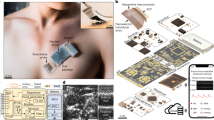
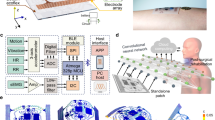
Abstract
Wearable electromyography devices can detect muscular activity for health monitoring and body motion tracking, but this approach is limited by weak and stochastic signals with a low spatial resolution. Alternatively, echomyography can detect muscle movement using ultrasound waves, but typically relies on complex transducer arrays, which are bulky, have high power consumption and can limit user mobility. Here we report a fully integrated wearable echomyography system that consists of a customized single transducer, a wireless circuit for data processing and an on-board battery for power. The system can be attached to the skin and provides accurate long-term wireless monitoring of muscles. To illustrate its capabilities, we use this system to detect the activity of the diaphragm, which allows the recognition of different breathing modes. We also develop a deep learning algorithm to correlate the single-transducer radio-frequency data from forearm muscles with hand gestures to accurately and continuously track 13 hand joints with a mean error of only 7.9°.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available in the Article or its Supplementary Information.
Code availability
Code used in this work is available via GitHub at https://github.com/XiaoxiangGao/single_transducer_project.
References
Yu, Y. et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 5, eaaz7946 (2020).
Bandodkar, A. et al. Sweat-activated biocompatible batteries for epidermal electronic and microfluidic systems. Nat. Electron. 3, 554–562 (2020).
Liu, Y. et al. Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv. 2, e1601185 (2016).
Zhang, L. et al. Fully organic compliant dry electrodes self-adhesive to skin for long-term motion-robust epidermal biopotential monitoring. Nat. Commun. 11, 4683 (2020).
Yu, Y. et al. All-printed soft human-machine interface for robotic physicochemical sensing. Sci. Robot. 7, eabn0495 (2022).
Ershad, F. et al. Ultra-conformal drawn-on-skin electronics for multifunctional motion artifact-free sensing and point-of-care treatment. Nat. Commun. 11, 3823 (2020).
Moin, A. et al. A wearable biosensing system with in-sensor adaptive machine learning for hand gesture recognition. Nat. Electron. 4, 54–63 (2021).
Reaz, M. B. I., Hussain, M. S. & Mohd-Yasin, F. Techniques of EMG signal analysis: detection, processing, classification and applications. Biol. Proced. Online 8, 11–35 (2006).
McKeown, M. J. & Radtke, R. Phasic and tonic coupling between EEG and EMG demonstrated with independent component analysis. J. Clin. Neurophysiol. 18, 45–57 (2001).
Klotz, T., Gizzi, L. & Röhrle, O. Investigating the spatial resolution of EMG and MMG based on a systemic multi-scale model. Biomech. Model. Mechanobiol. 21, 983–997 (2022).
Luo, Y. M., Moxham, J. & Polkey, M. I. Diaphragm electromyography using an oesophageal catheter: current concepts. Clin. Sci. 115, 233–244 (2008).
Simão, M., Mendes, N., Gibaru, O. & Neto, P. A review on electromyography decoding and pattern recognition for human-machine interaction. IEEE Access 7, 39564–39582 (2019).
Sun, Z., Xi, X., Yuan, C., Yang, Y. & Hua, X. Surface electromyography signal denoising via EEMD and improved wavelet thresholds. Math. Biosci. Eng. 17, 6945–6962 (2020).
Solnik, S., DeVita, P., Rider, P., Long, B. & Hortobágyi, T. Teager–Kaiser operator improves the accuracy of EMG onset detection independent of signal-to-noise ratio. Acta Bioeng. Biomech. 10, 65–68 (2008).
Eskes, M. et al. sEMG-assisted inverse modelling of 3D lip movement: a feasibility study towards person-specific modelling. Sci. Rep. 7, 17729 (2017).
Wakefield, R. J. et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J. Rheumatol. 32, 2485–2487 (2005).
Sconfienza, L. M. et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur. Radiol. 28, 5338–5351 (2018).
Wang, C. et al. Continuous monitoring of deep-tissue haemodynamics with stretchable ultrasonic phased arrays. Nat. Biomed. Eng. 5, 749–758 (2021).
Hu, H. et al. A wearable cardiac ultrasound imager. Nature 613, 667–675 (2023).
Hu, H. et al. Stretchable ultrasonic arrays for the three-dimensional mapping of the modulus of deep tissue. Nat. Biomed. Eng. 7, 1321–1334 (2023).
Wang, C. et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2, 687–695 (2018).
Wang, F. et al. Flexible Doppler ultrasound device for the monitoring of blood flow velocity. Sci. Adv. 7, eabi9283 (2021).
Gao, X. et al. A photoacoustic patch for three-dimensional imaging of hemoglobin and core temperature. Nat. Commun. 13, 7757 (2022).
AlMohimeed, I. & Ono, Y. Ultrasound measurement of skeletal muscle contractile parameters using flexible and wearable single-element ultrasonic sensor. Sensors 20, 3616 (2020).
Wang, Z. et al. Ultrasonography and electromyography based hand motion intention recognition for a trans-radial amputee: a case study. Med. Eng. Phys. 75, 45–48 (2020).
Ortenzi, V., Tarantino, S., Castellini, C. & Cipriani, C. Ultrasound imaging for hand prosthesis control: a comparative study of features and classification methods. In 2015 IEEE International Conference on Rehabilitation Robotics (ICORR) 1–6 (IEEE, 2015).
Oakley, C. G. Calculation of ultrasonic transducer signal-to-noise ratios using the KLM model. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 44, 1018–1026 (1997).
Yang, X., Yan, J., Fang, Y., Zhou, D. & Liu, H. Simultaneous prediction of wrist/hand motion via wearable ultrasound sensing. IEEE Trans. Neural Syst. Rehabil. Eng. 28, 970–977 (2020).
Xue, X. et al. Development of a wearable ultrasound transducer for sensing muscle activities in assistive robotics applications. Biosensors 13, 134 (2023).
McCool, F. D. & Tzelepis, G. E. Dysfunction of the diaphragm. N. Engl. J. Med. 366, 932–942 (2012).
Tuinman, P. R. et al. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients—a narrative review. Intensive Care Med. 46, 594–605 (2020).
DiNino, E., Gartman, E. J., Sethi, J. M. & McCool, F. D. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 69, 431–435 (2014).
Guarracino, F. et al. Lung, heart, vascular, and diaphragm ultrasound examination of COVID-19 patients: a comprehensive approach. J. Cardiothorac. Vasc. Anesth. 35, 1866–1874 (2021).
Gottesman, E. & McCool, F. D. Ultrasound evaluation of the paralyzed diaphragm. Am. J. Resp. Crit. Care Med. 155, 1570–1574 (1997).
Summerhill, E. M., El-Sameed, Y. A., Glidden, T. J. & McCool, F. D. Monitoring recovery from diaphragm paralysis with ultrasound. Chest 133, 737–743 (2008).
Pirompanich, P. & Romsaiyut, S. Use of diaphragm thickening fraction combined with rapid shallow breathing index for predicting success of weaning from mechanical ventilator in medical patients. J. Intensive Care 6, 6 (2018).
Krisanaprakornkit, T., Sriraj, W., Piyavhatkul, N. & Laopaiboon, M. Meditation therapy for anxiety disorders. Cochrane Database Syst. Rev. 10.1002/14651858.CD004998.pub2 (2006).
Dries, D. J., McGonigal, M. D., Malian, M. S., Bor, B. J. & Sullivan, C. Protocol-driven ventilator weaning reduces use of mechanical ventilation, rate of early reintubation, and ventilator-associated pneumonia. J. Trauma 56, 943–952 (2004).
Roux, F., D’Ambrosio, C. & Mohsenin, V. Sleep-related breathing disorders and cardiovascular disease. Am. J. Med. 108, 396–402 (2000).
Conrad, A. et al. Psychophysiological effects of breathing instructions for stress management. Appl. Psychophysiol. Biofeedback 32, 89–98 (2007).
Pfaltz, M. C., Michael, T., Grossman, P., Blechert, J. & Wilhelm, F. H. Respiratory pathophysiology of panic disorder: an ambulatory monitoring study. Psychosom. Med. 71, 869–876 (2009).
Julious, S. A. Sample Sizes for Clinical Trials (Chapman and Hall/CRC, 2023).
Barnes, P. J. Small airway fibrosis in COPD. Int. J. Biochem. Cell Biol. 116, 105598 (2019).
Jones, R. L., Noble, P. B., Elliot, J. G. & James, A. L. Airway remodelling in COPD: it’s not asthma! Respirology 21, 1347–1356 (2016).
Pallotti, A., Orengo, G. & Saggio, G. Measurements comparison of finger joint angles in hand postures between an sEMG armband and a sensory glove. Biocybern. Biomed. Eng. 41, 605–616 (2021).
Kim, K. K. et al. A substrate-less nanomesh receptor with meta-learning for rapid hand task recognition. Nat. Electron. 6, 64–75 (2022).
Zheng, Y.-P., Chan, M., Shi, J., Chen, X. & Huang, Q.-H. Sonomyography: monitoring morphological changes of forearm muscles in actions with the feasibility for the control of powered prosthesis. Med. Eng. Phys. 28, 405–415 (2006).
Dhawan, A. S. et al. Proprioceptive sonomyographic control: a novel method for intuitive and proportional control of multiple degrees-of-freedom for individuals with upper extremity limb loss. Sci. Rep. 9, 9499 (2019).
Kotses, H., Harver, A. & Humphries, C. T. Home monitoring in asthma self-management. J. Asthma 43, 649–655 (2006).
Côté, J., Cartier, A., Malo, J. L., Rouleau, M. & Boulet, L. P. Compliance with peak expiratory flow monitoring in home management of asthma. Chest 113, 968–972 (1998).
Donaldson, G., Seemungal, T. A., Bhowmik, A. & Wedzicha, J. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 57, 847–852 (2002).
Goligher, E. C. et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am. J. Resp. Crit. Care Med. 197, 204–213 (2018).
Thille, A. W., Harrois, A., Schortgen, F., Brun-Buisson, C. & Brochard, L. Outcomes of extubation failure in medical intensive care unit patients. Crit. Care Med. 39, 2612–2618 (2011).
Li, S., Chen, Z. & Yan, W. Application of bedside ultrasound in predicting the outcome of weaning from mechanical ventilation in elderly patients. BMC Pulm. Med. 21, 217 (2021).
DiNino, E., Gartman, E. J., Sethi, J. M. & McCool, F. D. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 69, 423–427 (2014).
Liu, Y., Zhang, S. & Gowda, M. NeuroPose: 3D hand pose tracking using EMG wearables. In Proc. Web Conference 2021 1471–1482 (ACM, 2021).
Côté-Allard, U. et al. Deep learning for electromyographic hand gesture signal classification using transfer learning. IEEE Trans. Neural Syst. Rehabil. Eng. 27, 760–771 (2019).
Jensen, J. A. Field: a program for simulating ultrasound systems. In 10th Nordic-Baltic Conference on Biomedical Imaging 351–353 (Citeseer, 1996).
Shahgholi, L. et al. Diaphragm depth in normal subjects. Muscle Nerve 49, 666–668 (2014).
Acknowledgements
The system was based on research sponsored by the National Institutes of Health (NIH) grant no. 1R21EB025521-01 (S.X.), grant no. 1R21EB027303-01A1 (S.X.), grant no. 3R21EB027303-02S1 (S.X.) and grant no. 1R01 EB033464-01 (S.X.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. All the biological experiments were conducted in accordance with the ethical guidelines with the approval of the Institutional Review Board of the University of California San Diego.
Author information
Authors and Affiliations
Contributions
X.G. and S.X. conceived the study and designed the experiments. X.G., X.C., W.Y., S.Q., F.Z. and Z.L. performed the experiments. X.G. processed the data. M.L. designed the circuit. L.Y. fabricated the 3D-printed mould. J.L. recruited the patients. X.G., X.C., M.L., J.L. and S.X. wrote the manuscript. H. Hu, H. Huang, S.Z., Y.B., X.Y., Y.Z., J.M., X.W., G.P., C.L., R.W., R.S.W., J.W. and J.L. discussed the experimental results and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Electronics thanks Yuan Huang, Alina Rwei and Lizhi Xu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–13, Figs. 1–32, Tables 1–5, captions for for Supplementary Videos 1–3 and references.
Supplementary Video 1
Simultaneous measurements of EcMG and EMG signals as the fingers bend and the wrist rotates.
Supplementary Video 2
Real-time virtual bird control by correlating the pitch angle of the wrist with the height of the virtual bird.
Supplementary Video 3
Real-time robotic arm control by correlating the pitch angle of the wrist and finger joint angle with motions of the robotic arm.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, X., Chen, X., Lin, M. et al. A wearable echomyography system based on a single transducer. Nat Electron 7, 1035–1046 (2024). https://doi.org/10.1038/s41928-024-01271-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41928-024-01271-4
This article is cited by
-
Wearables that learn to read gestures on the move
Nature Sensors (2026)
-
Wearable Ultrasound Devices for Therapeutic Applications
Nano-Micro Letters (2026)
-
Wearable ultrasound technology
Nature Reviews Bioengineering (2025)
-
A simplified wearable device powered by a generative EMG network for hand-gesture recognition and gait prediction
Nature Sensors (2025)
-
Superior lead-free piezoceramics for wearable multimodal ultrasound imaging arrays
Nature Communications (2025)