Abstract
Practical brain–computer interfaces should be able to decipher brain signals and dynamically adapt to brain fluctuations. This, however, requires a decoder capable of flexible updates with energy-efficient decoding capabilities. Here we report a neuromorphic and adaptive decoder for brain–computer interfaces, which is based on a 128k-cell memristor chip. Our approach features a hardware-efficient one-step memristor decoding strategy that allows the interface to achieve software-equivalent decoding performance. Furthermore, we show that the system can be used for the real-time control of a drone in four degrees of freedom. We also develop an interactive update framework that allows the memristor decoder and the changing brain signals to adapt to each other. We illustrate the capabilities of this co-evolution of the brain and memristor decoder over an extended interaction task involving ten participants, which leads to around 20% higher accuracy than an interface without co-evolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data are provided with this paper. Other data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
The core code for memristor-based one-step decoding is publicly available via GitHub at https://github.com/zhengwuliu/Memristor_Co-evoutional_BCI. Other code that supports the findings of this study is available from the corresponding authors upon reasonable request.
References
Willett, F. R., Avansino, D. T., Hochberg, L. R., Henderson, J. M. & Shenoy, K. V. High-performance brain-to-text communication via handwriting. Nature 593, 249–254 (2021).
Anumanchipalli, G. K., Chartier, J. & Chang, E. F. Speech synthesis from neural decoding of spoken sentences. Nature 568, 493–498 (2019).
Metzger, S. L. et al. A high-performance neuroprosthesis for speech decoding and avatar control. Nature 620, 1037–1046 (2023).
Willett, F. R. et al. A high-performance speech neuroprosthesis. Nature 620, 1031–1036 (2023).
Flesher, S. N. et al. A brain–computer interface that evokes tactile sensations improves robotic arm control. Science 372, 831–836 (2021).
Lorach, H. et al. Walking naturally after spinal cord injury using a brain–spine interface. Nature 618, 126–133 (2023).
Silversmith, D. B. et al. Plug-and-play control of a brain–computer interface through neural map stabilization. Nat. Biotechnol. 39, 326–335 (2021).
Chiang, K.-J., Wei, C.-S., Nakanishi, M. & Jung, T.-P. Boosting template-based SSVEP decoding by cross-domain transfer learning. J. Neural Eng. 18, 016002 (2021).
Pascual-Leone, A., Amedi, A., Fregni, F. & Merabet, L. B. The plastic human brain cortex. Annu. Rev. Neurosci. 28, 377–401 (2005).
Luppi, A. I. et al. A synergistic core for human brain evolution and cognition. Nat. Neurosci. 25, 771–782 (2022).
Abbott, L. F. & Nelson, S. B. Synaptic plasticity: taming the beast. Nat. Neurosci. 3, 1178–1183 (2000).
Sur, M. & Rubenstein, J. L. R. Patterning and plasticity of the cerebral cortex. Science 310, 805–810 (2005).
Holz, E. M., Botrel, L., Kaufmann, T. & Kübler, A. Long-term independent brain–computer interface home use improves quality of life of a patient in the locked-in state: a case study. Arch. Phys. Med. Rehabil. 96, S16–S26 (2015).
Sussillo, D., Stavisky, S. D., Kao, J. C., Ryu, S. I. & Shenoy, K. V. Making brain–machine interfaces robust to future neural variability. Nat. Commun. 7, 13749 (2016).
Wen, S. et al. Rapid adaptation of brain–computer interfaces to new neuronal ensembles or participants via generative modelling. Nat. Biomed. Eng. 7, 546–558 (2023).
Renart, A. & Machens, C. K. Variability in neural activity and behavior. Curr. Opin. Neurobiol. 25, 211–220 (2014).
van Praag, H., Kempermann, G. & Gage, F. H. Neural consequences of enviromental enrichment. Nat. Rev. Neurosci. 1, 191–198 (2000).
Wolpaw, J. R. Brain–computer interfaces as new brain output pathways. J. Physiol. 579, 613–619 (2007).
Musk, E. An integrated brain–machine interface platform with thousands of channels. J. Med. Internet Res. 21, e16194 (2019).
Sahasrabuddhe, K. et al. The Argo: a high channel count recording system for neural recording in vivo. J. Neural Eng. 18, 015002 (2021).
Steinmetz, N. A. et al. Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
Grover, P. & Venkatesh, P. An information-theoretic view of EEG sensing. Proc. IEEE 105, 367–384 (2016).
Han, J. et al. A high-speed hybrid brain–computer interface with more than 200 targets. J. Neural Eng. 20, 016025 (2023).
Chen, Y. et al. Implementing a calibration-free SSVEP-based BCI system with 160 targets. J. Neural Eng. 18, 046094 (2021).
Horowitz, M. 1.1 Computing’s energy problem (and what we can do about it). In 2014 IEEE International Solid-State Circuits Conference Digest of Technical Papers (ISSCC) 10–14 (IEEE, 2014).
Dethier, J., Nuyujukian, P., Ryu, S. I., Shenoy, K. V. & Boahen, K. Design and validation of a real-time spiking-neural-network decoder for brain–machine interfaces. J. Neural Eng. 10, 036008 (2013).
Boi, F. et al. A bidirectional brain–machine interface featuring a neuromorphic hardware decoder. Front. Neurosci. 10, 563 (2016).
Shaikh, S., So, R., Sibindi, T., Libedinsky, C. & Basu, A. Real-time closed loop neural decoding on a neuromorphic chip. In 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER) 670–673 (IEEE, 2019).
Sharifshazileh, M., Burelo, K., Sarnthein, J. & Indiveri, G. An electronic neuromorphic system for real-time detection of high frequency oscillations (HFO) in intracranial EEG. Nat. Commun. 12, 3095 (2021).
Ambrogio, S. et al. Equivalent-accuracy accelerated neural-network training using analogue memory. Nature 558, 60–67 (2018).
Wan, W. et al. A compute-in-memory chip based on resistive random-access memory. Nature 608, 504–512 (2022).
Xia, Q. & Yang, J. J. Memristive crossbar arrays for brain-inspired computing. Nat. Mater. 18, 309–323 (2019).
Guo, Z. et al. An emerging NVM CIM accelerator with shared-path transpose read and bit-interleaving weight storage for efficient on-chip training in edge devices. In IEEE Transactions on Circuits and Systems II: Express Briefs 2645–2649 (IEEE, 2023).
Gao, B. et al. Memristor-based analogue computing for brain-inspired sound localization with in situ training. Nat. Commun. 13, 2026 (2022).
Zhang, W. et al. Edge learning using a fully integrated neuro-inspired memristor chip. Science 381, 1205–1211 (2023).
Liu, Z. et al. Neural signal analysis with memristor arrays towards high-efficiency brain–machine interfaces. Nat. Commun. 11, 4234 (2020).
Liu, Z. et al. Multichannel parallel processing of neural signals in memristor arrays. Sci. Adv. 6, eabc4797 (2020).
Yuan, R. et al. A neuromorphic physiological signal processing system based on VO2 memristor for next-generation human–machine interface. Nat. Commun. 14, 3695 (2023).
Lee, D. et al. Oxide based nanoscale analog synapse device for neural signal recognition system. In 2015 IEEE International Electron Devices Meeting (IEDM) 4.7.1–4.7.4 (IEEE, 2015).
Park, S. et al. Electronic system with memristive synapses for pattern recognition. Sci. Rep. 5, 10123 (2015).
Zhu, X., Wang, Q. & Lu, W. D. Memristor networks for real-time neural activity analysis. Nat. Commun. 11, 2439 (2020).
Gupta, I. et al. Real-time encoding and compression of neuronal spikes by metal-oxide memristors. Nat. Commun. 7, 12805 (2016).
Chen, X. et al. High-speed spelling with a noninvasive brain–computer interface. Proc. Natl Acad. Sci. USA 112, E6058–E6067 (2015).
Mahmood, M. et al. Fully portable and wireless universal brain–machine interfaces enabled by flexible scalp electronics and deep learning algorithm. Nat. Mach. Intell. 1, 412–422 (2019).
Chiang, K.-J. et al. A closed-loop adaptive brain-computer interface framework: improving the classifier with the use of error-related potentials. In 2021 10th International IEEE/EMBS Conference on Neural Engineering (NER) 487–490 (IEEE, 2021).
Chavarriaga, R., Sobolewski, A. & Millán, J. D. R. Errare machinale est: the use of error-related potentials in brain-machine interfaces. Front. Neurosci. 8, 208 (2014).
Chavarriaga, R. & Millan, J. D. R. Learning from EEG error-related potentials in noninvasive brain-computer interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 18, 381–388 (2010).
Nakanishi, M. et al. Enhancing detection of SSVEPs for a high-speed brain speller using task-related component analysis. IEEE Trans. Biomed. Eng. 65, 104–112 (2018).
Gulati, T., Guo, L., Ramanathan, D. S., Bodepudi, A. & Ganguly, K. Neural reactivations during sleep determine network credit assignment. Nat. Neurosci. 20, 1277–1284 (2017).
Müller, J. S. et al. A mathematical model for the two-learners problem. J. Neural Eng. 14, 036005 (2017).
Orsborn, A. L. et al. Closed-loop decoder adaptation shapes neural plasticity for skillful neuroprosthetic control. Neuron 82, 1380–1393 (2014).
Madduri, M. M., Burden, S. A. & Orsborn, A. L. Biosignal-based co-adaptive user-machine interfaces for motor control. Curr. Opin. Biomed. Eng. 27, 100462 (2023).
Shanechi, M. M. et al. Rapid control and feedback rates enhance neuroprosthetic control. Nat. Commun. 8, 13825 (2017).
Ahmadipour, P., Yang, Y., Chang, E. F. & Shanechi, M. M. Adaptive tracking of human ECoG network dynamics. J. Neural Eng. 18, 016011 (2021).
Meidahl, A. C. et al. Adaptive deep brain stimulation for movement disorders: the long road to clinical therapy. Mov. Disord. 32, 810–819 (2017).
Shin, U. et al. NeuralTree: a 256-channel 0.227-μJ/class versatile neural activity classification and closed-loop neuromodulation SoC. IEEE J. Solid-State Circuits 57, 3243–3257 (2022).
Xiao, X. et al. Discriminative canonical pattern matching for single-trial classification of ERP components. IEEE Trans. Biomed. Eng. 67, 2266–2275 (2019).
Luo, R. et al. Data augmentation of SSVEPs using source aliasing matrix estimation for brain-computer interfaces. IEEE Trans. Biomed. Eng. 70, 1775–1785 (2023).
Acknowledgements
This work was supported in part by the STI 2030-Major Projects 2022ZD0210200 (J.T.), National Natural Science Foundation of China (nos. 92264201 (J.T.), 62025111 (H.W.), 82330064 (M.X.), 62122059 (M.X.), 81925020 (D.M.), 62206198 (K.W.) and 62404187 (Z.L.)), the XPLORER Prize (H.W.), the Theme-based Research Scheme (TRS) project T45-701/22-R (N.W.), the Seed Fund for Basic Research from the University of Hong Kong (Z.L.) and the Center of Nanofabrication (Tsinghua University).
Author information
Authors and Affiliations
Contributions
J.T., M.X., D.M. and H.W. supervised the project. Z.L., J.M., J.T., M.X., D.M. and H.W. conceived the idea. Z.L., J.M., J.T. and M.X. designed the experiments and wrote the paper. Q.L., J.T., B.G., H.Q. and H.W. contributed to the design and fabrication of the memristor chip. Z.L., S.D. and Q.Q. designed the chip test system. Z.L., Y.X. and Y.L. tested and characterized the memristor devices. Z.L., J.M., J.T. and M.X. designed the interactive co-evolution scheme. Z.L. and J.T. designed the memristor-related algorithms and implemented them on the co-evolution BCI system. J.M., M.X. and W.C. designed the co-evolution BCI software at the PC end and implemented the electroencephalogram decoding algorithm. J.M., M.X., K.W. and W.C. designed the BCI paradigm. J.M. collected the brain signals for the simulated online experiments. Z.L. developed and implemented the one-step decoding models on memristor chips. Z.L. and J.M. performed the memristor-chip-based brain-controlled drone flight experiments. Z.L., J.M. and W.C. performed the brain–memristor decoder co-evolution experiments. P.Y., H.Z., N.W., B.H. and T.-P.J. discussed the results and benchmark. All authors commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Electronics thanks Shuo Gao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
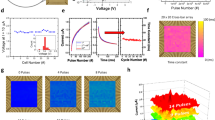
Extended Data Fig. 1 Analog resistive switching characteristics of memristors.
a, Direct-current (DC) I-V curves of a typical memristor device. The red and green lines plot the mean values of 50 consecutive switching cycles, which are indicated by grey lines. b, Analog resistive switching characteristics for 128 representative memristor devices under 10 consecutive pulsed SET and RESET cycles. The read voltage is fixed at 0.2 V. Data are presented as mean values ± s.d.
Extended Data Fig. 2 Binary masks for parameter pruning experiments.
a, Mask for parameters whose magnitudes are at the top 5%, b, Mask for parameters whose magnitudes are at the top 20%, b, Mask for parameters whose magnitudes are at the top 40%, d, Mask examples for single-channel decoding experiments. Here the activated channels for subjects A1-A5 are channels PO5, POz, PO6, O1 and O2, respectively.
Extended Data Fig. 3 Results of SU experiments for brain-memristor decoder co-evolution.
a, Decoding accuracies over 5 update cycles for the BCIs with (w/) and without (w/o) co-evolution. b, Adaptive memristor decoder map after each update cycle. c, Correlation coefficients between each decoder map and the final one. d, Correlation coefficients between the decoder maps for each channel.
Extended Data Fig. 4 Overall performance of the ErrP detector.
a, Average TPR, TNR and BACC as a function of the number of samples (n = 100, 200, 300, 500, 700, and 1000) in the training set, and the testing set contains 360 samples. The samples in the training set and testing set are randomly selected from the data collected in the ErrP calibration experiment without overlap. b, Average TPR, TNR and BACC as a function of the classification threshold. If the feature value of the DCPM-based classifier exceeds a predefined threshold, it labels the classification of “target”; otherwise, it labels the classification of “non-target”. These metrics are calculated from the leave-one-block-out cross-validation on the training data. c, Confusion matrix between the “target” class and the “non-target” class when the threshold is set as 0.5 in b. d, Grand averaged metrics on 10 blocks online data from all 10 subjects (n = 100). The error bars in a and d, and the shaded areas in b indicate the mean values ± s.d. e, Correct and incorrect evoked signals from channel FCZ (green and red lines, respectively). The shade areas in e indicate the 95% confidence intervals.
Extended Data Fig. 5 Accuracy evolution for BCIs with and without co-evolution for 10 subjects.
a-j, Decoding accuracy results for subjects B1-B10, respectively.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15 and Tables 1–4.
Supplementary Video 1
Real-time brain-controlled drone flight with the memristor-enabled neuromorphic BCI.
Source data
Source Data Figs. 2–4
Source data for Figs. 2–4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Mei, J., Tang, J. et al. A memristor-based adaptive neuromorphic decoder for brain–computer interfaces. Nat Electron 8, 362–372 (2025). https://doi.org/10.1038/s41928-025-01340-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41928-025-01340-2
This article is cited by
-
Mechanosensory neuron implemented by a single freestanding epitaxial SrTiO3 capacitor
npj Flexible Electronics (2026)
-
Non-Invasive Brain-Computer Interfaces: Converging Frontiers in Neural Signal Decoding and Flexible Bioelectronics Integration
Nano-Micro Letters (2026)
-
Mechanical Properties Analysis of Flexible Memristors for Neuromorphic Computing
Nano-Micro Letters (2026)
-
100 years of field-effect transistors
Nature Electronics (2025)
-
Bioinspired and biointegrated vision for artificial sight convergence
Nature Reviews Bioengineering (2025)