Abstract
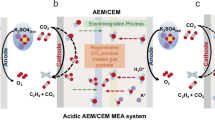
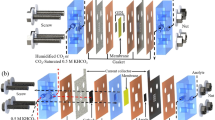
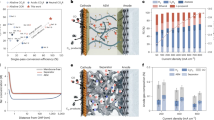
The electrochemical reduction of carbon dioxide (CO2) can produce value-added chemicals and fuels with reduced greenhouse gas emissions by leveraging renewable electricity. However, the economic viability of this technology is limited by high full-cell voltages due to the combination of various materials and processes between the anode and the cathode. Here we analyse voltage distributions within systems that are candidates for achieving energy-efficient CO2 electroreduction: CO2 reduction (CO2R) in acidic media, cascade CO2R-CO electroreduction and CO2 reactive capture. By measuring and resolving the individual contributions to the overall cell voltage, we show that the cascade approach is, at present, the most energy-efficient candidate for achieving economic and scalable CO2R. The membrane and cathode overpotentials dominate in direct CO2R approaches (acidic CO2R and CO2R with forward-biased bipolar membranes). By contrast, the reactive capture approach benefits from a low membrane overpotential (<0.2 V) as a result of the high proton conductivity of the cation exchange membrane, with remaining overpotentials at the cathode, anode and intrinsic Nernstian pH gradient. Applying these insights, we optimize the CO electroreduction system to reach a full-cell voltage of 1.95 V at 200 mA cm−2. Our findings offer a framework for steering the advance of more energy-efficient and scalable CO2R electrolysers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and Supplementary Information. Source data are provided with this paper.
References
O’Brien, C. P. et al. Single pass CO2 conversion exceeding 85% in the electrosynthesis of multicarbon products via local CO2 regeneration. ACS Energy Lett. 6, 2952–2959 (2021).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Ozden, A. et al. Carbon-efficient carbon dioxide electrolysers. Nat. Sustain. 5, 563–573 (2022).
Kibria, M. G. et al. Electrochemical CO2 reduction into chemical feedstocks: from mechanistic electrocatalysis models to system design. Adv. Mater. 31, 1807166 (2019).
Boulamanti, A. & Moya, J. A. Energy Efficiency and GHG Emissions: Prospective Scenarios for the Chemical and Petrochemical Industry Report No. 9789279657344 (EU Science Hub, 2017).
Li, L. et al. Stable, active CO2 reduction to formate via redox-modulated stabilization of active sites. Nat. Commun. 12, 5223 (2021).
Hansen, K. U. & Jiao, F. Creating the right environment. Nat. Energy 6, 1005–1006 (2021).
Burdyny, T. & Smith, W. A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 12, 1442–1453 (2019).
Moore, T. et al. Electrolyzer energy dominates separation costs in state-of-the-art CO2 electrolyzers: implications for single-pass CO2 utilization. Joule 7, 782–796 (2023).
Verma, S., Kim, B., Jhong, H. R. M., Ma, S. & Kenis, P. J. A. A gross-margin model for defining technoeconomic benchmarks in the electroreduction of CO2. ChemSusChem 9, 1972–1979 (2016).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng Chem. Res. 57, 2165–2177 (2018).
Sisler, J. et al. Ethylene electrosynthesis: a comparative techno-economic analysis of alkaline vs membrane electrode assembly vs CO2-CO-C2H4 tandems. ACS Energy Lett. 6, 997–1002 (2021).
Bard, A. J., Faulkner, L. R. & White, H. S. Electrochemical Methods: Fundamentals and Applications (Wiley, 2022).
Ohs, J. H., Sauter, U., Maass, S. & Stolten, D. The effect of the reference electrode position on the measurement of half cell polarization in proton-exchange membrane fuel cells. J. Electrochem. Soc. 159, F181 (2012).
Sorsa, O., Nieminen, J., Kauranen, P. & Kallio, T. Stable reference electrode in polymer electrolyte membrane electrolyser for three-electrode measurements. J. Electrochem. Soc. 166, F1326 (2019).
Zeng, R., Slade, R. C. T. & Varcoe, J. R. An experimental study on the placement of reference electrodes in alkaline polymer electrolyte membrane fuel cells. Electrochim. Acta 56, 607–619 (2010).
He, W. & Van Nguyen, T. Edge effects on reference electrode measurements in PEM fuel cells. J. Electrochem. Soc. 151, A185 (2004).
Salvatore, D. & Berlinguette, C. P. Voltage matters when reducing CO2 in an electrochemical flow cell. ACS Energy Lett. 5, 215–220 (2019).
Hansen, K. U., Cherniack, L. H. & Jiao, F. Voltage loss diagnosis in CO2 electrolyzers using five-electrode technique. ACS Energy Lett. 7, 4504–4511 (2022).
Bohn, L. et al. Reference electrode types for zero-gap CO2 electrolyzers: benefits and limitations. Adv. Sci. 11, 2402095 (2024).
Xu, Q. et al. Integrated reference electrodes in anion-exchange-membrane electrolyzers: impact of stainless-steel gas-diffusion layers and internal mechanical pressure. ACS Energy Lett. 6, 305–312 (2021).
Li, G. & Pickup, P. G. Measurement of single electrode potentials and impedances in hydrogen and direct methanol PEM fuel cells. Electrochim. Acta 49, 4119–4126 (2004).
Niu, S., Li, S., Du, Y., Han, X. & Xu, P. How to reliably report the overpotential of an electrocatalyst. ACS Energy Lett. 5, 1083–1087 (2020).
Wang, S., Lu, A. & Zhong, C.-J. Hydrogen production from water electrolysis: role of catalysts. Nano Convergence 8, 4 (2021).
Gabardo, C. M. et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3, 2777–2791 (2019).
Lee, G. et al. CO2 electroreduction to multicarbon products from carbonate capture liquid. Joule 7, 1277–1288 (2023).
Mitchell, J. B., Chen, L., Langworthy, K., Fabrizio, K. & Boettcher, S. W. Catalytic proton-hydroxide recombination for forward-bias bipolar membranes. ACS Energy Lett. 7, 3967–3973 (2022).
Xiao, H., Cheng, T., Goddard, W. A. & Sundararaman, R. Mechanistic explanation of the pH dependence and onset potentials for hydrocarbon products from electrochemical reduction of CO on Cu (111). J. Am. Chem. Soc. 138, 483–486 (2016).
Dinh, C. T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Ripatti, D. S., Veltman, T. R. & Kanan, M. W. Carbon monoxide gas diffusion electrolysis that produces concentrated C2 products with high single-pass conversion. Joule 3, 240–256 (2019).
Xia, R., Lv, J. J., Ma, X. & Jiao, F. Enhanced multi-carbon selectivity via CO electroreduction approach. J. Catal. 398, 185–191 (2021).
Ozden, A. et al. Energy- and carbon-efficient CO2/CO electrolysis to multicarbon products via asymmetric ion migration–adsorption. Nat. Energy 8, 179–190 (2023).
Alkayyali, T. et al. Pathways to reduce the energy cost of carbon monoxide electroreduction to ethylene. Joule 8, 1478–1500 (2024).
Muhammad, A. et al. in Handbook of Energy Materials (ed. Gupta, R.) 1–19 (Springer, 2023).
Wang, N. et al. Boride-derived oxygen-evolution catalysts. Nat. Commun. 12, 6089 (2021).
Acknowledgements
We gratefully acknowledge funding from the Government of Canada’s New Frontiers in Research Fund (CANSTOREnergy Project NFRFT-2022-00197), the Natural Sciences and Engineering Research Council of Canada and the Canada Research Chairs Program. We also received support from Suncor Canada. F.A. thanks Hatch, CGEN and the University of Toronto for their support through graduate scholarships.
Author information
Authors and Affiliations
Contributions
D.S., E.H.S. and R.K.M. supervised the project. F.A. designed the research. F.A., R.K.M. and A.S.Z. carried out all the experiments. F.A. and R.K.M. analysed the data and wrote the paper. M.Z. performed the COMSOL model simulation. R.K.M. and C.P.O’B. gave guidance on scenario selection and analyses. T.A., J.A. and G.L. assisted with the experiments. T.A. and F.L. assisted with the COMSOL simulation. R.D. and M.F. assisted with the experimental design. All authors contributed to the discussions and assisted during the preparation of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Feng Jiao, Hunter Simonson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1 and 2, Figs. 1–25 and Tables 1–10.
Supplementary Data 1
Source data for supplementary figures.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arabyarmohammadi, F., Miao, R.K., Zeraati, A.S. et al. Voltage distribution within carbon dioxide reduction electrolysers. Nat Sustain 8, 1592–1600 (2025). https://doi.org/10.1038/s41893-025-01643-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41893-025-01643-4