Abstract
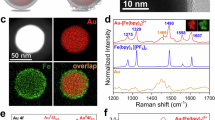
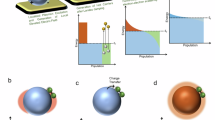
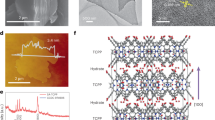
Steam methane reforming (SMR) is the major industrial process for hydrogen production. It currently relies on high-temperature operating conditions and is associated with high carbon intensity. Photocatalytic SMR could provide greener and potentially more efficient H2 production. Here we demonstrate a plasmonic photocatalytic approach based on a Cu–Rh antenna–reactor photocatalyst for highly reactive, selective and stable SMR due to plasmon-mediated hot carrier contributions. We observe that the photocatalyst is intrinsically stable in photocatalysis but deactivates under thermocatalysis; however, the thermally deactivated catalyst can be regenerated by resonant illumination. The regeneration mechanism is studied in detail and found to be caused by plasmon-induced associative desorption of oxygen and carbon species.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Additional datasets generated and/or analysed during the current study that are not included in the published Article and associated Supplementary Information, including the atomic coordinates for the JDFTx calculations, are available from the open-access Zenodo repository at https://doi.org/10.5281/zenodo.13377300 (ref. 66).
Code availability
Python codes for the EM simulation and hot carrier distribution calculations are available from the open-access Zenodo repository at https://doi.org/10.5281/zenodo.13377300 (ref. 66).
References
Guo, X. et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 344, 616–619 (2014).
Upham, D. C. et al. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science 358, 917–921 (2017).
Sushkevich, V. L., Palagin, D., Ranocchiari, M. & Van Bokhoven, J. A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356, 523–527 (2017).
Jin, Z. et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 367, 193–197 (2020).
Li, X., Xie, J., Rao, H., Wang, C. & Tang, J. Platinum- and CuOx-decorated TiO2 photocatalyst for oxidative coupling of methane to C2 hydrocarbons in a flow reactor. Angew. Chem. Int. Ed. 59, 19702–19707 (2020).
Yu, X. et al. Stoichiometric methane conversion to ethane using photochemical looping at ambient temperature. Nat. Energy 5, 511–519 (2020).
Morejudo, S. H. et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 353, 563–566 (2016).
Hu, A., Guo, J.-J., Pan, H. & Zuo, Z. Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis. Science 361, 668–672 (2018).
Nikolaidis, P. & Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 67, 597–611 (2017).
Kannah, R. Y. et al. Techno-economic assessment of various hydrogen production methods—a review. Bioresour. Technol. 319, 124175 (2021).
Schulz, H. Short history and present trends of Fischer–Tropsch synthesis. Appl. Catal. A 186, 3–12 (1999).
Dry, M. E. The Fischer–Tropsch process: 1950–2000. Catal. Today 71, 227–241 (2002).
Jiao, F. et al. Disentangling the activity–selectivity trade-off in catalytic conversion of syngas to light olefins. Science 380, 727–730 (2023).
Van Hook, J. P. Methane–steam reforming. Catal. Rev. 21, 1–51 (1980).
Meng, X. et al. Direct methane conversion under mild condition by thermo-, electro-, or photocatalysis. Chem 5, 2296–2325 (2019).
Li, Q., Ouyang, Y., Li, H., Wang, L. & Zeng, J. Photocatalytic conversion of methane: recent advancements and prospects. Angew. Chem. Int. Ed. 61, e202108069 (2022).
Li, X., Wang, C. & Tang, J. Methane transformation by photocatalysis. Nat. Rev. Mater. 7, 617–632 (2022).
Yoshida, H., Kato, S., Hirao, K., Nishimoto, J.-I. & Hattori, T. Photocatalytic steam reforming of methane over platinum-loaded semiconductors for hydrogen production. Chem. Lett. 36, 430–431 (2007).
Shimura, K. et al. Photocatalytic steam reforming of methane over sodium tantalate. J. Phys. Chem. C 114, 3493–3503 (2010).
Shimura, K., Kawai, H., Yoshida, T. & Yoshida, H. Bifunctional rhodium cocatalysts for photocatalytic steam reforming of methane over alkaline titanate. ACS Catal. 2, 2126–2134 (2012).
Yamamoto, A., Mizuba, S., Saeki, Y. & Yoshida, H. Platinum loaded sodium tantalate photocatalysts prepared by a flux method for photocatalytic steam reforming of methane. Appl. Catal. A 521, 125–132 (2016).
Song, H. et al. Visible-light-mediated methane activation for steam methane reforming under mild conditions: a case study of Rh/TiO2 catalysts. ACS Catal. 8, 7556–7565 (2018).
Han, B., Wei, W., Li, M., Sun, K. & Hu, Y. H. A thermo-photo hybrid process for steam reforming of methane: highly efficient visible light photocatalysis. Chem. Commun. 55, 7816–7819 (2019).
Linic, S., Christopher, P. & Ingram, D. B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 10, 911–921 (2011).
Brongersma, M. L., Halas, N. J. & Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 10, 25–34 (2015).
Aslam, U., Chavez, S. & Linic, S. Controlling energy flow in multimetallic nanostructures for plasmonic catalysis. Nat. Nanotechnol. 12, 1000–1005 (2017).
Robatjazi, H., Yuan, L., Yuan, Y. & Halas, N. J. in Emerging Trends in Chemical Applications of Lasers Vol. 1398, 363–387 (ACS Symposium Series, American Chemical Society, 2021).
Mukherjee, S. et al. Hot-electron-induced dissociation of H2 on gold nanoparticles supported on SiO2. J. Am. Chem. Soc. 136, 64–67 (2013).
Lou, M. et al. Direct H2S decomposition by plasmonic photocatalysis: efficient remediation plus sustainable hydrogen production. ACS Energy Lett. 7, 3666–3674 (2022).
Contreras, E. et al. Plasmon-assisted ammonia electrosynthesis. J. Am. Chem. Soc. 144, 10743–10751 (2022).
Christopher, P., Xin, H. & Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 3, 467–472 (2011).
Devasia, D., Wilson, A. J., Heo, J., Mohan, V. & Jain, P. K. A rich catalog of C–C bonded species formed in CO2 reduction on a plasmonic photocatalyst. Nat. Commun. 12, 2612 (2021).
Marimuthu, A., Zhang, J. & Linic, S. Tuning selectivity in propylene epoxidation by plasmon mediated photo-switching of Cu oxidation state. Science 339, 1590–1593 (2013).
Zhou, L. et al. Aluminum nanocrystals as a plasmonic photocatalyst for hydrogen dissociation. Nano Lett. 16, 1478–1484 (2016).
Swearer, D. F. et al. Heterometallic antenna–reactor complexes for photocatalysis. Proc. Natl Acad. Sci. USA 113, 8916–8920 (2016).
Sytwu, K. et al. Driving energetically unfavorable dehydrogenation dynamics with plasmonics. Science 371, 280–283 (2021).
Halas, N. J. et al. Multicomponent plasmonic photocatalysts consisting of a plasmonic antenna and a reactive catalytic surface: the antenna–reactor effect. US patent US10766024B2 (2020).
Yuan, Y. et al. Earth-abundant photocatalyst for H2 generation from NH3 with light-emitting diode illumination. Science 378, 889–893 (2022).
Zhou, L. et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 362, 69–72 (2018).
Zhou, L. et al. Light-driven methane dry reforming with single atomic site antenna–reactor plasmonic photocatalysts. Nat. Energy 5, 61–70 (2020).
Robatjazi, H. et al. Plasmon-induced selective carbon dioxide conversion on Earth-abundant aluminum–cuprous oxide antenna–reactor nanoparticles. Nat. Commun. 8, 27 (2017).
Robatjazi, H. et al. Plasmon-driven carbon–fluorine (C(sp3)–F)) bond activation with mechanistic insights into hot-carrier-mediated pathways. Nat. Catal. 3, 564–573 (2020).
Zhou, L. et al. Hot carrier multiplication in plasmonic photocatalysis. Proc. Natl Acad. Sci. USA 118, e2022109118 (2021).
Jones, G. et al. First principles calculations and experimental insight into methane steam reforming over transition metal catalysts. J. Catal. 259, 147–160 (2008).
Besenbacher, F. et al. Design of a surface alloy catalyst for steam reforming. Science 279, 1913–1915 (1998).
Marcinkowski, M. D. et al. Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C–H activation. Nat. Chem. 10, 325–332 (2018).
Hannagan, R. T. et al. First-principles design of a single-atom-alloy propane dehydrogenation catalyst. Science 372, 1444–1447 (2021).
Behrens, M. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897 (2012).
Behrens, M. Coprecipitation: an excellent tool for the synthesis of supported metal catalysts—from the understanding of the well known recipes to new materials. Catal. Today 246, 46–54 (2015).
Boudart, M. Turnover rates in heterogeneous catalysis. Chem. Rev. 95, 661–666 (1995).
Brown, A. M., Sundararaman, R., Narang, P., Goddard, W. A. & Atwater, H. A. Nonradiative plasmon decay and hot carrier dynamics: effects of phonons, surfaces, and geometry. ACS Nano 10, 957–966 (2016).
Zhou, L. et al. Response to comment on “Quantifying hot carrier and thermal contributions in plasmonic photocatalysis”. Science 364, eaaw9545 (2019).
Robatjazi, H. et al. Reply to: Distinguishing thermal from non-thermal contributions to plasmonic hydrodefluorination. Nat. Catal. 5, 247–250 (2022).
Mukherjee, S. et al. Hot electrons do the impossible: plasmon-induced dissociation of H2 on Au. Nano Lett. 13, 240–247 (2013).
Linic, S., Aslam, U., Boerigter, C. & Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 14, 567–576 (2015).
Lindstrom, C. D. & Zhu, X. Y. Photoinduced electron transfer at molecule–metal interfaces. Chem. Rev. 106, 4281–4300 (2006).
Kim, K. H., Watanabe, K., Mulugeta, D., Freund, H.-J. & Menzel, D. Enhanced photoinduced desorption from metal nanoparticles by photoexcitation of confined hot electrons using femtosecond laser pulses. Phys. Rev. Lett. 107, 047401 (2011).
Seemala, B. et al. Plasmon-mediated catalytic O2 dissociation on Ag nanostructures: hot electrons or near fields? ACS Energy Lett. 4, 1803–1809 (2019).
Stephens, R. E. & Malitson, I. H. Index of refraction of magnesium oxide. J. Res. Nat. Bur. Stand. 49, 249–252 (1952).
Edward, D. P. Handbook of Optical Constants of Solids (Academic Press, 1998).
Lindsay, A. L. & Bromley, L. A. Thermal conductivity of gas mixtures. Ind. Eng. Chem. 42, 1508–1511 (1950).
Touloukian, Y. S., Liley, P. E. & Saxena, S. C. Thermophysical Properties of Matter—the TPRC Data Series Vol. 3 (IFI/Plenum, 1970).
Touloukian, Y. S., Saxena, S. C. & Hestermans, P. Thermophysical Properties of Matter—the TPRC Data Series Vol. 11 (IFI/Plenum, 1975).
Crane, C. Flow of Fluids Through Valves, Fittings, and Pipe (Crane Company, 1988).
Sundararaman, R. et al. JDFTx: software for joint density-functional theory. SoftwareX 6, 278–284 (2017).
Yuan, Y. et al. Steam methane reforming using a regenerable antenna-reactor plasmonic photocatalyst. Zenodo https://doi.org/10.5281/zenodo.13377300 (2024).
Acknowledgements
This article is based on work supported by the Robert A. Welch Foundation under grants C-1220 (to N.J.H.) and C-1222 (to P.N.) and by the Air Force Office of Scientific Research via the Department of Defense Multidisciplinary University Research Initiative under AFOSR Award number FA9550-15-1-0022. We acknowledge B. Chen from the Shared Equipment Authority at Rice University for providing valuable insights and assistance with processing the XPS data.
Author information
Authors and Affiliations
Contributions
Y.Y., P.N. and N.J.H. initiated the project. Y.Y. developed the photocatalyst, performed the characterization, studied the catalysis and analysed the data. J.Z. performed the theoretical simulations. A.B. performed the scanning transmission electron microscopy. H.R. helped to interpret the data. P.N. and N.J.H. supervised the research. All authors contributed to preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The antenna–reactor concept is protected under US patents US10766024B2 and US11958043B2, with additional coverage pending in published US patent application US20210023541A1. The antenna–reactor concept is also described in the international patent application WO 2018/231398 A2. Syzygy Plasmonics owns several patents relating to the photoreactor platform. N.J.H. and P.N. are co-founders of Syzygy Plasmonics. N.J.H., P.N. and H.R. have interest in Syzygy Plasmonics and hold equity in the company. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Jennifer Dionne, Hisao Yoshida and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Note 1 and Figs. 1–10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, Y., Zhou, J., Bayles, A. et al. Steam methane reforming using a regenerable antenna–reactor plasmonic photocatalyst. Nat Catal 7, 1339–1349 (2024). https://doi.org/10.1038/s41929-024-01248-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-024-01248-8
This article is cited by
-
Plasmonic Ni-doped W18O49 with dual active sites drives efficient methanol dehydration to dimethyl ether
Nature Communications (2025)
-
Near-infrared photon-triggered CH4-to-CH3OH conversion over plasmonic oxyselenides
Nature Communications (2025)
-
Light-induced pressurization effect in Au–Ni heterostructures for plasmonic photocatalysis
Nature Communications (2025)