Abstract
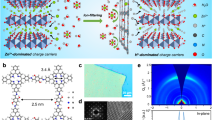
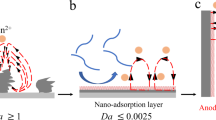
Rechargeable aqueous zinc-ion batteries (AZIBs), renowned for their safety, high energy density and rapid charging, are prime choices for grid-scale energy storage. Historically, ion-shuttling models centring on ion-migration behaviour have dominated explanations for charge/discharge processes in aqueous batteries, like classical ion insertion/extraction and pseudocapacitance mechanisms. However, these models struggle to account for the exceptional performance of AZIBs compared to other aqueous metal-ion batteries. Here we present a catalysis model elucidating the Zn2+ anomaly in aqueous batteries, explaining it through the concept of adsorption in catalysis. Such behaviour can serve the charge/discharge role, predominantly dictated by solvated metal cations and cathode materials. First-principles calculations suggest optimal adsorption/desorption behaviour (water dissociation process) with the Zn2+–vanadium nitride (VN) combination. Experimentally, AZIBs implementing VN cathodes demonstrate fast-charging kinetics, showing a capacity of 577.1 mAh g−1 at a current density of 300,000 mA g−1. The grasp of catalysis steps within AZIBs can drive solutions beyond state-of-the-art fast-charging batteries.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper.
References
Jiang, L. et al. Building aqueous K-ion batteries for energy storage. Nat. Energy 4, 495–503 (2019).
Kundu, D., Adams, B. D., Duffort, V., Vajargah, S. H. & Nazar, L. F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 1, 16119 (2016).
Xu, C., Li, B., Du, H. & Kang, F. Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew. Chem. Int. Ed. 51, 933–935 (2012).
Li, C., Jin, S., Archer, L. A. & Nazar, L. F. Toward practical aqueous zinc-ion batteries for electrochemical energy storage. Joule 6, 1733–1738 (2022).
Liu, Y., Zhu, Y. & Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 4, 540–550 (2019).
Choi, C. et al. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 5, 5–19 (2019).
Michelbacher, C. J. et al. Enabling Fast Charging: a Technology Gap Assessment No. INL/EXT-17-41638 (US Department of Energy, 2017).
Tsiropoulos, I., Siskos, P. & Capros, P. The cost of recharging infrastructure for electric vehicles in the EU in a climate neutrality context: factors influencing investments in 2030 and 2050. Appl. Energy 322, 119446 (2022).
Li, W., Dahn, J. R. & Wainwright, D. S. Rechargeable lithium batteries with aqueous electrolytes. Science 264, 1115–1118 (1994).
Zhang, N. et al. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 49, 4203–4219 (2020).
Jia, X., Liu, C., Neale, Z. G., Yang, J. & Cao, G. Active materials for aqueous zinc ion batteries: synthesis, crystal structure, morphology and electrochemistry. Chem. Rev. 120, 7795–7866 (2020).
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Simon, P., Gogotsi, Y. & Dunn, B. Where do batteries end and supercapacitors begin? Science 343, 1210–1211 (2014).
Yuan, Y. et al. Understanding intercalation chemistry for sustainable aqueous zinc–manganese dioxide batteries. Nat. Sustain. 5, 890–898 (2022).
Wu, X. et al. Diffusion-free Grotthuss topochemistry for high-rate and long-life proton batteries. Nat. Energy 4, 123–130 (2019).
Fang, G. et al. Simultaneous cationic and anionic redox reactions mechanism enabling high‐rate long‐life aqueous zinc‐ion battery. Adv. Funct. Mater. 29, 1905267 (2019).
Wang, L., Huang, K. W., Chen, J. & Zheng, J. Ultralong cycle stability of aqueous zinc-ion batteries with zinc vanadium oxide cathodes. Sci. Adv. 5, eaax4279 (2019).
Park, M. J., Yaghoobnejad Asl, H. & Manthiram, A. Multivalent-ion versus proton insertion into battery electrodes. ACS Energy Lett. 5, 2367–2375 (2020).
Wan, F. et al. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 9, 1656 (2018).
Chao, D. et al. Roadmap for advanced aqueous batteries: from design of materials to applications. Sci. Adv. 6, eaba4098 (2020).
Choi, D., Blomgren, G. E. & Kumta, P. N. Fast and reversible surface redox reaction in nanocrystalline vanadium nitride supercapacitors. Adv. Mater. 18, 1178–1182 (2006).
Ge, J., Fan, L., Rao, A. M., Zhou, J. & Lu, B. Surface-substituted Prussian blue analogue cathode for sustainable potassium-ion batteries. Nat. Sustain. 5, 225–234 (2021).
Luo, J. Y., Cui, W. J., He, P. & Xia, Y. Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2, 760–765 (2010).
Pastel, G. R. et al. A sobering examination of the feasibility of aqueous aluminum batteries. Energy Environ. Sci. 15, 2460–2469 (2022).
Cao, L. et al. Fluorinated interphase enables reversible aqueous zinc battery chemistries. Nat. Nanotechnol. 16, 902–910 (2021).
Li, K. & Xue, D. Estimation of electronegativity values of elements in different valence states. J. Phys. Chem. A 110, 11332–11337 (2006).
Sproul, G. Electronegativity and bond type: predicting bond type. J. Chem. Educ. 78, 387 (2001).
Kinraide, T. B. & Yermiyahu, U. A scale of metal ion binding strengths correlating with ionic charge, Pauling electronegativity, toxicity and other physiological effects. J. Inorg. Biochem. 101, 1201–1213 (2007).
Miranda-Quintana, R. A., Martinez Gonzalez, M. & Ayers, P. W. Electronegativity and redox reactions. Phys. Chem. Chem. Phys. 18, 22235–22243 (2016).
Tan, S. et al. Revealing the origin of highly efficient polysulfide anchoring and transformation on anion‐substituted vanadium nitride host. Adv. Funct. Mater. 31, 2008034 (2020).
Li, J. et al. Multi‐scale investigations of δ‐Ni0.25V2O5·nH2O cathode materials in aqueous zinc‐ion batteries. Adv. Energy Mater. 10, 2000058 (2020).
Chen, W. et al. Two-dimensional quantum-sheet films with sub-1.2-nm channels for ultrahigh-rate electrochemical capacitance. Nat. Nanotechnol. 17, 153–158 (2022).
Rudolph, W. W., Brooker, M. H. & Tremaine, P. R. Raman spectroscopy of aqueous ZnSO4 solutions under hydrothermal conditions: solubility, hydrolysis and sulfate ion pairing. J. Solution Chem. 28, 621–630 (1999).
Zhao, H. et al. In operando synchrotron studies of NH4+ preintercalated V2O5·nH2O nanobelts as the cathode material for aqueous rechargeable zinc batteries. ACS Nano 14, 11809–11820 (2020).
Zuo, S. et al. Direct detection and visualization of the H+ reaction process in a VO2 cathode for aqueous zinc-ion batteries. J. Phys. Chem. Lett. 12, 7076–7084 (2021).
Dai, Y. et al. Quicker and more Zn2+ storage predominantly from the interface. Adv. Mater. 33, 2100359 (2021).
Liu, Y. et al. In-situ electrochemically activated surface vanadium valence in V2C MXene to achieve high capacity and superior rate performance for Zn-ion batteries. Adv. Funct. Mater. 31, 2008033 (2020).
Deng, S. et al. Electrochemically induced metal-organic-framework-derived amorphous V2O5 for superior rate aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 59, 22002–22006 (2020).
Wang, L. et al. Ultrahigh-rate and ultralong-life aqueous batteries enabled by special pair-dancing proton transfer. Sci. Adv. 9, eadf4589 (2023).
Li, M. et al. Universal multifunctional hydrogen bond network construction strategy for enhanced aqueous Zn2+/proton hybrid batteries. Nano Energy 100, 107539 (2022).
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (52172233, 51832004, 21905218, 51872218 and 52072285; L.M.), the Natural Science Foundation of Guangdong Province (no. 2021A1515010144; L.M.), the National Key Research and Development Program of China (2020YFA0715000; L.M.), the Foshan Xianhu Laboratory of the Advanced Energy Science and Technology Guangdong Laboratory (XHT2020-003; L.M.) and the Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City (520LH055; L.M.). The computational study was supported by the Marsden Fund Council from Government funding (21-UOA-237; Z.W.) and Catalyst: Seeding General Grant (22-UOA-031-CGS; Z.W.), managed by Royal Society Te Apārangi. All DFT calculations were carried out on the New Zealand eScience Infrastructure (NeSI) high-performance computing facilities. G.I.N.W. is supported by a James Cook Research Fellowship from New Zealand Government funding, administered by the Royal Society Te Apārangi. This research also used resources of the Advanced Photon Source, a US DOE Office of Science User Facility, operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02- 06CH11357.
Author information
Authors and Affiliations
Contributions
J. Lu, K.A., Z.W. and L.M. supervised this project. Y.D., R.L., C.Z. and Jiantao Li conceived the ideas. Y.D. designed the materials and experiments. R.L., C.Z. and Y.M. carried out theoretical calculations. Jiantao Li and Y.R. conducted XAS and high-energy XRD characterizations. Y.Y. and C.Y. designed the figures and participated in the draft writing. Z.C. performed materials synthesis and tested the electrochemical performance. J.Z. carried out SEM and EIS measurements. Jinghao Li carried out basic characterizations such as XRD and XPS. R.Y. performed the STEM characterization. L.C. carried out UPS tests. S.Z., G.H. and P.R.S. carried out the X-ray micro-CT characterizations. Q.A. provided help with SEM, XRD and UPS tests. Y.D., R.L., C.Z., G.I.N.W. and Jiantao Li wrote the manuscript. All authors discussed the results and assisted with manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Stefan Freunberger, Yan Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Figs. 1–35, Tables 1–4, notes 1–7 and references 1–170.
Source data
Source Data Figs. 1–5
Unprocessed statistical source data.
Atomic Coordinates
Atomic coordinates of the optimized computational models.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, Y., Lu, R., Zhang, C. et al. Zn2+-mediated catalysis for fast-charging aqueous Zn-ion batteries. Nat Catal 7, 776–784 (2024). https://doi.org/10.1038/s41929-024-01169-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-024-01169-6
This article is cited by
-
Inorganic Interface Engineering for Stabilizing Zn Metal Anode
Nano-Micro Letters (2026)
-
Dissolution, solvation and diffusion in low-temperature zinc electrolyte design
Nature Reviews Chemistry (2025)
-
Enhanced hydrogen peroxide photosynthesis via charge-complementary π-electron sites
Nature Communications (2025)
-
Hydrogel Electrolytes-Based Rechargeable Zinc-Ion Batteries under Harsh Conditions
Nano-Micro Letters (2025)
-
An updated review on the potential of V₂O₅-based materials for zinc-ion batteries
Ionics (2025)