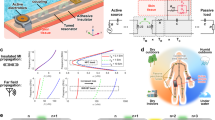
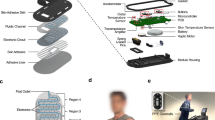
Abstract
Recent advances in materials, mechanics and design have led to the development of ultrathin, lightweight electronic devices that can conformally interface with human skin. With few exceptions, these devices rely on electrical power to support sensing, wireless communication and signal conditioning. Unfortunately, most sources of such power consist of batteries constructed using hazardous materials, often with form factors that frustrate incorporation into skin-like, or epidermal, electronic devices. Here we report a biocompatible, sweat-activated battery technology that can be embedded within a soft, microfluidic platform. The battery can be used in a detachable electronic module that contains wireless communication and power management systems, and is capable of continuous on-skin recording of physiological signals. To illustrate the practical utility of our approach, we show using human trials that the sweat-activated batteries can operate hybrid microfluidic/microelectronic systems that simultaneously monitor heart rate, sweat chloride and sweat pH.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
Code availability
Custom-developed firmware for the electronic module and LabView data acquisition software for hardware characterization are available from the corresponding authors upon reasonable request.
References
Ray, T. R. et al. Bio-integrated wearable systems: a comprehensive review. Chem. Rev. 119, 5461–5533 (2019).
Song, Y., Min, J. & Gao, W. Wearable and implantable electronics: moving toward precision therapy. ACS Nano 13, 12280–12286 (2019).
Bariya, M., Nyein, H. Y. Y. & Javey, A. Wearable sweat sensors. Nat. Electron. 1, 160 (2018).
Choi, J. et al. Soft, skin-integrated multifunctional microfluidic systems for accurate colorimetric analysis of sweat biomarkers and temperature. ACS Sens. 4, 379–388 (2019).
Caldara, M., Colleoni, C., Guido, E., Re, V. & Rosace, G. Optical monitoring of sweat pH by a textile fabric wearable sensor based on covalently bonded litmus-3-glycidoxypropyltrimethoxysilane coating. Sens. Actuators B 222, 213–220 (2016).
Reeder, J. T. et al. Resettable skin interfaced microfluidic sweat collection devices with chemesthetic hydration feedback. Nat. Commun. 10, 5513 (2019).
Yang, Y. et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 38, 217–224 (2020).
Kim, J. et al. Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci. 5, 1800880 (2018).
Khan, Y. et al. Organic multi-channel optoelectronic sensors for wearable health monitoring. IEEE Access 7, 128114–128124 (2019).
Kafi, M. A., Paul, A., Vilouras, A., Hosseini, E. S. & Dahiya, R. S. Chitosan-graphene oxide based ultra-thin and flexible sensor for diabetic wound monitoring. IEEE Sens. J. 13, 6794–6801 (2019).
Lee, K. et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng. 4, 148–158 (2020).
Imani, S. et al. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 7, 11650 (2016).
Yin, L. et al. Highly stable battery pack via insulated, reinforced, buckling-enabled interconnect array. Small 14, 1800938 (2018).
Liu, R. et al. Shape memory polymers for body motion energy harvesting and self‐powered mechanosensing. Adv. Mater. 30, 1705195 (2018).
Mokhtari, F., Foroughi, J., Zheng, T., Cheng, Z. & Spinks, G. M. Triaxial braided piezo fiber energy harvesters for self-powered wearable technologies. J. Mater. Chem. A 7, 8245–8257 (2019).
Bandodkar, A. J. Wearable biofuel cells: past, present and future. J. Electrochem. Soc. 164, H3007–H3014 (2017).
Chen, X. et al. Stretchable and flexible buckypaper-based lactate biofuel cell for wearable electronics. Adv. Funct. Mater. 29, 1905785 (2019).
Yu, Y. et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 5, eaaz7946 (2020).
Hu, X. et al. Nacre-inspired crystallization and elastic “brick-and-mortar” structure for a wearable perovskite solar module. Energy Environ. Sci. 12, 979–987 (2019).
O’Connor, T. F. et al. Wearable organic solar cells with high cyclic bending stability: materials selection criteria. Sol. Energy Mater. Sol. Cells 144, 438–444 (2016).
Li, C. et al. Flexible perovskite solar cell-driven photo-rechargeable lithium-ion capacitor for self-powered wearable strain sensors. Nano Energy 60, 247–256 (2019).
Ostfeld, A. E., Gaikwad, A. M., Khan, Y. & Arias, A. C. High-performance flexible energy storage and harvesting system for wearable electronics. Sci. Rep. 6, 26122 (2016).
Huang, X. et al. Epidermal radio frequency electronics for wireless power transfer. Microsyst. Nanoeng. 2, 16052 (2016).
Bito, J., Hester, J. G. & Tentzeris, M. M. Ambient RF energy harvesting from a two-way talk radio for flexible wearable wireless sensor devices utilizing inkjet printing technologies. IEEE Trans. Microw. Theory Tech. 63, 4533–4543 (2015).
Rose, D. P. et al. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. 62, 1457–1465 (2015).
Bandodkar, A. J. et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 5, eaav3294 (2019).
Niu, S. et al. A wireless body area sensor network based on stretchable passive tags. Nat. Electron. 2, 361–368 (2019).
Liu, W., Song, M. S., Kong, B. & Cui, Y. Flexible and stretchable energy storage: recent advances and future perspectives. Adv. Mater. 29, 1603436 (2017).
Zhang, Y., Zhao, Y., Ren, J., Weng, W. & Peng, H. Advances in wearable fiber‐shaped lithium‐ion batteries. Adv. Mater. 28, 4524–4531 (2016).
Liu, Q.-C. et al. A flexible and wearable lithium–oxygen battery with record energy density achieved by the interlaced architecture inspired by bamboo slips. Adv. Mater. 28, 8413–8418 (2016).
Kumar, R. et al. All‐printed, stretchable Zn‐Ag2O rechargeable battery via hyperelastic binder for self‐powering wearable electronics. Adv. Energy Mater. 7, 1602096 (2017).
Ji, D. et al. Atomically transition metals on self‐supported porous carbon flake arrays as binder‐free air cathode for wearable zinc−air batteries. Adv. Mater. 31, 1808267 (2019).
Li, H. et al. An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energy Environ. Sci. 11, 941–951 (2018).
Berchmans, S. et al. An epidermal alkaline rechargeable Ag–Zn printable tattoo battery for wearable electronics. J. Mater. Chem. A 2, 15788–15795 (2014).
Yang, Y. et al. Waterproof, ultrahigh areal‐capacitance, wearable supercapacitor fabrics. Adv. Mater. 29, 1606679 (2017).
Rajendran, V., Mohan, A. M. V., Jayaraman, M. & Nakagawa, T. All-printed, interdigitated, freestanding serpentine interconnects based flexible solid state supercapacitor for self powered wearable electronics. Nano Energy 65, 104055 (2019).
Lee, K. B. Urine-activated paper batteries for biosystems. J. Micromech. Microeng. 15, S210–S214 (2005).
Koo, Y., Sankar, J. & Yun, Y. High performance magnesium anode in paper-based microfluidic battery, powering on-chip fluorescence assay. Biomicrofluidics 8, 054104 (2014).
Ortega, L., Llorella, A., Esquivel, J. P. & Sabaté, N. Self-powered smart patch for sweat conductivity monitoring. Microsyst. Nanoeng. 5, 3 (2019).
She, D., Tsang, M. & Allen, M. Biodegradable batteries with immobilized electrolyte for transient MEMS. Biomed. Microdevices 21, 17 (2019).
Liu, G. et al. A wearable conductivity sensor for wireless real-time sweat monitoring. Sens. Actuators B 227, 35–42 (2016).
Emrich, H. M. et al. Sweat composition in relation to rate of sweating in patients with cystic fibrosis of the pancreas. Pediatr. Res. 2, 464–478 (1968).
Buono, M. J., Ball, K. D. & Kolkhorst, F. W. Sodium ion concentration vs. sweat rate relationship in humans. J. Appl. Physiol. 103, 990–994 (2007).
Bandodkar, A. J. et al. Soft, skin-interfaced microfluidic systems with passive galvanic stopwatches for precise chronometric sampling of sweat. Adv. Mater. 31, 1902109 (2019).
Kong, Y., Wang, C., Yang, Y., Too, C. O. & Wallace, G. G. A battery composed of a polypyrrole cathode and a magnesium alloy anode—toward a bioelectric battery. Synth. Met. 162, 584–589 (2012).
Williams, G. & McMurray, H. N. Localized corrosion of magnesium in chloride-containing electrolyte studied by a scanning vibrating electrode technique. J. Electrochem. Soc. 155, C340–C349 (2008).
Huang, W. et al. Nanostructural and electrochemical evolution of the solid-electrolyte interphase on CuO nanowires revealed by cryogenic-electron microscopy and impedance spectroscopy. ACS Nano 13, 737–744 (2018).
Cong, G., Wang, W., Lai, N.-C., Liang, Z. & Lu, Y.-C. A high-rate and long-life organic–oxygen battery. Nat. Mater. 18, 390 (2019).
Peng, G. S., Chen, K. H., Fang, H. C., Chao, H. & Chen, S. Y. EIS study on pitting corrosion of 7150 aluminum alloy in sodium chloride and hydrochloric acid solution. Mater. Corros. 61, 783–789 (2010).
Brett, C. M. A. On the electrochemical behaviour of aluminium in acidic chloride solution. Corros. Sci. 33, 203–210 (1992).
Choi, D.-H., Kim, J. S., Cutting, G. R. & Searson, P. C. Wearable potentiometric chloride sweat sensor: the critical role of the salt bridge. Anal. Chem. 88, 12241–12247 (2016).
Choi, J. et al. Soft, skin-mounted microfluidic systems for measuring secretory fluidic pressures generated at the surface of the skin by eccrine sweat glands. Lab Chip 17, 2572–2580 (2017).
Twine, N. B. et al. Open nanofluidic films with rapid transport and no analyte exchange for ultra-low sample volumes. Lab Chip 18, 2816–2825 (2018).
Wang, R. et al. Accuracy of wrist-worn heart rate monitors. JAMA Cardiol. 2, 104–106 (2017).
Emaminejad, S. et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA 114, 4625–4630 (2017).
Acknowledgements
This research was funded by the Air Force Research Laboratory (AFRL) Human Signatures Branch through Core funds provided to Northwestern University under contract FA8650-14-D-6516. S.W., T.H. and S.M. acknowledge support from the ‘Top Open’ programme (Tsinghua University, P. R. China), the visiting scholar programme (grant no. 201706235005, China Scholarship Council) and the Indo-US Science and Technology Forum (grant no. SERB-IUSSTF-2017/192) respectively. This work utilized the Northwestern University Micro/Nano Fabrication Facility (NUFAB), which is partially supported by the Soft and Hybrid Nanotechnology Experimental (SHyNE) resource (NSF ECCS-1542205), the Materials Research Science and Engineering Center (DMR-1720139), the State of Illinois and Northwestern University.
Author information
Authors and Affiliations
Contributions
J.A.R., A.J.B., R.G. and S.P.L. conceived the project, designed the studies and analysed and interpreted the data. A.J.B. designed and developed the batteries, microfluidics, pH sensor and chloride sensor. S.P.L. designed and developed the electronics. W.L. developed the firmware. A.J.B., I.H., S.W., T.H., S.M. and N.N. worked on testing and optimizing the batteries. S.P.L., C.-J.S. and P.G. worked on fabricating and testing of the electronics. A.J.B. and J.C. worked on fabricating the microfluidics. J.K. assisted in optical studies. W.J.J., J.T.R. and R.T. assisted in testing the devices. A.J.B., S.P.L., W.J.J., I.H., R.G. and J.A.R. composed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.A.R., S.P.L., W.L. and R.G. are cofounders and/or employees of Epicore Biosystems, Inc., a company that pursues commercialization of microfluidic devices for wearable applications.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, discussion, equations and Table 1.
Rights and permissions
About this article
Cite this article
Bandodkar, A.J., Lee, S.P., Huang, I. et al. Sweat-activated biocompatible batteries for epidermal electronic and microfluidic systems. Nat Electron 3, 554–562 (2020). https://doi.org/10.1038/s41928-020-0443-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41928-020-0443-7
This article is cited by
-
Implantable bioelectronic devices for photoelectrochemical and electrochemical modulation of cells and tissues
Nature Reviews Bioengineering (2025)
-
Leaf-based energy harvesting and storage utilizing hygroscopic iron hydrogel for continuous power generation
Nature Communications (2025)
-
Smart closed-loop drug delivery systems
Nature Reviews Bioengineering (2025)
-
Current advances in multi-strategy collaborative sensitization for self-powered electrochemical biosensors and their applications in disease biomarker detection
Microchimica Acta (2025)
-
An Energy-Autonomous Wearable Fabric Powered by High-Power Density Sweat-Activated Batteries for Health Monitoring
Advanced Fiber Materials (2025)