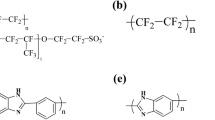
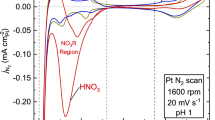
Abstract
Electrochemical interfaces between polymer electrolytes and electrodes are central to electrochemical devices in the global transition towards renewable energy. Here we show that the adsorption and desorption of sulfonates in Nafion on Pt(111) involve distinct elementary steps, with the latter proceeding through a coupled cation–electron transfer. Adsorbed sulfonates not only block a fraction of surface Pt sites but, more importantly, generate two additional types of surface adsorbate, OHNafion and ONafion, which exhibit distinct kinetic properties from adsorbed OH and O on bare Pt(111), respectively. The impact of the adsorption of sulfonate groups in Nafion on the activity of the oxygen reduction reaction (ORR) on Pt cannot be rationalized by existing thermodynamic descriptors. The reduced ORR activity on the Nafion-covered Pt(111) is caused by the kinetically hindered *O→*OH conversion and *OH reduction on sites close to adsorbed sulfonates.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Jacobse, L., Huang, Y.-F., Koper, M. T. M. & Rost, M. J. Correlation of surface site formation to nanoisland growth in the electrochemical roughening of Pt(111). Nat. Mater. 17, 277–282 (2018).
Ojha, K., Arulmozhi, N., Aranzales, D. & Koper, M. T. M. Double layer at the Pt(111)-aqueous electrolyte interface: potential of zero charge and anomalous Gouy-Chapman screening. Angew. Chem. Int. Ed. 59, 711–715 (2020).
Rizo, R. et al. Investigating the presence of adsorbed species on Pt steps at low potentials. Nat. Commun. 13, 2550 (2022).
McCrum, I. T. & Koper, M. T. M. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nat. Energy 5, 891–899 (2020).
Luo, M. & Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2, 17059 (2017).
Zhu, J., Hu, L., Zhao, P., Lee, L. Y. S. & Wong, K. Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 120, 851–918 (2020).
Li, Y. et al. Modifying the electrocatalyst-ionomer interface via sulfonated poly(ionic liquid) block copolymers to enable high-performance polymer electrolyte fuel cells. ACS Energy Lett. 5, 1726–1731 (2020).
Lan, C. et al. Research progress of proton-exchange membrane fuel cell cathode nonnoble metal M-Nx/C-type oxygen reduction catalysts. Acta Phys. Chim. Sin. 39, 2210036 (2022).
Lazaridis, T., Stühmeier, B. M., Gasteiger, H. A. & El-Sayed, H. A. Capabilities and limitations of rotating disk electrodes versus membrane electrode assemblies in the investigation of electrocatalysts. Nat. Catal. 5, 363–373 (2022).
Peng, X. et al. Using operando techniques to understand and design high performance and stable alkaline membrane fuel cells. Nat. Commun. 11, 3561 (2020).
Wang, Q., Cha, C. S., Lu, J. & Zhuang, L. The electrochemistry of ‘solid/water’ interfaces involved in PEM-H2O reactors: Part I. The ‘Pt/water’ interfaces. Phys. Chem. Chem. Phys. 11, 679–687 (2009).
Wang, Y.-C. et al. Identification of the active triple-phase boundary of a non-Pt catalyst layer in fuel cells. Sci. Adv. 8, eadd8873 (2022).
Li, Y., Intikhab, S., Malkani, A., Xu, B. & Snyder, J. Ionic liquid additives for the mitigation of Nafion specific adsorption on platinum. ACS Catal. 10, 7691–7698 (2020).
Kodama, K. et al. Effect of the side-chain structure of perfluoro-sulfonic acid ionomers on the oxygen reduction reaction on the surface of Pt. ACS Catal. 8, 694–700 (2017).
Xu, Y. et al. Boosting oxygen reduction at Pt(111)|proton exchange ionomer interfaces through tuning the microenvironment water activity. ACS Appl. Mater. Interfaces 16, 4540–4549 (2024).
Subbaraman, R., Strmcnik, D., Stamenkovic, V. & Markovic, N. M. Three phase interfaces at electrified metal-solid electrolyte systems 1. Study of the Pt(hkl)-Nafion interface. J. Phys. Chem. C 114, 8414–8422 (2010).
Kusoglu, A. & Weber, A. Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 117, 987–1104 (2017).
Ahmed, M. et al. Unprecedented structural sensitivity toward average terrace width: Nafion adsorption at Pt{hkl} electrodes. J. Phys. Chem. C 115, 17020–17027 (2011).
Kodama, K. & Motobayashi, K. Adsorption of ionomer and ionic liquid on model Pt catalysts for polymer electrolyte fuel cells. Electrochem. Sci. Adv. 3, e2100183 (2022).
Climent, V., Gómez, R., Orts, J. M. & Feliu, J. M. Thermodynamic analysis of the temperature dependence of OH adsorption on Pt(111) and Pt(100) electrodes in acidic media in the absence of specific anion adsorption. J. Phys. Chem. B 110, 11344–11351 (2006).
Berna, A., Climent, V. & Feliu, J. New understanding of the nature of OH adsorption on Pt(111) electrodes. Electrochem. Commun. 9, 2789–2794 (2007).
Herrero, E., Mostany, J., Feliu, J. M. & Lipkowski, J. Thermodynamic studies of anion adsorption at the Pt(111) electrode surface in sulfuric acid solutions. J. Electroanal. Chem. 534, 79–89 (2002).
Koper, M. T. M. & Lukkien, J. J. Modeling the butterfly: the voltammetry of (√3 × √3)R30° and p(2 × 2) overlayers on (111) electrodes. J. Electroanal. Chem. 485, 161–165 (2000).
Koper, M. T. M. & Lukkien, J. J. Modeling the butterfly: influence of lateral interactions and adsorption geometry on the voltammetry at (111) and (100) electrodes. Surf. Sci. 498, 105–115 (2002).
Kolics, A. & Wieckowski, A. Adsorption of bisulfate and sulfate anions on a Pt(111) electrode. J. Phys. Chem. B 105, 2588–2595 (2001).
Zuo, X. Q. et al. pH effect on acetate adsorption at Pt(111) electrode. Electrochem. Commun. 89, 6–9 (2018).
Cui, H., Xu, Y.-J., Pan, S.-Y. & Chen, Y.-X. Effects of solution pH and preparation conditions on the electrochemical behaviors of Pt(111)-Nafion interface. Electrochim. Acta 475, 143652 (2024).
Naegeli, R., Redepenning, J. & Anson, F. C. Influence of supporting electrolyte concentration and composition on formal potentials and entropies of redox couples incorporated in Nafion coatings on electrodes. J. Phys. Chem. 90, 6227–6232 (2002).
Yang, Y. et al. Inverse kinetic isotope effects in the oxygen reduction reaction at platinum single crystals. Nat. Chem. 15, 271–277 (2022).
Funtikov, A. M., Stimming, U. & Vogel, R. Anion adsorption from sulfuric acid solutions on Pt(111) single crystal electrodes. J. Electroanal. Chem. 428, 147–153 (1997).
Gisbert, R., García, G. & Koper, M. T. M. Adsorption of phosphate species on poly-oriented Pt and Pt(111) electrodes over a wide range of pH. Electrochim. Acta 55, 7961–7968 (2010).
Luo, M. & Koper, M. T. M. A kinetic descriptor for the electrolyte effect on the oxygen reduction kinetics on Pt(111). Nat. Catal. 5, 615–623 (2022).
Gómez-Marín, A. M., Clavilier, J. & Feliu, J. M. Sequential Pt(111) oxide formation in perchloric acid: an electrochemical study of surface species inter-conversion. J. Electroanal. Chem. 688, 360–370 (2013).
Briega-Martos, V., Herrero, E. & Feliu, J. M. Effect of pH and water structure on the oxygen reduction reaction on platinum electrodes. Electrochim. Acta 241, 497–509 (2017).
Shinozaki, K., Morimoto, Y., Pivovar, B. S. & Kocha, S. S. Suppression of oxygen reduction reaction activity on Pt-based electrocatalysts from ionomer incorporation. J. Power Sources 325, 745–751 (2016).
Jomori, S., Komatsubara, K., Nonoyama, N., Kato, M. & Yoshida, T. An experimental study of the effects of operational history on activity changes in a PEMFC. J. Electrochem. Soc. 160, F1067–F1073 (2013).
Subbaraman, R., Strmcnik, D., Paulikas, A. P., Stamenkovic, V. R. & Markovic, N. M. Oxygen reduction reaction at three‐phase interfaces. ChemPhysChem 11, 2825–2833 (2010).
Shinozaki, K., Zack, J. W., Pylypenko, S., Pivovar, B. S. & Kocha, S. S. Oxygen reduction reaction measurements on platinum electrocatalysts utilizing rotating disk electrode technique. J. Electrochem. Soc. 162, F1384–F1396 (2015).
Park, Y.-C., Kakinuma, K., Uchida, H., Watanabe, M. & Uchida, M. Effects of short-side-chain perfluorosulfonic acid ionomers as binders on the performance of low Pt loading fuel cell cathodes. J. Power Sources 275, 384–391 (2015).
Garsany, Y. et al. Improving PEMFC performance using short-side-chain low-equivalent-weight PFSA ionomer in the cathode catalyst layer. J. Electrochem. Soc. 165, F381–F391 (2018).
Marković, N. M., Schmidt, T. J., Stamenković, V. & Ross, P. N. Oxygen reduction reaction on Pt and Pt bimetallic surfaces: a selective review. Fuel Cells 1, 105–116 (2001).
Marković, N. M., Gasteiger, H. A., Grgur, B. N. & Ross, P. N. Oxygen reduction reaction on Pt(111): effects of bromide. J. Electroanal. Chem. 467, 157–163 (1999).
Stamenkovic, V. R. et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315, 493–497 (2007).
Greeley, J. et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 1, 552–556 (2009).
Stephens, I. E. L., Bondarenko, A. S., Grønbjerg, U., Rossmeisl, J. & Chorkendorff, I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 5, 6744–6762 (2012).
Gu, Y., Xi, B., Zhang, H., Ma, Y. & Xiong, S. Activation of main-group Sb atomic sites for oxygen reduction catalysis. Angew. Chem. Int. Ed. 61, e202202200 (2022).
Luo, M. et al. PdMo bimetallene for oxygen reduction catalysis. Nature 574, 81–85 (2019).
Stamenkovic, V. R., Mun, B. S., Mayrhofer, K. J., Ross, P. N. & Markovic, N. M. Effect of surface composition on electronic structure, stability and electrocatalytic properties of Pt-transition metal alloys: Pt-skin versus Pt-skeleton surfaces. J. Am. Chem. Soc. 128, 8813–8819 (2006).
Bandarenka, A. S., Hansen, H. A., Rossmeisl, J. & Stephens, I. E. L. Elucidating the activity of stepped Pt single crystals for oxygen reduction. Phys. Chem. Chem. Phys. 16, 13625–13629 (2014).
Casalongue, H. S. et al. Direct observation of the oxygenated species during oxygen reduction on a platinum fuel cell cathode. Nat. Commun. 4, 2817 (2013).
Wakisaka, M., Suzuki, H., Mitsui, S., Uchida, H. & Watanabe, M. Identification and quantification of oxygen species adsorbed on Pt(111) single-crystal and polycrystalline Pt electrodes by photoelectron spectroscopy. Langmuir 25, 1897–1900 (2009).
Drnec, J. et al. Initial stages of Pt(111) electrooxidation: dynamic and structural studies by surface X-ray diffraction. Electrochim. Acta 224, 220–227 (2017).
Sakong, S. et al. Influence of local inhomogeneities and the electrochemical environment on the oxygen reduction reaction on Pt-based electrodes: a DFT study. J. Phys. Chem. C 124, 27604–27613 (2020).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Cheng, T. et al. Mechanism and kinetics of the electrocatalytic reaction responsible for the high cost of hydrogen fuel cells. Phys. Chem. Chem. Phys. 19, 2666–2673 (2017).
Ye, Y. F. et al. Dramatic differences in carbon dioxide adsorption and initial steps of reduction between silver and copper. Nat. Commun. 10, 1875 (2019).
Cheng, T., Xiao, H. & Goddard, W. A. Full atomistic reaction mechanism with kinetics for CO reduction on Cu(100) from ab initio molecular dynamics free-energy calculations at 298 K. Proc. Natl Acad. Sci. USA 114, 1795–1800 (2017).
Xiao, H., Cheng, T., Goddard, W. A. & Sundararaman, R. Mechanistic explanation of the pH dependence and onset potentials for hydrocarbon products from electrochemical reduction of CO on Cu (111). J. Am. Chem. Soc. 138, 483–486 (2016).
Goldsmith, Z. K., Andrade, M. F. C. & Selloni, A. Effects of applied voltage on water at a gold electrode interface from ab initio molecular dynamics. Chem. Sci. 12, 5865–5873 (2021).
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985).
Nosé, S. Constant temperature molecular dynamics methods. Prog. Theor. Phys. Suppl. 103, 1–46 (1991).
Laio, A. & Parrinello, M. Escaping free-energy minima. Proc. Natl Acad. Sci. USA 99, 12562–12566 (2002).
Acknowledgements
This work is supported by the National Key R&D Program of China (no. 2021YFA1501003). M.L. acknowledges the support of the National Natural Science Foundation of China under grant no. 22379002, and X.C. acknowledges the support of the National Natural Science Foundation of China under grant no. 22278002. We thank X. Chen from Beijing Institute of Technology for technical assistance and useful discussions.
Author information
Authors and Affiliations
Contributions
K.Z., M.L. and B.X. conceived the idea and designed the experiments. K.Z. conducted the electrochemical measurements and in situ IR experiments. Y.Z. performed molecular dynamics simulations. X.C. conducted parts of the electrochemical measurements. K.Z., M.L., Y.Z. and B.X. analysed experimental and computational data, and co-wrote the paper, with input from all other co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Chiyoung Jung, Kensaku Kodama and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–22, Tables 1–3, Notes 1–5 and refs. 1–12.
Supplementary Data 1
Atomic coordinates of calculation
Source data
Source Data Fig. 1
Adsorption and desorption of sulfonate groups on Nafion-covered Pt(111).
Source Data Fig. 2
Coupled cation-electron transfer in the sulfonate desorption.
Source Data Fig. 3
Isotopic labelling experiments.
Source Data Fig. 4
Non-Nernstian shift behaviour of the sulfonate adsorption peak.
Source Data Fig. 5
Identification of OHNafion and ONafion species.
Source Data Fig. 6
Kinetic properties of OHnormal and OHNafion.
Source Data Fig. 7
Impact of Nafion on the ORR activities.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, K., Luo, M., Zhang, Y. et al. Coupled cation–electron transfer at the Pt(111)/perfluoro-sulfonic acid ionomer interface and its impact on the oxygen reduction reaction kinetics. Nat Catal 8, 46–57 (2025). https://doi.org/10.1038/s41929-024-01279-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-024-01279-1
This article is cited by
-
Single-atom cobalt on N-doped reduced graphene oxide pushes the oxygen reduction reaction toward 4-electron pathway
npj 2D Materials and Applications (2025)
-
Boosted high-throughput D⁺ transfer from D₂O to unsaturated bonds via Pdδ+ cathode for solvent-free deuteration
Nature Communications (2025)
-
A molecular design strategy to enhance hydrogen evolution on platinum electrocatalysts
Nature Energy (2025)
-
Resolving non-covalent interactions between surface hydroxyl on Cu and interfacial water in alkaline CO electroreduction
Nature Catalysis (2025)