Abstract
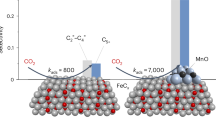
Alkane dehydrogenation as a direct route to produce olefins receives widespread attention from industry and academia. However, high temperatures (>550 °C) are often needed to break C–H bonds, leading to deleterious side reactions in the alkane dehydrogenation process. Here we reduce the reaction temperature of n-butane dehydrogenation by fabricating a robust and regenerable Ir1–Cu1 dual-atom catalyst. The so-prepared system shows a turnover frequency of 2.45 s−1 at 450 °C, which is 6.3 times higher than the single-atom Ir1/ND@G catalyst, while, at he same time, achieving a high C4 olefin selectivity of 98%. Importantly, key for the success of the Ir1–Cu1 dual-atom catalyst are the sterically favourable geometric configuration and the modulated electronic property, which can lower the reaction barrier for C–H activation, shift the rate-determining step and facilitate the desorption of the product. Thus, a remarkable activity can be achieved for n-butane dehydrogenation at relatively low temperature (≤450 °C).

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. The optimized computational models and MD trajectories are available in the Supplementary Data. Source data are provided with this paper.
References
Sattler, J. J., Ruiz-Martinez, J., Santillan-Jimenez, E. & Weckhuysen, B. M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 114, 10613–10653 (2014).
Chen, S. et al. Propane dehydrogenation: catalyst development, new chemistry, and emerging technologies. Chem. Soc. Rev. 50, 3315–3354 (2021).
Otroshchenko, T., Jiang, G., Kondratenko, V. A., Rodemerck, U. & Kondratenko, E. V. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 50, 473–527 (2021).
Zhao, Z.-J., Chiu, C.-c & Gong, J. Molecular understandings on the activation of light hydrocarbons over heterogeneous catalysts. Chem. Sci. 6, 4403–4425 (2015).
Chang, X., Lu, Z., Wang, X., Zhao, Z. J. & Gong, J. Tracking C–H bond activation for propane dehydrogenation over transition metal catalysts: work function shines. Chem. Sci. 14, 6414–6419 (2023).
Liu, L. et al. Regioselective generation and reactivity control of subnanometric platinum clusters in zeolites for high-temperature catalysis. Nat. Mater. 18, 866–873 (2019).
Liu, L. et al. Structural modulation and direct measurement of subnanometric bimetallic PtSn clusters confined in zeolites. Nat. Catal. 3, 628–638 (2020).
Ryoo, R. et al. Rare-earth-platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 585, 221–224 (2020).
Motagamwala, A. H., Almallahi, R., Wortman, J., Igenegbai, V. O. & Linic, S. Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science 373, 217 (2021).
Peng, M. et al. Fully exposed cluster catalyst (FECC): toward rich surface sites and full atom utilization efficiency. ACS Cent. Sci. 7, 262–273 (2021).
Chen, X. et al. Structure-dependence and metal-dependence on atomically dispersed Ir catalysts for efficient n-butane dehydrogenation. Nat. Commun. 14, 2588 (2023).
Chen, X. et al. Regulating coordination number in atomically dispersed Pt species on defect-rich graphene for n-butane dehydrogenation reaction. Nat. Commun. 12, 2664 (2021).
Peng, M. et al. Antisintering Pd1 catalyst for propane direct dehydrogenation with in situ active sites regeneration ability. ACS Catal. 12, 2244–2252 (2022).
Yang, Z. et al. Coking-resistant iron catalyst in ethane dehydrogenation achieved through siliceous zeolite modulation. J. Am. Chem. Soc. 142, 16429–16436 (2020).
Ma, R. et al. Insights into the nature of selective nickel sites on Ni/Al2O3 catalysts for propane dehydrogenation. ACS Catal. 12, 12607–12616 (2022).
Sharma, L. et al. Atomically dispersed tin-modified gamma-alumina for selective propane dehydrogenation under H2S co-feed. ACS Catal. 11, 13472–13482 (2021).
Wang, W. et al. Single Co sites in ordered SiO2 channels for boosting nonoxidative propane dehydrogenation. ACS Catal. 12, 2632–2638 (2022).
Deng, Y. et al. Few-atom Pt ensembles enable efficient catalytic cyclohexane dehydrogenation for hydrogen production. J. Am. Chem. Soc. 144, 3535–3542 (2022). 8.
Chen, X., Jia, Z., Huang, F., Diao, J. & Liu, H. Atomically dispersed metal catalysts on nanodiamond and its derivatives: synthesis and catalytic application. Chem. Commun. 57, 11591–11603 (2021).
Cai, X. et al. Towards a library of atomically dispersed catalysts. Mater. Des. 210, 110080 (2021).
Dong, C. et al. Fully exposed palladium cluster catalysts enable hydrogen production from nitrogen heterocycles. Nat. Catal. 5, 485–493 (2022).
Liu, P., Huang, X., Mance, D. & Copéret, C. Atomically dispersed iridium on MgO(111) nanosheets catalyses benzene–ethylene coupling towards styrene. Nat. Catal. 4, 968–975 (2021).
Shao, X. et al. Iridium single-atom catalyst performing a quasi-homogeneous hydrogenation transformation of CO2 to formate. Chem 5, 693–705 (2019).
Ghijsen, J. et al. Electronic-structure of Cu2O and CuO. Phys. Rev. B 38, 11322–11330 (1988).
Ertl, G., Hierl, R., Knözinger, H., Thiele, N. & Urbach, H. P. XPS study of copper aluminate catalysts. Appl. Surf. Sci. 5, 49–64 (1980).
Robert, T., Bartel, M. & Offergeld, G. Characterization of oxygen species adsorbed on copper and nickel oxides by X-ray photoelectron spectroscopy. Surf. Sci. 33, 123–130 (1972).
Atanasoska, L., Atanasoski, R. & Trasatti, S. XPS and AES study of mixed layers of RuO2 and IrO2. Vacuum 40, 91–94 (1990).
Duckers, K. & Bonzel, H. P. Core and valence level spectroscopy with Y-M-Zeta radiation—CO and K on (110) surfaces of Ir, Pt and Au. Surf. Sci. 213, 25–48 (1989).
Lee, B.-H. et al. Reversible and cooperative photoactivation of single-atom Cu/TiO2 photocatalysts. Nat. Mater. 18, 620–626 (2019).
Tang, X. et al. Direct oxidation of methane to oxygenates on supported single Cu atom catalyst. Appl. Catal. B 285, 119827 (2021).
Fan, C. et al. The influence of Si/Al ratio on the catalytic property and hydrothermal stability of Cu-SSZ-13 catalysts for NH3-SCR. Appl. Catal. A Gen. 550, 256–265 (2018).
Lian, Z., Si, C., Jan, F., Zhi, S. & Li, B. Coke deposition on Pt-based catalysts in propane direct dehydrogenation: kinetics, suppression, and elimination. ACS Catal. 11, 9279–9292 (2021).
Jin, R. X. et al. Low temperature oxidation of ethane to oxygenates by oxygen over iridium-cluster catalysts. J. Am. Chem. Soc. 141, 18921–18925 (2019).
Hu, Q. et al. Facile synthesis of sub-nanometric copper clusters by double confinement enables selective reduction of carbon dioxide to methane. Angew. Chem. Int. Ed. 59, 19054–19059 (2020).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Perdew, J. P. & Wang, Y. Accurate and simple analytic representation of the electron–gas correlation energy. Phys. Rev. B 45, 13244–13249 (1992).
Methfessel, M. & Paxton, A. T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 40, 3616–3621 (1989).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Jonsson, H., Mills, G. & Jacobsen, K. W. in Classical and Quantum Dynamics in Condensed Phase Simulations (eds Berne, B. J. et al.) 385–404 (World Scientific, 1998).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Acknowledgements
This work was supported by the National Key R&D Program of China (grant numbers 2022YFA1504500, 2022YFB4003100 and 2021YFA1502802), the National Natural Science Foundation of China (grant numbers 92145301, U21B2092, 22232001, 21961160722, 91845201 and 22072162), China Postdoctoral Science Foundation (grant number 2024M763338), Chinese Academy of Sciences (grant number 172GJHZ2022028MI), Shenyang Young Talents Program (grant number RC210435), Dalian National Lab for Clean Energy (DNL Cooperation Fund 202001) and China Petroleum & Chemical Corporation (grant number 420043-2). The XAS experiments were conducted in Beijing Synchrotron Radiation Facility (BSRF). D.M. acknowledges support from the Tencent Foundation through the XPLORER PRIZE and New Cornerstone Investigator Program. X. Cai acknowledges the support from the NTU Presidential Postdoctoral Fellowship (grant number 03INS001828C230).
Author information
Authors and Affiliations
Contributions
H.L. and D.M. conceived the research. X. Chen conducted material synthesis and carried out the catalytic performance test. M.W. conducted the X-ray absorption fine structure spectroscopic measurements and analysed the data. Y.H. performed the DFT calculations. X. Cai contributed to the aberration-corrected high-angle annular dark-field scanning transmission electron microscopy. M.W. and P.M. conducted the X-ray photoelectron spectroscopy measurements. J.D. performed some of the synthesis experiments. The paper was primarily written by X. Chen, D.X., H.L. and D.M. All authors contributed to discussions and paper review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Sonia Bocanegra and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28, Notes 1–3, Tables 1–24 and References.
Supplementary Data
The optimized computational models and MD trajectories.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Wang, M., He, Y. et al. A highly efficient and regenerable Ir1–Cu1 dual-atom catalyst for low-temperature alkane dehydrogenation. Nat Catal 8, 436–447 (2025). https://doi.org/10.1038/s41929-025-01328-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01328-3