Abstract
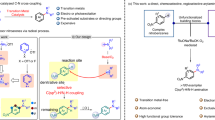
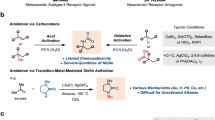
Tertiary nitroalkanes, as well as their reduced products, α-tertiary amines, play an essential role in drug discovery as either key synthetic precursors or final motifs in targeted molecules. Existing methods to prepare tertiary nitro compounds generally rely on polar-bond disconnections, in which strong bases or highly active electrophiles are needed. Here we report the development of an anomeric nitroamide-based reagent that enables selective metal-hydride hydrogen atom transfer-based Co-catalysed alkene hydronitration for the preparation of valuable tertiary nitro compounds. This mild, scalable reaction shows broad functional group tolerance. Its synthetic application is demonstrated via late-stage nitration of complex alkenes derived from drugs and natural products, and simplifying the synthesis of a rare naturally occurring nitro sugar. Simple access to isotopically labelled 15N-containing nitro compounds is also disclosed. The anomeric nitroamide reagent was deemed safe by energetic measurements and its reactivity rationalized based on X-ray crystallographic analysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under the deposition numbers CCDC 2385494 (16), 2329539 (27), 2329540 (35A), 2383725 (35B), 2380454 (36), 2373356 (39), 2376555 (40), 2376557 (41), 2377801 (42), 2377800 (43), 2375144 (48), 2376556 (50), 2373359 (51), 2383726 (56), 2377632 (58), 2375142 (60), 2382868 (62), 2380180 (63) and 2382867 (67). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Ballini, R. & Palmieri, A. Nitroalkanes: Synthesis, Reactivity, and Applications (Wiley, 2021).
Qiu, H. et al. Enantioselective synthesis and biological evaluation of pyrrolidines bearing quaternary stereogenic centers. J. Med. Chem. 66, 9866–9880 (2023).
Mergelsberg, I. et al. Process and intermediates for the synthesis of 8-[{1-(3,5-bis(thrifluoromethyl)phenyl)ethoxy}methyl]-8-phenyl-1,7-diazadpiro[4,5]decan-2-one compounds. Patent WO2008118328A2 (2008).
Ganguly, A. K., Sarre, O. Z. & Reimann, H. Chemistry of everninomicin antibiotics. III. Evernitrose, a naturally occurring nitro sugar from everninomicins. J. Am. Chem. Soc. 90, 7129–7130 (1968).
Liu, Y. et al. Lycodine-type alkaloids from Lycopodiastrum casuarinoides and their cholinesterase inhibitory activities. Fitoterapia 130, 203–209 (2018).
Ballini, R. & Palmieri, A. Synthetic procedures for the preparation of nitroalkanes. Adv. Synth. Catal. 360, 2240–2266 (2018).
Trost, B. M. & Surivet, J.-P. Asymmetric alkylation of nitroalkanes. Angew. Chem. Int. Ed. 39, 3122–3124 (2000).
Kim, R. S., Dinh-Nguyen, L. V., Shimkin, K. W. & Watson, D. A. Copper-catalyzed propargylation of nitroalkanes. Org. Lett. 22, 8106–8110 (2020).
Rezazadeh, S. et al. Photoredox-nickel dual-catalyzed C-alkylation of secondary nitroalkanes: access to sterically hindered α-tertiary amines. J. Am. Chem. Soc. 145, 4707–4715 (2023).
Fu, H., Qiao, T., Carceller, J. M., MacMillan, S. N. & Hyster, T. K. Asymmetric C-alkylation of nitroalkanes via enzymatic photoredox catalysis. J. Am. Chem. Soc. 145, 787–793 (2023).
Gilbert, K. E. & Borden, W. T. Peracid oxidation of aliphatic amines: general synthesis of nitroalkanes. J. Org. Chem. 44, 659–661 (1979).
Lajunen, M. & Hiukka, R. Acid-catalyzed hydrolysis of bridged bi- and tricyclic compounds. 25. Comparison of the hydrations of 2-methyl-2-norbornene and 2-methylenenorbornane with those of 1-methylcyclohexene and methylenecyclohexane. J. Org. Chem. 51, 1522–1525 (1986).
Isayama, S. & Mukaiyama, T. A new method for preparation of alcohols from olefins with molecular oxygen and phenylsilane by the use of bis(acetylacetonato)cobalt(II). Chem. Lett. 18, 1071–1074 (2006).
Gaspar, B. & Carreira, E. M. Catalytic hydrochlorination of unactivated olefins with para-toluenesulfonyl chloride. Angew. Chem. Int. Ed. 47, 5758–5760 (2008).
Barker, T. J. & Boger, D. L. Fe(III)/NaBH4-mediated free radical hydrofluorination of unactivated alkenes. J. Am. Chem. Soc. 134, 13588–13591 (2012).
Ma, X. & Herzon, S. B. Non-classical selectivities in the reduction of alkenes by cobalt-mediated hydrogen atom transfer. Chem. Sci. 6, 6250–6255 (2015).
Elfert, J., Frye, N. L., Rempel, I., Daniliuc, C. G. & Studer, A. Iron-catalyzed radical Markovnikov hydrohalogenation and hydroazidation of alkenes. Nat. Commun. 15, 7230 (2024).
Waser, J., Nambu, H. & Carreira, E. M. Cobalt-catalyzed hydroazidation of olefins: convenient access to alkyl azides. J. Am. Chem. Soc. 127, 8294–8295 (2005).
Qin, T. et al. Cobalt-catalyzed radical hydroamination of alkenes with N-fluorobenzenesulfonimides. Angew. Chem. Int. Ed. 60, 25949–25957 (2021).
Miao, H., Guan, M., Xiong, T., Zhang, G. & Zhang, Q. Cobalt-catalyzed enantioselective hydroamination of arylalkenes with secondary amines. Angew. Chem. Int. Ed. 62, e202213913 (2023).
Gaspar, B. & Carreira, E. M. Mild cobalt-catalyzed hydrocyanation of olefins with tosyl cyanide. Angew. Chem. Int. Ed. 46, 4519–4522 (2007).
Crossley, S. W. M., Obradors, C., Martinez, R. M. & Shenvi, R. A. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. Chem. Rev. 116, 8912–9000 (2016).
Tilney-Bassett, J. F. & Waters, W. A. 610. Properties and reactions of free alkyl radicals in solution. Part IX. Synthesis and reactions of some tertiary nitroalkanes. J. Chem. Soc. https://doi.org/10.1039/JR9570003129 (1957).
Nishiwaki, Y., Sakaguchi, S. & Ishii, Y. An efficient nitration of light alkanes and the alkyl side-chain of aromatic compounds with nitrogen dioxide and nitric acid catalyzed by N-hydroxyphthalimide. J. Org. Chem. 67, 5663–5668 (2002).
Chatterjee, J., Coombes, R. G., Barnes, J. R. & Fildes, M. J. Mechanism of reaction of nitrogen dioxide with alkenes in solution. J. Chem. Soc., Perkin Trans. 2 https://doi.org/10.1039/P29950001031 (1995).
Lewis, R. J. & Moodie, R. B. The nitration of styrenes by nitric acid in dichloromethane. J. Chem. Soc., Perkin Trans. 2 https://doi.org/10.1039/A605782I (1997).
Gui, J. et al. Practical olefin hydroamination with nitroarenes. Science 348, 886–891 (2015).
Bhunia, A., Bergander, K., Daniliuc, C. G. & Studer, A. Fe-catalyzed anaerobic Mukaiyama-type hydration of alkenes using nitroarenes. Angew. Chem. Int. Ed. 60, 8313–8320 (2021).
Patra, S., Mosiagin, I., Giri, R. & Katayev, D. Organic nitrating reagents. Synthesis 54, 3432–3472 (2022).
Calvo, R., Zhang, K., Passera, A. & Katayev, D. Facile access to nitroarenes and nitroheteroarenes using N-nitrosaccharin. Nat. Commun. 10, 3410 (2019).
Jia, F., Li, A. & Hu, X. Dinitro-5,5-dimethylhydantoin: an arene nitration reagent. Org. Lett. 25, 4605–4609 (2023).
Zhang, K., Jelier, B., Passera, A., Jeschke, G. & Katayev, D. Synthetic diversity from a versatile and radical nitrating reagent. Chem. Eur. J. 25, 12929–12939 (2019).
Arnatt, C. K. & Zhang, Y. Facile synthesis of 2,3,5,6-tetrabromo-4-methyl-nitrocyclohexa-2,5-dien-1-one, a mild nitration reagent. Tetrahedron Lett. 53, 1592–1594 (2012).
Wang, Y. et al. Discovery of N–X anomeric amides as electrophilic halogenation reagents. Nat. Chem. 16, 1539–1545 (2024).
Glover, S. A. Anomeric amides—structure, properties and reactivity. Tetrahedron 54, 7229–7271 (1998).
Xu, X., Wang, M., Peng, L. & Guo, C. Nickel-catalyzed asymmetric propargylation for the synthesis of axially chiral 1,3-disubstituted allenes. J. Am. Chem. Soc. 144, 21022–21029 (2022).
Mimura, M. et al. Synthesis and evaluation of (piperidinomethylene)bis(phosphonic acid) derivatives as anti-osteoporosis agents. Chem. Pharm. Bull. 41, 1971–1986 (1993).
von Philipsborn, W. & Müller, D.-C. R. 15N-NMR spectroscopy—new methods and applications. Angew. Chem. Int. Ed. 25, 383–413 (1986).
Benson, L. M., Naylor, S. & Tomlinson, A. J. Investigation of Maillard reaction products using 15N isotope studies and analysis by electrospray ionization–mass spectrometry. Food Chem. 62, 179–183 (1998).
von Morze, C. et al. 15N-carnitine, a novel endogenous hyperpolarized MRI probe with long signal lifetime. Magn. Reson. Med. 85, 1814–1820 (2021).
Xia, W. et al. Advances of stable isotope technology in food safety analysis and nutrient metabolism research. Food Chem. 408, 135191 (2023).
Lloyd-Jones, G. C. & Muñoz, M. P. Isotopic labelling in the study of organic and organometallic mechanism and structure: an account. J. Label. Compd. Radiopharm. 50, 1072–1087 (2007).
Dayie, K. T. Key labeling technologies to tackle sizeable problems in RNA structural biology. Int. J. Mol. Sci. 9, 1214–1240 (2008).
Mariotti, A., Landreau, A. & Simon, B. 15N isotope biogeochemistry and natural denitrification process in groundwater: application to the chalk aquifer of northern France. Geochim. Cosmochim. Acta 52, 1869–1878 (1988).
May, D. S. et al. 15N stable isotope labeling and comparative metabolomics facilitates genome mining in cultured cyanobacteria. ACS Chem. Biol. 15, 758–765 (2020).
Finkelstein, P. et al. Nitrogen atom insertion into indenes to access isoquinolines. Chem. Sci. 14, 2954–2959 (2023).
Bartholomew, G. L. et al. 14N to 15N isotopic exchange of nitrogen heteroaromatics through skeletal editing. J. Am. Chem. Soc. 146, 2950–2958 (2024).
Xing, Q., Chandrachud, P. P., Tillett, K. & Lopchuk, J. M. Regioselective hydroamination of unactivated olefins with diazirines as a diversifiable nitrogen source. Nat. Commun. 15, 6049 (2024).
Miller, M. et al. 3-Pyridinylindazole compounds as inhibitors of leucine-rich repeat kinase enzyme activity and their preparation. Patent WO2014137728 (2014).
Ganguly, A. K. & Szmulewicz, S. Structure of everninomicin C. J. Antibiot. 28, 710–712 (1975).
Clode, D. M., Horton, D. & Weckerle, W. Reaction of derivatives of methyl 2,3-O-benzylidene-6-deoxy-α-l-mannopyranoside with butyllithium: synthesis of methyl 2, 6-dideoxy-4-O-methyl-α-l-erytho-hexopyranosid-3-ulose. Carbohydr. Res. 49, 305–314 (1976).
Yoshimura, J., Matsuzawa, M., Sato, K.-i & Nagasawa, Y. The synthesis of evernitrose and 3-epi-evernitrose. Carbohydr. Res. 76, 67–78 (1979).
Touney, E. E., Foy, N. J. & Pronin, S. V. Catalytic radical–polar crossover reactions of allylic alcohols. J. Am. Chem. Soc. 140, 16982–16987 (2018).
Zhou, X.-L. et al. Cobalt-catalyzed intermolecular hydrofunctionalization of alkenes: evidence for a bimetallic pathway. J. Am. Chem. Soc. 141, 7250–7255 (2019).
Wilson, C. V. & Holland, P. L. Mechanism of alkene hydrofunctionalization by oxidative cobalt(salen) catalyzed hydrogen atom transfer. J. Am. Chem. Soc. 146, 2685–2700 (2024).
Shigehisa, H., Aoki, T., Yamaguchi, S., Shimizu, N. & Hiroya, K. Hydroalkoxylation of unactivated olefins with carbon radicals and carbocation species as key intermediates. J. Am. Chem. Soc. 135, 10306–10309 (2013).
Nakagawa, M., Matsuki, Y., Nagao, K. & Ohmiya, H. A triple photoredox/cobalt/brønsted acid catalysis enabling Markovnikov hydroalkoxylation of unactivated alkenes. J. Am. Chem. Soc. 144, 7953–7959 (2022).
Shigehisa, H. et al. Catalytic synthesis of saturated oxygen heterocycles by hydrofunctionalization of unactivated olefins: unprotected and protected strategies. J. Am. Chem. Soc. 138, 10597–10604 (2016).
Zhang, B. et al. Cobalt-catalyzed Markovnikov-selective radical hydroacylation of unactivated alkenes with acylphosphonates. J. Am. Chem. Soc. 143, 4955–4961 (2021).
Wu, J. & Ma, Z. Metal-hydride hydrogen atom transfer (MHAT) reactions in natural product synthesis. Org. Chem. Front. 8, 7050–7076 (2021).
Sperry, J. B. et al. Thermal stability assessment of peptide coupling reagents commonly used in pharmaceutical manufacturing. Org. Process Res. Dev. 22, 1262–1275 (2018).
Stein, C. et al. Anomeric amide-enabled alkene–arene and alkene–alkene aminative coupling. Angew. Chem. Int. Ed. 64, e202418141 (2025).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Acknowledgements
Financial support for this work was provided by the National Institutes of Health (grant no. GM-118176, to P.S.B.). We thank L. Pasternack and G. J. Kroon (Scripps Research) for assistance with NMR spectroscopy; J. Lee, B. Sanchez and Q. N. Wong (Scripps Research ASF) for HRMS; and A. Pollatos for proofreading.
Author information
Authors and Affiliations
Contributions
Y.W. and P.S.B. conceptualized the study and developed the reagents. Y.W. conducted reaction optimization. Y.W. and M.M.B. contributed to the substrate scope and analysed the data. L.N.G. performed computational experiments. P.F.R. performed the safety experiments. J.B.B. and M.G. performed the X-ray crystallographic analysis. Y.W., M.M.B. and P.S.B. wrote the paper. P.S.B. supervised this work. All authors contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Qian Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–34, Figs. 1–14, experimental procedures, product characterization and NMR spectra.
Supplementary Data 1
Crystallographic data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Bogner, M.M., Bailey, J.B. et al. Cobalt-catalysed alkene hydronitration enabled by anomeric nitroamide. Nat Catal 8, 457–464 (2025). https://doi.org/10.1038/s41929-025-01336-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01336-3