Abstract
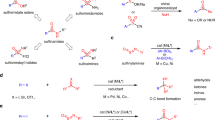
Sulfur stereogenic molecules have a significant impact on drug development. Among them, sulfilimines are chiral molecules bearing S(IV) stereocentres, which exhibit great value in chemistry and biology but have so far been synthetically challenging to achieve. Similarly, it has also been a challenge to control the stereochemistry in Chan–Lam coupling, which has been widely used to construct C–N, C–O and C–S bonds by coupling nucleophiles with boronic acids using copper complexes. Here we report a highly chemoselective and enantioselective Chan–Lam S-arylation of sulfenamides with arylboronic acids to deliver an array of thermodynamically disfavoured aryl sulfilimines containing a sulfur stereocentre. A copper catalyst from a 2-pyridyl N-phenyl dihydroimidazole ligand has been designed that enables effective enantiocontrol by means of a well-defined chiral environment and high reactivity that outcompetes the background racemic transformation. A combined experimental and computational study establishes the reaction mechanism and unveils the origin of chemoselectivity and stereoselectivity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Detailed experimental procedures, characterization data, NMR spectra of compounds, detailed computational results and calculated structures are available within the Supplementary Information and related files. The X-ray crystallographic coordinates for the structure reported in this study have been deposited at the CCDC under deposition number CCDC 2215359 (for 3ba). These data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/data_request/cif. Any further relevant data are available from the authors upon request.
References
Lücking, U. Sulfoximines: a neglected opportunity in medicinal chemistry. Angew. Chem. Int. Ed. 52, 9399–9408 (2013).
Lücking, U. New opportunities for the utilization of the sulfoximine group in medicinal chemistry from the drug designer’s perspective. Chem. Eur. J. 28, e202201993 (2022).
Kaiser, D., Klose, I., Oost, R., Neuhaus, J. & Maulide, N. Bond-forming and -breaking reactions at sulfur(IV): sulfoxides, sulfonium salts, sulfur ylides, and sulfinate salts. Chem. Rev. 119, 8701–8780 (2019).
Wojaczynska, E. & Wojaczynski, J. Modern stereoselective synthesis of chiral sulfinyl compounds. Chem. Rev. 120, 4578–4611 (2020).
Gilchrist, T. L. & Moody, C. J. The chemistry of sulfilimines. Chem. Rev. 77, 409–435 (1977).
Taylor, P. C. Sulfimides (sulfilimines): applications in stereoselective synthesis. Sulfur Rep. 21, 241–280 (1999).
Bizet, V., Hendriks, C. M. & Bolm, C. Sulfur imidations: access to sulfimides and sulfoximines. Chem. Soc. Rev. 44, 3378–3390 (2015).
Vanacore, R. et al. A sulfilimine bond identified in collagen IV. Science 325, 1230–1234 (2009).
Lin, S. et al. Redox-based reagents for chemoselective methionine bioconjugation. Science 355, 597–602 (2017).
Christian, A. H. et al. A physical organic approach to tuning reagents for selective and stable methionine bioconjugation. J. Am. Chem. Soc. 141, 12657–12662 (2019).
Xu, K. et al. An ultrasensitive cyclization-based fluorescent probe for imaging native HOBr in live cells and zebrafish. Angew. Chem. Int. Ed. 55, 12751–12754 (2016).
Lücking, U. Neglected sulfur(VI) pharmacophores in drug discovery: exploration of novel chemical space by the interplay of drug design and method development. Org. Chem. Front. 6, 1319–1324 (2019).
Zhang, X., Wang, F. & Tan, C.-H. Asymmetric synthesis of S(IV) and S(VI) stereogenic centers. JACS Au 3, 700–714 (2023).
Takada, H. et al. Catalytic asymmetric sulfimidation. J. Org. Chem. 62, 6512–6518 (1997).
Tomooka, C. S. & Carreira, E. M. Enantioselective nitrogen transfer to sulfides from nitridomanganese(V) complexes. Helv. Chim. Acta 85, 3773–3784 (2002).
Armstrong, A., Edmonds, I. D. & Swarbrick, M. E. Efficient nitrogen transfer from aldehyde-derived N-acyloxaziridines. Tetrahedron Lett. 44, 5335–5338 (2003).
Collet, F., Dodd, R. H. & Dauban, P. Stereoselective rhodium-catalyzed imination of sulfides. Org. Lett. 10, 5473–5476 (2008).
Wang, J., Frings, M. & Bolm, C. Enantioselective nitrene transfer to sulfides catalyzed by a chiral iron complex. Angew. Chem. Int. Ed. 52, 8661–8665 (2013).
Uchida, T. & Katsuki, T. Asymmetric nitrene transfer reactions: sulfimidation, aziridination and C–H amination using azide compounds as nitrene precursors. Chem. Rec. 14, 117–129 (2014).
Lebel, H., Piras, H. & Bartholoméüs, J. Rhodium-catalyzed stereoselective amination of thioethers with N-mesyloxycarbamates: DMAP and bis(DMAP)CH2Cl2 as key additives. Angew. Chem. Int. Ed. 53, 7300–7304 (2014).
Yoshitake, M., Hayashi, H. & Uchida, T. Ruthenium-catalyzed asymmetric N-acyl nitrene transfer reaction: imidation of sulfide. Org. Lett. 22, 4021–4025 (2020).
Annapureddy, R. R. et al. Silver-catalyzed enantioselective sulfimidation mediated by hydrogen bonding interactions. Angew. Chem. Int. Ed. 60, 7920–7926 (2021).
Greenwood, N. S., Champlin, A. T. & Ellman, J. A. Catalytic enantioselective sulfur alkylation of sulfenamides for the asymmetric synthesis of sulfoximines. J. Am. Chem. Soc. 144, 17808–17814 (2022).
Kikuchi, K., Furukawa, N., Moriyama, M. & Oae, S. Nucleophilic substitution of tricoordinate sulfur atom of sulfonium salt with retention of configuration. Different stereochemistry of substitution by amidate anions. Bull. Chem. Soc. Jpn 58, 1934–1941 (1985).
Takada, H., Oda, M., Oyamada, A., Ohe, K. & Uemura, S. Catalytic diastereoselective sulfimidation of diaryl sulfides and application of chiral sulfimides to asymmetric allylic alkylation. Chirality 12, 299–312 (2000).
Tsuzuki, S. & Kano, T. Asymmetric synthesis of chiral sulfimides through the O-alkylation of enantioenriched sulfinamides and addition of carbon nucleophiles. Angew. Chem. Int. Ed. 62, e202300637 (2023).
West, M. J., Fyfe, J. W. B., Vantourout, J. C. & Watson, A. J. B. Mechanistic development and recent applications of the Chan–Lam amination. Chem. Rev. 119, 12491–12523 (2019).
Chen, J. Q., Li, J. H. & Dong, Z. B. A review on the latest progress of Chan-Lam coupling reaction. Adv. Synth. Catal. 362, 3311–3331 (2020).
King, A. E., Brunold, T. C. & Stahl, S. S. Mechanistic study of copper-catalyzed aerobic oxidative coupling of arylboronic esters and methanol: insights into an organometallic oxidase reaction. J. Am. Chem. Soc. 131, 5044–5045 (2009).
King, A. E., Ryland, B. L., Brunold, T. C. & Stahl, S. S. Kinetic and spectroscopic studies of aerobic copper(II)-catalyzed methoxylation of arylboronic esters and insights into aryl transmetalation to copper(II). Organometallics 31, 7948–7957 (2012).
Vantourout, J. C., Miras, H. N., Isidro-Llobet, A., Sproules, S. & Watson, A. J. Spectroscopic studies of the Chan–Lam amination: a mechanism-inspired solution to boronic ester reactivity. J. Am. Chem. Soc. 139, 4769–4779 (2017).
Bose, S., Dutta, S. & Koley, D. Entering chemical space with theoretical underpinning of the mechanistic pathways in the Chan–Lam amination. ACS Catal. 12, 1461–1474 (2022).
Pooventhiran, T., Khilari, N. & Koley, D. Mechanistic avenues in the Chan-Lam-based etherification reaction: a computational exploration. Chem. Eur. J. 29, e202302983 (2023).
Hardouin Duparc, V., Bano, G. L. & Schaper, F. Chan–Evans–Lam couplings with copper iminoarylsulfonate complexes: scope and mechanism. ACS Catal. 8, 7308–7325 (2018).
Liang, Q. et al. Synthesis of sulfilimines enabled by copper-catalyzed S-arylation of sulfenamides. J. Am. Chem. Soc. 145, 6310–6318 (2023).
Chen, Y. et al. Synthesis of sulfilimines via selective S–C bond formation in water. Org. Lett. 25, 2134–2138 (2023).
Jutand, A. & Grimaud, L. Role of fluoride ions in palladium-catalyzed cross-coupling reactions. Synthesis 49, 1182–1189 (2016).
Akutagawa, K., Furukawa, N. & Oae, S. Preparation of N-(arylsulfonyl)sulfoximines by oxidation of N-(arylsulfonyl)sulfilimines with sodium hypochlorite in a two-phase system. J. Org. Chem. 49, 2282–2284 (2002).
Siemeister, G. et al. BAY 1000394, a novel cyclin-dependent kinase inhibitor, with potent antitumor activity in mono- and in combination treatment upon oral application. Mol. Cancer Ther. 11, 2265–2273 (2012).
Kim, E. et al. Novel compound and pharmaceutical composition comprising same as active ingredient. US patent 2020/0190024 A1 (2020).
Albrecht, B. K. et al. Modulators of methyl modifying enzymes, compositions and uses thereof. US patent 9206128 B2 (2015).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Petersson, G. A. & Al-Laham, M. A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 94, 6081–6090 (1991).
Andrae, D., Häußermann, U., Dolg, M., Stoll, H. & Preuß, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 77, 123–141 (1990).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
McLean, A. D. & Chandler, G. S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J. Chem. Phys. 72, 5639–5648 (1980).
Krishnan, R., Binkley, J. S., Seeger, R. & Pople, J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980).
Cossi, M., Rega, N., Scalmani, G. & Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 24, 669–681 (2003).
Kozuch, S. & Shaik, S. How to conceptualize catalytic cycles? The energetic span model. Acc. Chem. Res. 44, 101–110 (2011).
Kitaura, K. & Morokuma, K. A new energy decomposition scheme for molecular interactions within the Hartree‐Fock approximation. Int. J. Quantum Chem. 10, 325–340 (1976).
Bickelhaupt, F. M. & Houk, K. N. Analyzing reaction rates with the distortion/interaction-activation strain model. Angew. Chem. Int. Ed. 56, 10070–10086 (2017).
Horn, P. R. & Head-Gordon, M. Alternative definitions of the frozen energy in energy decomposition analysis of density functional theory calculations. J. Chem. Phys. 144, 084118 (2016).
Horn, P. R., Mao, Y. & Head-Gordon, M. Defining the contributions of permanent electrostatics, Pauli repulsion, and dispersion in density functional theory calculations of intermolecular interaction energies. J. Chem. Phys. 144, 114107 (2016).
Horn, P. R., Mao, Y. & Head-Gordon, M. Probing non-covalent interactions with a second generation energy decomposition analysis using absolutely localized molecular orbitals. Phys. Chem. Chem. Phys. 18, 23067–23079 (2016).
Shao, Y. et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol. Phys. 113, 184–215 (2015).
Thomas, A. A. et al. Mechanistically guided design of ligands that significantly improve the efficiency of CuH-catalyzed hydroamination reactions. J. Am. Chem. Soc. 140, 13976–13984 (2017).
Chan, L., Morris, G. M. & Hutchison, G. R. Understanding conformational entropy in small molecules. J. Chem. Theory Comput. 17, 2099–2106 (2021).
Acknowledgements
T.J. thanks the National Natural Science Foundation of China (U23A20528), Guangdong Basic and Applied Basic Research Foundation (2021B1515120046 and 2022B1515120075), the Science and Technology Innovation Commission of Shenzhen Municipality (JCYJ20220818101404010 and 20220815113214003) and the High Level of Special Funds (G03050K003) for financial support. M.C.K. thanks the National Institutes of Health (NIH; R35 GM131902) for financial support and Advanced Cyberinfrastructure Coordination Ecosystem: Services and Support (ACCESS; TG-CHE120052) for computational support. We are grateful to Y. Yu and X. Chang (both at SUSTech) for High Resolution Mass Spectrum and X-ray crystallography, respectively. We also acknowledge the assistance of SUSTech Core Research Facilities.
Author information
Authors and Affiliations
Contributions
T.J. conceived and supervised the project. Q.L., X.Z. and Z.X. performed the experiments. M.C.K. directed the computational study. M.E.R. carried out the computational study. T.J., Q.L. and X.Z. analysed the data. All authors participated in writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Taichi Kano, Debasis Koley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–333, Tables 1–21, Methods, notes and references.
Supplementary Data 1
Crystallographic data for compound 3ba.
Supplementary Data 2
Cif check report for 3ba.
Supplementary Data 3
Computational data.
Supplementary Data 4
Computational data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Q., Zhang, X., Rotella, M.E. et al. Enantioselective Chan–Lam S-arylation of sulfenamides. Nat Catal 7, 1010–1020 (2024). https://doi.org/10.1038/s41929-024-01213-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-024-01213-5
This article is cited by
-
Copper-catalysed asymmetric cross-coupling reactions tolerant of highly reactive radicals
Nature Chemistry (2026)
-
Chiral anionic ProPhenol ligand enabled nickel catalyzed enantioselective synthesis of sulfinamides
Science China Chemistry (2026)
-
Assembly of (hetero)aryl sulfilimines via copper-catalyzed enantioselective S-arylation of sulfenamides with (hetero)aryl Iodides
Nature Communications (2025)
-
Catalytic synthesis of chiral sulfinimidate esters via oxidative esterification of sulfenamides
Nature Communications (2025)