Abstract
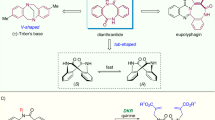
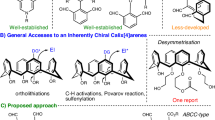
Chiral macrocycles play critical roles across medicinal chemistry and materials science, yet their catalytic asymmetric synthesis remains challenging. Existing methods predominantly rely on intramolecular cyclization of linear precursors and asymmetric resolution of racemic macrocycles, often requiring complex synthesis while offering limited structural diversity. Here, inspired by non-ribosomal cyclopeptide biosynthesis, we present a catalytic metallic dipole relay strategy for the construction of axially chiral macrolactones. This approach enables concise enantioselective synthesis through stepwise strain release in biaryl lactones and dynamic kinetic resolution mediated by π-allyl-Pd dipoles. The method demonstrates broad applicability to medium (up to 91% yield with 93% enantiomeric excess) and large (up to 93% yield with 99% enantiomeric excess and >19:1 diastereomeric ratio) ring systems under mild conditions. By establishing stereochemical control during both medium-ring formation and subsequent macrocyclization, this strategy overcomes traditional limitations in the generation of axial chirality while extending the methodology of transition-metal-catalysed asymmetric cyclization.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings in this work are available within this article and its Supplementary Information or from the authors upon reasonable request. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2271260 (3a), 2271264 (4a), 2325057 (6a), 2325060 (8a) and 2362166 (11g). Copies of the data can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures/.
References
Driggers, E. M., Hale, S. P., Lee, J. & Terrett, N. K. The exploration of macrocycles for drug discovery—an underexploited structural class. Nat. Rev. Drug Discov. 7, 608–624 (2008).
Seiple, I. B. et al. A platform for the discovery of new macrolide antibiotics. Nature 533, 338–345 (2016).
Morimoto, M. et al. Advances in supramolecular host-mediated reactivity. Nat. Catal. 3, 969–984 (2020).
Wu, Q. et al. Poly[n]catenanes: synthesis of molecular interlocked chains. Science 358, 1434–1439 (2017).
Levine, D. P. Vancomycin: a history. Clin. Infect. Dis. 42, 5–12 (2006).
Li, Y. et al. Iron catalyzed asymmetric hydrogenation of ketones. J. Am. Chem. Soc. 136, 4031–4039 (2014).
Mortensen, K. T., Osberger, T. J., King, T. A., Sore, H. F. & Spring, D. R. Strategies for the diversity-oriented synthesis of macrocycles. Chem. Rev. 119, 10288–10317 (2019).
Lv, X.-K. et al. Carbene organic catalytic planar enantioselective macrolactonization. Nat. Commun. 15, 958 (2024).
Yang, G., He, Y., Wang, T., Li, Z. & Wang, J. Atroposelective synthesis of planar-chiral indoles via carbene catalyzed macrocyclization. Angew. Chem. Int. Ed. 63, e202316739 (2024).
Yu, S.-Z., Shen, G.-S., He, F.-Q. & Yang, X.-Y. Asymmetric synthesis of planar-chiral macrocycles via organocatalyzed enantioselective macrocyclization. Chem. Commun. 58, 7293–7296 (2022).
Ding, Q., Wang, Q.-Y., He, H. & Cai, Q. Asymmetric synthesis of (−)-pterocarine and (−)-galeon via chiral phase transfer-catalyzed atropselective formation of diarylether cyclophane skeleton. Org. Lett. 19, 1804–1807 (2017).
Tong, S. et al. Catalytic enantioselective synthesis and switchable chiroptical property of inherently chiral macrocycles. J. Am. Chem. Soc. 142, 14432–14436 (2020).
Araki, T., Noguchi, K. & Tanaka, K. Enantioselective synthesis of planar-chiral carba-paracyclophanes: rhodium-catalyzed [2+2+2] cycloaddition of cyclic diynes with terminal monoynes. Angew. Chem. Int. Ed. 52, 5617–5621 (2013).
Kanda, K., Koike, T., Endo, K. & Shibata, T. The first asymmetric Sonogashira coupling for the enantioselective generation of planar chirality in paracyclophanes. Chem. Commun. 2009, 1870–1872 (2009).
Wang, D., Shao, Y.-B., Chen, Y., Xue, X.-S. & Yang, X. Enantioselective synthesis of planar-chiral macrocycles through asymmetric electrophilic aromatic amination. Angew. Chem. Int. Ed. 61, e202201064 (2022).
Dong, Z., Li, J., Yao, T. & Zhao, C. Palladium-catalyzed enantioselective C–H olefination to access planar-chiral cyclophanes by dynamic kinetic resolution. Angew. Chem. Int. Ed. 62, e202315603 (2023).
Gagnon, C. et al. Biocatalytic synthesis of planar chiral macrocycles. Science 367, 917–921 (2020).
Zou, S., Yu, B. & Huang, H. Enantioselective ring-closing aminomethylamination of aminodienes enabled by modified Trost ligands. Chem. Catal. 2, 2034–2048 (2022).
Xiong, Q., Xiao, L., Dong, X.-Q. & Wang, C.-J. Asymmetric synthesis of chiral aza-macrodiolides via iridium-catalyzed cascade allylation/macrolactonization. Org. Lett. 24, 2579–2584 (2022).
López, R. & Palomo, C. Planar chirality: a mine for catalysis and structure discovery. Angew. Chem. Int. Ed. 61, e202113504 (2022).
Marsault, E. & Peterson, M. L. Macrocycles are great cycles: applications, opportunities and challenges of synthetic macrocycles in drug discovery. J. Med. Chem. 54, 1961–2004 (2011).
Iyoda, M., Yamakawa, J. & Rahman, M. J. Conjugated macrocycles: concepts and applications. Angew. Chem. Int. Ed. 50, 10522–10553 (2011).
Kopp, F. & Marahiel, M. A. Macrocyclization strategies in polyketide and nonribosomal peptide biosynthesis. Nat. Prod. Rep. 24, 735–749 (2007).
Xu, X. & Doyle, M. P. The [3+3]-cycloaddition alternative for heterocycle syntheses: catalytically generated metalloenolcarbenes as dipolar adducts. Acc. Chem. Res. 47, 1396–1405 (2014).
Zhang, M.-M., Qu, B.-L., Shi, B., Xiao, W.-J. & Lu, L.-Q. High-order dipolar annulations with metal-containing reactive dipoles. Chem. Soc. Rev. 51, 4146–4174 (2022).
Zhang, J. et al. Transition metal-catalyzed asymmetric cyclizations involving allyl or propargyl heteroatom-dipole precursors. Chin. J. Org. Chem. 42, 3051–3101 (2022).
Trost, B. M., Huang, Z. & Murhade, G. M. Catalytic palladium-oxyallyl cycloaddition. Science 362, 564–568 (2018).
Yang, L.-C. et al. Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions. Nat. Chem. 12, 860–868 (2020).
Ohmatsu, K., Imagawa, N. & Ooi, T. Ligand-enabled multiple absolute stereocontrol in metal-catalysed cycloaddition for construction of contiguous all-carbon quaternary stereocentres. Nat. Chem. 6, 47–51 (2014).
Cheng, Q., Zhang, H.-J., Yue, W.-J. & You, S.-L. Palladium-catalyzed highly stereoselective dearomative [3+2] cycloaddition of nitrobenzofurans. Chem 3, 428–436 (2017).
Zheng, Y., Qin, T. & Zi, W.-W. Enantioselective inverse electron demand (3+2) cycloaddition of palladium-oxyallyl enabled by a hydrogen-bond-donating ligand. J. Am. Chem. Soc. 143, 1038–1045 (2021).
Peng, Y., Huo, X., Luo, Y., Wu, L. & Gong, L.-Z. Enantio- and diastereodivergent synthesis of spirocycles through dual-metal-catalyzed [3+2] annulation of 2-vinyloxiranes with nucleophilic dipoles. Angew. Chem. Int. Ed. 60, 24941–24949 (2021).
Ding, W.-W., Zhou, Y., Han, Z.-Y. & Gong, L.-Z. Asymmetric cascade carbonylation/annulation of benzyl bromides, CO, and vinyl benzoxazinanones enabled by Pd/chiral Lewis-base relay catalysis. J. Org. Chem. 88, 5187–5193 (2023).
Yu, C., Huang, H., Li, X., Zhang, Y. & Wang, W. Dynamic kinetic resolution of biaryl lactones via a chiral bifunctional amine thiourea-catalyzed highly atropo-enantioselective transesterification. J. Am. Chem. Soc. 138, 6956–6959 (2016).
Beleh, O. M., Miller, E., Toste, F. D. & Miller, S. J. Catalytic dynamic kinetic resolutions in tandem to construct two-axis terphenyl atropisomers. J. Am. Chem. Soc. 142, 16461–16470 (2020).
Ruan, L.-X., Sun, B., Liu, J.-M. & Shi, S.-L. Dynamic kinetic asymmetric arylation and alkenylation of ketones. Science 379, 662–670 (2023).
Reyes, R. L., Iwai, T. & Sawamura, M. Construction of medium-sized rings by gold catalysis. Chem. Rev. 121, 8926–8947 (2021).
Cheng, J. K., Xiang, S.-H., Li, S., Ye, L. & Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 121, 4805–4902 (2021).
Carmona, J. A., Rodríguez-Franco, C., Fernández, R., Hornillos, V. & Lassaletta, J. M. Atroposelective transformation of axially chiral (hetero)biaryls. From desymmetrization to modern resolution strategies. Chem. Soc. Rev. 50, 2968–2983 (2021).
Xiao, Y.-Q. et al. Taming chiral quaternary stereocenters via remote H-bonding stereoinduction in palladium-catalyzed (3+2) cycloadditions. Angew. Chem. Int. Ed. 62, e202212444 (2023).
Wang, B.-C. et al. Synthesis of S(IV)-stereogenic chiral thio-oxazolidinones via palladium-catalyzed asymmetric [3+2] annulations. Angew. Chem. Int. Ed. 63, e202319728 (2024).
Trost, B. M., Machacek, M. R. & Aponick, A. Predicting the stereochemistry of diphenylphosphino benzoic acid (DPPBA)-based palladium-catalyzed asymmetric allylic alkylation reactions: a working model. Acc. Chem. Res. 39, 747–760 (2006).
Zhang, Y.-C., Zhang, X. & Ma, S.-M. Stretchable chiral pockets for palladium-catalyzed highly chemo- and enantioselective allenylation. Nat. Commun. 12, 2416 (2021).
Yang, L.-C. et al. Construction of nine-membered heterocycles through palladium-catalyzed formal [5+4] cycloaddition. Angew. Chem. Int. Ed. 56, 2927–2931 (2017).
Li, M.-M. et al. Sequential visible-light photoactivation and palladium catalysis enabling enantioselective [4+2] cycloadditions. J. Am. Chem. Soc. 139, 14707–14713 (2017).
Acknowledgements
This work was supported by the National Key R&D Program of China (2023YFA1507203 to W.-J.X. and L.-Q.L., and 2022YFA1506100 to Z.Z.), the National Natural Science Foundation of China (22471089 to L.-Q.L., 22271113 to L.-Q.L., 22203034 to Z.Z. and 92256301 to W.-J.X.) and Founding from Central China Normal University and Wuhan Institute of Photochemistry and Technology (L.-Q.L. and W.-J.X.). We thank F.-F. Pan (CCNU) for X-ray crystallographic analysis assistance. We acknowledge W.-B. Liu in Wuhan University for helpful discussions.
Author information
Authors and Affiliations
Contributions
B.-L.Q. and L.-Q.L. conceived the work, B.-L.Q., L.H. and J.-W.S. designed and conducted the experiments, M.X. performed the DFT calculation under the supervision of Z.Z. W.-J.X. and L.-Q.L. supervised and directed the research, and all authors wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Rajarshi Samanta, Jian Wang, Yong Wang and Xiaoyu Yang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, discussion, references, Tables 1–9 and Figs. 1–14.
Supplementary Data 1
Crystallographic data for compound 3a.
Supplementary Data 2
Crystallographic data for compound 4a.
Supplementary Data 3
Crystallographic data for compound 6a.
Supplementary Data 4
Crystallographic data for compound 8a.
Supplementary Data 5
Crystallographic data for compound 11g.
Supplementary Data 6
Checkcif file for compound 3a.
Supplementary Data 7
Checkcif file for compound 4a.
Supplementary Data 8
Checkcif file for compound 6a.
Supplementary Data 9
Checkcif file for compound 8a.
Supplementary Data 10
Checkcif file for compound 11g.
Supplementary Data 11
The atomic coordinates of optimized structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qu, BL., Xiao, M., He, L. et al. Enantioselective macrocyclization via catalytic metallic dipole relay. Nat Catal 8, 368–377 (2025). https://doi.org/10.1038/s41929-025-01322-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01322-9