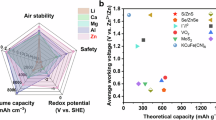
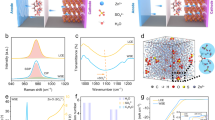
Abstract
Aqueous zinc batteries (AZBs) are promising for sustainable energy storage due to their safety and affordability. Conventional ‘lean-water’ electrolytes improve cell cyclability and the electrochemical stability window by stabilizing the interface; however, ionic transport in the bulk is limited and the use of high-concentration salt jeopardizes their practical adaptability. Here we report a dual-salt electrolyte involving ZnSO4 and Zn(ClO4)2 that decouples the interfacial chemistry from the bulk. Specifically, SO42− ions populate the Zn/electrolyte interface, whereas ClO4− anions dominate in the bulk. Strongly hydrated SO42− stabilizes interfacial water, while weakly hydrated ClO4− disrupts bulk hydrogen-bond networks, suppressing electrolyte freezing and enabling fast Zn2+ transport. In the absence of high salt concentrations and organic solvents, our decoupled electrolyte achieves a high ionic conductivity of 15.1 mS cm⁻1 and Zn plating/stripping reversibility of 99.97% at −40 °C. Assembled Zn//NaV3O8 pouch cells under practical configurations show a daily self-discharge rate of 0.13%, retain 93% capacity after 900 cycles at 25 °C, and deliver full capacity retention over 3,000 cycles at −40 °C. This decoupled dual-salt electrolyte advances the practical deployment of AZBs and offers a strategy for rational and sustainable electrolyte design beyond aqueous systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information, which can also be available from the corresponding author upon request. Source data are provided with this paper and are also available in figshare at https://doi.org/10.6084/m9.figshare.29941601 (ref. 65).
References
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2016).
Bauer, C. et al. Charging sustainable batteries. Nat. Sustain. 5, 176–178 (2022).
Yang, C. et al. All-temperature zinc batteries with high-entropy aqueous electrolyte. Nat. Sustain. 6, 325–335 (2023).
Larcher, D. & Tarascon, J. M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2015).
Innocenti, A., Bresser, D., Garche, J. & Passerini, S. A critical discussion of the current availability of lithium and zinc for use in batteries. Nat. Commun. 15, 4068 (2024).
Han, D. et al. A non-flammable hydrous organic electrolyte for sustainable zinc batteries. Nat. Sustain. 5, 205–213 (2021).
Liang, Y. & Yao, Y. Designing modern aqueous batteries. Nat. Rev. Mater. 8, 109–122 (2022).
Wang, F. et al. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 17, 543–549 (2018).
Dong, D., Wang, T., Sun, Y., Fan, J. & Lu, Y.-C. Hydrotropic solubilization of zinc acetates for sustainable aqueous battery electrolytes. Nat. Sustain. 6, 1474–1484 (2023).
Ji, X. & Nazar, L. F. Best practices for zinc metal batteries. Nat. Sustain. 7, 98–99 (2024).
Blanc, L. E., Kundu, D. & Nazar, L. F. Scientific challenges for the implementation of Zn-ion batteries. Joule 4, 771–799 (2020).
Yuan, L. et al. Regulation methods for the Zn/electrolyte interphase and the effectiveness evaluation in aqueous Zn-ion batteries. Energy Environ. Sci. 14, 5669–5689 (2021).
Wang, Y. et al. Electrolyte engineering enables high performance zinc-ion batteries. Small 18, 2107033 (2022).
Jiang, H. et al. Chloride electrolyte enabled practical zinc metal battery with a near-unity Coulombic efficiency. Nat. Sustain. 6, 806–815 (2023).
Zhang, C. et al. A ZnCl2 water-in-salt electrolyte for a reversible Zn metal anode. Chem. Commun. 54, 14097–14099 (2018).
Zhu, Y. et al. Concentrated dual-cation electrolyte strategy for aqueous zinc-ion batteries. Energy Environ. Sci. 14, 4463–4473 (2021).
Wang, Y. et al. Solvent control of water O-H bonds for highly reversible zinc ion batteries. Nat. Commun. 14, 2720 (2023).
Wang, W. et al. Regulating interfacial reaction through electrolyte chemistry enables gradient interphase for low-temperature zinc metal batteries. Nat. Commun. 14, 5443 (2023).
Wu, Z., Li, Y. & Liu, J. Coulombic efficiency for practical zinc metal batteries: critical analysis and perspectives. Small Methods 8, 2300660 (2023).
Liu, S. et al. Zinc ion batteries: bridging the gap from academia to industry for grid-scale energy storage. Angew. Chem. Int. Ed. 63, e202400045 (2024).
Shi, X. et al. A weakly solvating electrolyte towards practical rechargeable aqueous zinc-ion batteries. Nat. Commun. 15, 302 (2024).
Cho, Y. & Gabbar, H. A. Review of energy storage technologies in harsh environment. Saf. Extreme Environ. 1, 11–25 (2019).
Zhang, N. et al. Critical review on low‐temperature Li‐ion/metal batteries. Adv. Mater. 34, 2107899 (2022).
Zhang, Q. et al. Modulating electrolyte structure for ultralow temperature aqueous zinc batteries. Nat. Commun. 11, 4463 (2020).
Lyu, Y. et al. Organic pH buffer for dendrite‐free and shuttle‐free Zn‐I2 batteries. Angew. Chem. Int. Ed. 135, e202303011 (2023).
Wan, J. et al. Hydrated eutectic electrolyte induced bilayer interphase for high-performance aqueous Zn-ion batteries with 100 °C wide-temperature range. Adv. Mater. 36, e2310623 (2024).
Dong, Y. et al. Non-concentrated aqueous electrolytes with organic solvent additives for stable zinc batteries. Chem. Sci. 12, 5843–5852 (2021).
Zhang, Q. et al. Chaotropic anion and fast-kinetics cathode enabling low-temperature aqueous Zn batteries. ACS Energy Lett. 6, 2704–2712 (2021).
Sun, T., Zheng, S., Du, H. & Tao, Z. Synergistic effect of cation and anion for low-temperature aqueous zinc-ion battery. Nano-Micro Lett. 13, 204 (2021).
You, C. et al. Design strategies for anti-freeze electrolytes in aqueous energy storage devices at low temperatures. Adv. Funct. Mater. 34, 2403616 (2024).
Yu, X. et al. Unlocking dynamic solvation chemistry and hydrogen evolution mechanism in aqueous zinc batteries. J. Am. Chem. Soc. 146, 17103–17113 (2024).
Ru, M. T. et al. On the salt-induced activation of lyophilized enzymes in organic solvents: effect of salt kosmotropicity on enzyme activity. J. Am. Chem. Soc. 122, 1565–1571 (2000).
Takenaka, N., Ko, S., Kitada, A. & Yamada, A. Liquid Madelung energy accounts for the huge potential shift in electrochemical systems. Nat. Commun. 15, 1319 (2024).
Zhang, R. et al. Weakly solvating aqueous-based electrolyte facilitated by a soft co-solvent for extreme temperature operations of zinc-ion batteries. Energy Environ. Sci. 17, 4569–4581 (2024).
Tan, H. et al. Breaking the ice: Hofmeister effect-inspired hydrogen bond network reconstruction in hydrogel electrolytes for high-performance zinc-ion batteries. Small 21, e2410746 (2025).
Huang, S., Hou, L., Li, T., Jiao, Y. & Wu, P. Antifreezing hydrogel electrolyte with ternary hydrogen bonding for high-performance zinc-ion batteries. Adv. Mater. 34, e2110140 (2022).
Pestova, O. N. et al. Structural inhomogeneity in electrolyte solutions: the calcium perchlorate–water system. J. Solut. Chem. 46, 1854–1870 (2017).
Brandán, S. A. Theoretical study of the structure and vibrational spectra of chromyl perchlorate, CrO2(ClO4)2. J. Mol. Struct. 908, 19–25 (2009).
Liu, X. et al. Boosting SO2-tolerant catalytic reduction of NOx via selective adsorption and activation of reactants over Ce4+–SO42– pair sites. ACS Catal. 12, 11306–11317 (2022).
Wang, X. et al. Probing of photocatalytic surface sites on SO42−/TiO2 solid acids by in situ FT-IR spectroscopy and pyridine adsorption. J. Photochem. Photobiol. A 179, 339–347 (2006).
Huang, Z. et al. Effects of anion carriers on capacitance and self-discharge behaviors of zinc ion capacitors. Angew. Chem. Int. Ed. 133, 1024–1034 (2020).
Deng, W. et al. The mechanism and regulation of the electrosorption selectivity of inorganic anions during capacitive deionization. New J. Chem. 45, 16722–16731 (2021).
Xu, K. et al. Steering CO2 electroreduction selectivity towards CH4 and C2H4 on a tannic acid-modified Cu electrode. Mater. Chem. Front. 7, 1395–1402 (2023).
Ataka, K.-i & Osawa, M. In situ infrared study of water−sulfate coadsorption on gold(111) in sulfuric acid solutions. Langmuir 14, 951–959 (1998).
Yamada, Y. et al. Hydrate-melt electrolytes for high-energy-density aqueous batteries. Nat. Energy 1, 16129 (2016).
Ko, S. et al. Electrode potential influences the reversibility of lithium-metal anodes. Nat. Energy 7, 1217–1224 (2022).
Kim, S. C. et al. Potentiometric measurement to probe solvation energy and its correlation to lithium battery cyclability. J. Am. Chem. Soc. 143, 10301–10308 (2021).
Murphy, L. R., Meek, T. L., Allred, A. L. & Allen, L. C. Evaluation and test of Pauling’s electronegativity scale. J. Phys. Chem. A 104, 5867–5871 (2000).
Fugel, M. et al. Revisiting a historical concept by using quantum crystallography: are phosphate, sulfate and perchlorate anions hypervalent? Chem. Eur. J. 25, 6523–6532 (2019).
Islam, S. et al. Triggering the theoretical capacity of Na1.1V3O7.9 nanorod cathode by polypyrrole coating for high-energy zinc-ion batteries. Chem. Eng. J. 446, 137069 (2022).
Li, G. et al. Developing cathode materials for aqueous zinc ion batteries: challenges and practical prospects. Adv. Funct. Mater. 34, 2301291 (2023).
Kim, Y. et al. Corrosion as the origin of limited lifetime of vanadium oxide-based aqueous zinc ion batteries. Nat. Commun. 13, 2371 (2022).
Liu, D. S. et al. Manipulating OH−-mediated anode-cathode cross-communication toward long-life aqueous zinc-vanadium batteries. Angew. Chem. Int. Ed. 62, e202215385 (2023).
Zhong, Y. et al. An in-depth study of heterometallic interface chemistry: bi-component layer enables highly reversible and stable Zn metal anodes. Energy Storage Mater. 55, 575–586 (2023).
Zhu, S. et al. Cathodic Zn underpotential deposition: an evitable degradation mechanism in aqueous zinc-ion batteries. Sci. Bull. 67, 1882–1889 (2022).
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004).
Sousa da Silva, A. W. & Vranken, W. F. Sousa da Silva, A. W. & Vranken, W. F. ACPYPE—AnteChamber PYthon Parser interface. BMC Res. Notes 5, 367 (2012).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Li, G. et al. Decoupled dual-salt electrolyte for practical aqueous zinc batteries. figshare https://doi.org/10.6084/m9.figshare.29941601 (2025).
Acknowledgements
This work was supported by the Australian Research Council (FL210100050 Z.G. and DE240100159 S.Z.). G.L. was supported by scholarships from the China Scholarship Council (grant no. 202006750014 to G.L.). J.A.Y. acknowledges the high-performance computing facilities provided by National Computational Infrastructure (NCI) Australia. Components of this research were undertaken on the powder diffraction and SAXS/wide-angle X-ray scattering beamlines at the Australian Synchrotron, part of ANSTO, through the merit-based beamtime proposals (M21781 to S.Z., M21924 to G.L.). We thank Q. Gu for invaluable support and expertise in our powder diffraction experiments; K. Davey for valuable insights and contributions to this work, and we remember him with deep gratitude; C. Wang for providing valuable guidance on the conceptual development of this paper; C. Zhang for valuable assistance with the QCM measurements; and T. Lu for support with the Kelvin probe force microscopy measurements.
Author information
Authors and Affiliations
Contributions
G.L., S.Z. and Z.G. conceived the research idea. G.L. and S.Z. designed the experiments. G.L., Q.C. and L.M. performed electrochemical measurements and material characterizations. J.A.Y. conducted the theoretical calculations. All authors discussed the results and contributed to the data analysis. G.L. and S.Z. wrote the paper with contributions from all authors. H.J. and Z.G. assisted in revising the paper. S.Z. and Z.G. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Daliang Han, Guanjie He and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–87 and Tables 1–3.
Source data
Source Data Figs. 1–5
Statistical source data for Figs. 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, G., Cai, Q., Zhang, S. et al. Decoupled dual-salt electrolyte for practical aqueous zinc batteries. Nat Sustain 8, 1349–1359 (2025). https://doi.org/10.1038/s41893-025-01646-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41893-025-01646-1
This article is cited by
-
Aqueous batteries beating the cold
Nature Sustainability (2025)