H. & Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 3, 467–472 (2011).
Zhang, C., Hu, Z. & Deng, B. Silver nanoparticles in aquatic environments: physiochemical behavior and antimicrobial mechanisms. Water Res. 88, 403–427 (2016).
Qi, D. et al. Polymeric membranes with selective solution-diffusion for intercepting volatile organic compounds during solar-driven water remediation. Adv. Mater. 32, e2004401 (2020).
Shih, M.-C. An overview of arsenic removal by pressure-driven membrane processes. Desalination 172, 85–97 (2005).
Conley, D. J. et al. Ecology. Controlling eutrophication: nitrogen and phosphorus. Science 323, 1014–1015 (2009).
Ben-Sasson, M. et al. In situ formation of silver nanoparticles on thin-film composite reverse osmosis membranes for biofouling mitigation. Water Res. 62, 260–270 (2014).
Yang, Z., Guo, H., Yao, Z. K., Mei, Y. & Tang, C. Y. Hydrophilic silver nanoparticles induce selective nanochannels in thin film nanocomposite polyamide membranes. Environ. Sci. Technol. 53, 5301–5308 (2019).
Gan, Q. et al. Does surface roughness necessarily increase the fouling propensity of polyamide reverse osmosis membranes by humic acid? Environ. Sci. Technol. 57, 2548–2556 (2023).
Acknowledgements
This work was financially supported by grants from the Research Grants Council (GRF HKU 17204220 to C.Y.T.) and the Innovation and Technology Fund (GHP/181/20GD to C.Y.T.) of the Hong Kong Special Administrative Region, China. Partial support was also received from the Seed Funding for Strategic Interdisciplinary Research Scheme (102010174 to C.Y.T.) and Outstanding Researcher Award (102010224 to C.Y.T.) at The University of Hong Kong, and the Scientific Research Startup Fund (QD2023012C to H.G.) of Tsinghua Shenzhen International Graduate School.
Author information
Authors and Affiliations
Contributions
Q.G. and H.G. developed the concept and designed the experiments and simulations. W.L. conducted the detection of various micropollutants. Q.X. performed the detection of anions. Z.Y. provided help with the fabrication and experiments. C.Y.T. secured funding and supervised the research project. Q.G., H.G., M.E. and C.Y.T. wrote and revised the manuscript. All co-authors participated in discussions and provided feedback on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Zhongyi Jiang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–43, discussion and Tables 1–7.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gan, Q., Liu, W., Xiao, Q. et al. Plasmonic nanoheating for versatile water purification membranes. Nat Sustain 8, 1190–1198 (2025). https://doi.org/10.1038/s41893-025-01636-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41893-025-01636-3