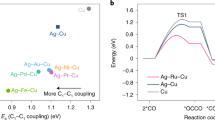
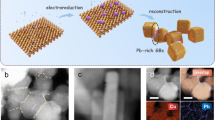
Abstract
N-propanol is an important industrial solvent but the current industrial routes for its production rely on fossil fuels and generate high carbon dioxide emissions. Replacing fossil processes with electrochemical systems powered using renewable energy offers one route to reduce the carbon intensity of n-propanol manufacture. The electrosynthesis of n-propanol via carbon monoxide electroreduction relies on the coupling of C1 and C2 intermediates, and these are preferentially stabilized on different sites. Here we pursued the synthesis of catalysts in which a high-oxygen-affinity metal (such as Sn in the best catalysts herein) is present in dilute quantities within a Cu matrix. The Sn–Cu catalyst is then formed into a catalyst/carbon/ionomer heterojunction architecture that reverses electro-osmotic drag to concentrate the n-propanol produced. We achieve n-propanol electrosynthesis from carbon monoxide with a Faradaic efficiency of 47 ± 3% and a concentration of 30 wt% at an energy efficiency of 24%. We report stable n-propanol electrosynthesis for 120 h in a membrane-electrode assembly electrolyser.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data supporting the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Leow, W. R. et al. Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science 368, 1228–1233 (2020).
Use of Energy Explained: Energy Use in Industry (US Energy Information Administration (EIA), 2019); www.eia.gov/energyexplained/use-of-energy/industry.php
Achakulwisut, P. et al. Global fossil fuel reduction pathways under different climate mitigation strategies and ambitions. Nat. Commun. 14, 5425 (2023).
Propanol Market Size, Share & Trends Analysis Report by Product by Application (N-propanol, Isopropyl Alcohol), by Region, and Segment Forecasts, 2023–2030 (Grand View Research, 2023); https://www.grandviewresearch.com/industry-analysis/propanol-market
Lepore, A. W. et al. Catalytic dehydration of biomass derived 1-propanol to propene over M-ZSM-5 (M = H, V, Cu, or Zn). Ind. Eng. Chem. Res. 56, 4302–4308 (2017).
Wang, X. et al. Efficient electrosynthesis of n-propanol from carbon monoxide using a Ag-Ru-Cu catalyst. Nat. Energy 7, 170–176 (2022).
Klabunde, J., Bischoff, C. & Papa, A. J. Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, 2018).
Motte, J. et al. Environmental performance assessment of a novel process concept for propanol production from widely available and wasted methane sources. Ind. Eng. Chem. Res. 61, 11071–11079 (2022).
Gehrmann, S. & Tenhumberg, N. Production and use of sustainable C2-C4 alcohol – an industrial perspective. Chem. Ing. Tech. 92, 1444–1458 (2020).
Nabil, S. K., McCoy, S. & Kibria, M. G. Comparative life cycle assessment of electrochemical upgrading of CO2 to fuels and feedstocks. Green Chem. 23, 867–880 (2021).
Shin, H., Hansen, K. U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 4, 911–919 (2021).
Jeong, S. et al. Facet-defined dilute metal alloy nanorods for efficient electroreduction of CO2 to n-propanol. J. Am. Chem. Soc. 146, 4508–4520 (2024).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Ozden, A. et al. Cascade CO2 electroreduction enables efficient carbonate-free production of ethylene. Joule 5, 706–719 (2021).
Jouny, M., Hutchings, G. S. & Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Nat. Catal. 2, 1062–1070 (2019).
Jin, J. et al. Constrained C2 adsorbate orientation enables CO-to-acetate electroreduction. Nature 617, 724–729 (2023).
Zhu, P. & Wang, H. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 4, 943–951 (2021).
Pang, Y. et al. Efficient electrocatalytic conversion of carbon monoxide to propanol using fragmented copper. Nat. Catal. 2, 251–258 (2019).
Niu, W. et al. Pb-rich Cu grain boundary sites for selective CO-to-n-propanol electroconversion. Nat. Commun. 14, 4882 (2023).
Pelayo Garcíade Arquer, F. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Wang, X. et al. Efficient upgrading of CO to C3 fuel using asymmetric C-C coupling active sites. Nat. Commun. 10, 5186 (2019).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Hori, Y., Takahashi, R., Yoshinami, Y. & Murata, A. Electrochemical reduction of CO at a copper electrode. J. Phys. Chem. B 101, 7075–7081 (1997).
Pablo-García, S. et al. Mechanistic routes toward C3 products in copper-catalysed CO2 electroreduction. Catal. Sci. Technol. 12, 409–417 (2022).
Vasileff, A., Xu, C., Jiao, Y., Zheng, Y. & Qiao, S.-Z. Surface and interface engineering in copper-based bimetallic materials for selective CO2 electroreduction. Chem 4, 1809–1831 (2018).
Kepp, K. P. A quantitative scale of oxophilicity and thiophilicity. Inorg. Chem. 55, 9461–9470 (2016).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Hansen, H., Shi, C., Lausche, A., Peterson, A. & Nørskov, J. K. Bifunctional alloys for the electroreduction of CO2 and CO. Phys. Chem. Chem. Phys. 18, 9194–9201 (2016).
Guo, Y., Wang, M., Zhu, Q., Xiao, D. & Ma, D. Ensemble effect for single-atom, small cluster and nanoparticle catalysts. Nat. Catal. 5, 766–776 (2022).
Hai, X. et al. Geminal-atom catalysis for cross-coupling. Nature 622, 754–760 (2023).
Millar, G. J., Rochester, C. H. & Waugh, K. C. An FTIR study of the adsorption of formic acid and formaldehyde on potassium-promoted Cu/SiO2 catalysts. J. Catal. 155, 52–58 (1995).
Katayama, Y. et al. An in situ surface-enhanced infrared absorption spectroscopy study of electrochemical CO2 reduction: selectivity dependence on surface C-bound and O-bound reaction intermediates. J. Phys. Chem. C 123, 5951–5963 (2018).
Popova, G. Y., Andrushkevich, T., Chesalov, Y. A. & Stoyanov, E. In situ FTIR study of the adsorption of formaldehyde, formic acid, and methyl formiate at the surface of TiO2 (anatase). Kinet. Catal. 41, 805–811 (2000).
Liu, Y.-Y. et al. Insight into the effect of the d-orbital energy of copper ions in metal–organic frameworks on the selectivity of electroreduction of CO2 to CH4. ACS Catal. 12, 2749–2755 (2022).
Li, X. et al. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 4, 690–699 (2019).
Yao, K. et al. Mechanistic insights into OC–COH coupling in CO2 electroreduction on fragmented copper. J. Am. Chem. Soc. 144, 14005–14011 (2022).
Li, F. et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule-metal catalyst interfaces. Nat. Catal. 3, 75–82 (2020).
Gunathunge, C. M., Ovalle, V. J., Li, Y., Janik, M. J. & Waegele, M. M. Existence of an electrochemically inert CO population on Cu electrodes in alkaline pH. ACS Catal. 8, 7507–7516 (2018).
Fielicke, A., Gruene, P., Meijer, G. & Rayner, D. M. The adsorption of CO on transition metal clusters: a case study of cluster surface chemistry. Surf. Sci. 603, 1427–1433 (2009).
An, H. et al. Sub-second time-resolved surface-enhanced Raman spectroscopy reveals dynamic CO intermediates during electrochemical CO2 reduction on copper. Angew. Chem. Int. Ed. 60, 16576–16584 (2021).
Li, H., Wei, P., Gao, D. & Wang, G. In situ Raman spectroscopy studies for electrochemical CO2 reduction over Cu catalysts. Curr. Opin. Green Sustain. Chem. 36, 100589 (2022).
Wei, P. et al. Coverage-driven selectivity switch from ethylene to acetate in high-rate CO2/CO electrolysis. Nat. Nanotechnol. 18, 299–306 (2023).
Wang, N. et al. Suppressing the liquid product crossover in electrochemical CO2 reduction. SmartMat 2, 12–16 (2021).
Miao, R. K. et al. Electroosmotic flow steers neutral products and enables concentrated ethanol electroproduction from CO2. Joule 5, 2742–2753 (2021).
Robb, A. et al. Concentrated ethanol electrosynthesis from CO2 via a porous hydrophobic adlayer. ACS Appl. Mater. Inter. 14, 4155–4162 (2022).
Li, J. et al. Copper adparticle enabled selective electrosynthesis of n-propanol. Nat. Commun. 9, 4614 (2018).
Luc, W., Rosen, J. & Jiao, F. An Ir-based anode for a practical CO2 electrolyzer. Catal. Today 288, 79–84 (2017).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558 (1993).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Blöchl, P. E., Jepsen, O. & Andersen, O. K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 49, 16223 (1994).
Grimme, S. Semiempirical GGA‐type density functional constructed with a long‐range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976).
Montoya, J. H., Shi, C., Chan, K. & Nørskov, J. K. Theoretical insights into a CO dimerization mechanismin CO2 electroreduction. J. Phys. Chem. Lett. 6, 2032–2037 (2015).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Acknowledgements
We acknowledge funding support from the Natural Sciences and Engineering Research Council (NSERC) of Canada. This research used synchrotron resources of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy, Office of Science by Argonne National Laboratory, and was supported by the US Department of Energy under contract no. DE-AC02-06CH11357 as well as by the Canadian Light Source and its funding partners. DFT calculations were performed on the Niagara supercomputer at the SciNet HPC Consortium. SciNet is funded by: the Canada Foundation for Innovation; the Government of Ontario; the Ontario Research Fund—Research Excellence and the University of Toronto. X.W. acknowledges the Zhejiang University Excellent Doctoral Dissertation Funding. W.N. acknowledges financial support from the Swiss National Science Foundation (SNSF) Postdoctoral Mobility Fellowship (grant no. P500PN_202906).
Author information
Authors and Affiliations
Contributions
E.H.S. and D.S. supervised the project. Y.C. conceived the idea, designed and conducted the experiments, and wrote the paper. X.W. carried out the experiments and contributed to data analysis and paper writing. X.-Y.L. performed the DFT calculations. R.K.M. performed experiments for the CCIH catalyst system, TEA and LCA. J.D. contributed to XAS data analysis. Z.Z. and C.L. contributed to in situ ATR-SEIRAS measurements. S.C. contributed to material characterizations. J.E.H. contributed to in situ Raman measurements. J.W. contributed to TEA. W.N. contributed to XAS measurements. P.O. assisted with DFT calculations. Z.G. and Y.X. contributed to CCIH catalyst system. B.X. and Y.H. contributed to data analysis and discussions and paper preparation. All authors discussed the results and assisted with paper preparation.
Corresponding authors
Ethics declarations
Competing interests
There is a US provisional patent application (63/584,253) titled ‘Processes and systems for the electrochemical reduction of carbon monoxide to propanol, cathode catalysts and cathodes used in the same’ filed by the authors Y.C., X.W., R.K.M., D.S. and E.H.S. and their institutions. The other authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Guiyan Zang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–5, Figs. 1–55 and Tables 1–10.
Supplementary Data 1
The atomic coordinates of the optimized models.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Wang, X., Li, XY. et al. Electrified synthesis of n-propanol using a dilute alloy catalyst. Nat Catal 8, 239–247 (2025). https://doi.org/10.1038/s41929-025-01301-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01301-0