Abstract
The oxygen evolution reaction is a key process in many energy technologies, but improving its efficiency remains challenging due to the energy scaling relationships that limit the reaction kinetics on conventional single-active-site solid catalysts. Here we report a cooperative solid–molecular mechanism for oxygen evolution on NiFe-based hydroxide electrocatalysts. By identifying the critical interfacial species and understanding their dynamics, we find that molecular FeO42− species, derived from the dissolution of Fe from the solid catalyst, act as molecular co-catalysts that participate in the critical O–O bond-formation step along with solid sites. This synergistic mechanism, involving both solid and molecular active species, circumvents the typical scaling limitations observed for solid catalysts alone. Our findings reveal an unconventional solid–molecular mechanism that governs electrocatalysis at the solid–liquid interface and suggest a strategy for transcending scaling constraints through cooperative multi-site catalysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper. The atomic coordinates of the optimized computational models are also provided. Other data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Nørskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009).
Thorarinsdottir, A. E., Veroneau, S. S. & Nocera, D. G. Self-healing oxygen evolution catalysts. Nat. Commun. 13, 1243 (2022).
Nong, H. N. et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature 587, 408–413 (2020).
Gorlin, M. et al. Oxygen evolution reaction dynamics, Faradaic charge efficiency, and the active metal redox states of Ni–Fe oxide water splitting electrocatalysts. J. Am. Chem. Soc. 138, 5603–5614 (2016).
Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017).
Zou, S. et al. Fe (oxy)hydroxide oxygen evolution reaction electrocatalysis: intrinsic activity and the roles of electrical conductivity, substrate, and dissolution. Chem. Mater. 27, 8011–8020 (2015).
Guo, J. et al. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 8, 264–272 (2023).
Wang, X. et al. Pivotal role of reversible NiO6 geometric conversion in oxygen evolution. Nature 611, 702–708 (2022).
Yang, H. et al. Intramolecular hydroxyl nucleophilic attack pathway by a polymeric water oxidation catalyst with single cobalt sites. Nat. Catal. 5, 414–429 (2022).
Righi, G. et al. On the origin of multihole oxygen evolution in haematite photoanodes. Nat. Catal. 5, 888–899 (2022).
Bai, L., Hsu, C.-S., Alexander, D. T. L., Chen, H. M. & Hu, X. Double-atom catalysts as a molecular platform for heterogeneous oxygen evolution electrocatalysis. Nat. Energy 6, 1054–1066 (2021).
Li, J. et al. Reaction kinetics and interplay of two different surface states on hematite photoanodes for water oxidation. Nat. Commun. 12, 255 (2021).
Zhang, W. & Cao, R. Switching the O–O bond-formation mechanism by controlling water activity. Chem 7, 1981–1982 (2021).
Roy, C. et al. Impact of nanoparticle size and lattice oxygen on water oxidation on NiFeOxHy. Nat. Catal. 1, 820–829 (2018).
Dickens, C. F., Kirk, C. & Nørskov, J. K. Insights into the electrochemical oxygen evolution reaction with ab initio calculations and microkinetic modeling: beyond the limiting potential volcano. J. Phys. Chem. C 123, 18960–18977 (2019).
Montoya, J. H. et al. Materials for solar fuels and chemicals. Nat. Mater. 16, 70–81 (2017).
Kondo, M., Tatewaki, H. & Masaoka, S. Design of molecular water oxidation catalysts with earth-abundant metal ions. Chem. Soc. Rev. 50, 6790–6831 (2021).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Velasco-Vélez, J.-J. et al. Surface electron–hole rich species active in the electrocatalytic water oxidation. J. Am. Chem. Soc. 143, 12524–12534 (2021).
Craig, M. J. et al. Universal scaling relations for the rational design of molecular water oxidation catalysts with near-zero overpotential. Nat. Commun. 10, 4993 (2019).
Wang, N. et al. Doping shortens the metal/metal distance and promotes OH coverage in non-noble acidic oxygen evolution reaction catalysts. J. Am. Chem. Soc. 145, 7829–7836 (2023).
Zhang, N. & Chai, Y. Lattice oxygen redox chemistry in solid-state electrocatalysts for water oxidation. Energy Environ. Sci. 14, 4647–4671 (2021).
Hong, W. T. et al. Charge-transfer-energy-dependent oxygen evolution reaction mechanisms for perovskite oxides. Energy Environ. Sci. 10, 2190–2200 (2017).
Chen, F.-Y., Wu, Z.-Y., Adler, Z. & Wang, H. Stability challenges of electrocatalytic oxygen evolution reaction: from mechanistic understanding to reactor design. Joule 5, 1704–1731 (2021).
Samira, S. et al. Dynamic surface reconstruction unifies the electrocatalytic oxygen evolution performance of nonstoichiometric mixed metal oxides. JACS Au 1, 2224–2241 (2021).
Wen, Y. et al. Stabilizing highly active Ru sites by suppressing lattice oxygen participation in acidic water oxidation. J. Am. Chem. Soc. 143, 6482–6490 (2021).
Zhao, S. et al. Structural transformation of highly active metal–organic framework electrocatalysts during the oxygen evolution reaction. Nat. Energy 5, 881–890 (2020).
Chung, D. Y. et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nat. Energy 5, 222–230 (2020).
Kuai, C. et al. Phase segregation reversibility in mixed-metal hydroxide water oxidation catalysts. Nat. Catal. 3, 743–753 (2020).
Kuai, C. et al. Revealing the dynamics and roles of iron incorporation in nickel hydroxide water oxidation catalysts. J. Am. Chem. Soc. 143, 18519–18526 (2021).
Trotochaud, L., Young, S. L., Ranney, J. K. & Boettcher, S. W. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 136, 6744–6753 (2014).
Corrigan, D. A. The catalysis of the oxygen evolution reaction by iron impurities in thin film nickel oxide electrodes. J. Electrochem. Soc. 134, 377 (1987).
Klaus, S., Cai, Y., Louie, M. W., Trotochaud, L. & Bell, A. T. Effects of Fe electrolyte impurities on Ni(OH)2/NiOOH structure and oxygen evolution activity. J. Phys. Chem. C 119, 7243–7254 (2015).
Michael, J. D. et al. Alkaline electrolyte and Fe impurity effects on the performance and active-phase structure of NiOOH thin films for OER catalysis applications. J. Phys. Chem. C 119, 11475–11481 (2015).
Stevens, M. B., Trang, C. D. M., Enman, L. J., Deng, J. & Boettcher, S. W. Reactive Fe-sites in Ni/Fe (oxy)hydroxide are responsible for exceptional oxygen electrocatalysis activity. J. Am. Chem. Soc. 139, 11361–11364 (2017).
Akbashev, A. R. et al. Probing the stability of SrIrO3 during active water electrolysis via operando atomic force microscopy. Energy Environ. Sci. 16, 513–522 (2023).
Bao, F. et al. Host, suppressor, and promoter—the roles of Ni and Fe on oxygen evolution reaction activity and stability of NiFe alloy thin films in alkaline media. ACS Catal. 11, 10537–10552 (2021).
Hu, C. et al. Surface-enhanced Raman spectroscopic evidence of key intermediate species and role of NiFe dual-catalytic center in water oxidation. Angew. Chem. Int. Ed. 60, 19774–19778 (2021).
Mefford, J. T. et al. Correlative operando microscopy of oxygen evolution electrocatalysts. Nature 593, 67–73 (2021).
Peng, L. et al. Atomic cation-vacancy engineering of NiFe-layered double hydroxides for improved activity and stability towards the oxygen evolution reaction. Angew. Chem. Int. Ed. 60, 24612–24619 (2021).
Chen, J. et al. Interfacial interaction between FeOOH and Ni–Fe LDH to modulate the local electronic structure for enhanced OER electrocatalysis. ACS Catal. 8, 11342–11351 (2018).
Friebel, D. et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305–1313 (2015).
Hausmann, J. N. & Menezes, P. W. Effect of surface-adsorbed and intercalated (oxy)anions on the oxygen evolution reaction. Angew. Chem. Int. Ed. 61, e202207279 (2022).
Li, N. et al. Influence of iron doping on tetravalent nickel content in catalytic oxygen evolving films. Proc. Natl Acad. Sci. USA 114, 1486–1491 (2017).
Xiao, H., Shin, H. & Goddard, W. A. III Synergy between Fe and Ni in the optimal performance of (Ni,Fe)OOH catalysts for the oxygen evolution reaction. Proc. Natl Acad. Sci. USA 115, 5872–5877 (2018).
Kuai, C. et al. Fully oxidized Ni–Fe layered double hydroxide with 100% exposed active sites for catalyzing oxygen evolution reaction. ACS Catal. 9, 6027–6032 (2019).
Hu, A. et al. Manipulating interfacial dissolution–redeposition dynamics to resynthesize electrode surface chemistry. ACS Energy Lett. 7, 2588–2594 (2022).
Zhang, Y., Hu, A., Maxey, E., Li, L. & Lin, F. Spatiotemporal visualization and chemical identification of the metal diffusion layer at the electrochemical interface. J. Electrochem. Soc. 169, 100512 (2022).
Hu, A. et al. Uncovering phase transformation, morphological evolution, and nanoscale color heterogeneity in tungsten oxide electrochromic materials. J. Mater. Chem. A 8, 20000–20010 (2020).
Zhang, Y. et al. Operando characterization and regulation of metal dissolution and redeposition dynamics near battery electrode surface. Nat. Nanotechnol. 18, 790–797 (2023).
Feng, C., She, X., Xiao, Y. & Li, Y. Direct detection of Fe(VI) water oxidation intermediates in an aqueous solution. Angew. Chem. Int. Ed. 62, e202218738 (2023).
Hunter, B. M. et al. Trapping an iron(VI) water-splitting intermediate in nonaqueous media. Joule 2, 747–763 (2018).
Yu, X. & Licht, S. Advances in electrochemical Fe(VI) synthesis and analysis. J. Appl. Electrochem. 38, 731–742 (2008).
Kemner, K. M. et al. XAS investigations of Fe(VI). J. Synchrotron Radiat. 8, 949–951 (2001).
Saveleva, V. A. et al. Operando evidence for a universal oxygen evolution mechanism on thermal and electrochemical iridium oxides. J. Phys. Chem. Lett. 9, 3154–3160 (2018).
Drevon, D. et al. Uncovering the role of oxygen in Ni-Fe(OxHy) electrocatalysts using in situ soft X-ray absorption spectroscopy during the oxygen evolution reaction. Sci. Rep. 9, 1532 (2019).
Reith, L. et al. In situ detection of iron in oxidation states ≥ IV in cobalt-iron oxyhydroxide reconstructed during oxygen evolution reaction. Adv. Energy Mater. 13, 2203886 (2023).
Lee, S., Bai, L. & Hu, X. Deciphering iron-dependent activity in oxygen evolution catalyzed by nickel-iron layered double hydroxide. Angew. Chem. Int. Ed. 59, 8072–8077 (2020).
Lee, S., Banjac, K., Lingenfelder, M. & Hu, X. Oxygen isotope labeling experiments reveal different reaction sites for the oxygen evolution reaction on nickel and nickel iron oxides. Angew. Chem. Int. Ed. 58, 10295–10299 (2019).
Medford, A. J. et al. Assessing the reliability of calculated catalytic ammonia synthesis rates. Science 345, 197–200 (2014).
Glowacki, A. T. XRF-MAPS (US Department of Energy, 2020)
Webb, S. M. SIXPack a graphical user interface for XAS analysis using IFEFFIT. Phys. Scripta T115, 1011–1014 (2005).
Newville, M. IFEFFIT: interactive XAFS analysis and FEFF fitting. J. Synchrotron Rad. 8, 322–324 (2001).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter. 54, 11169–11186 (1996).
Mathew, K., Kolluru, V. S. C., Mula, S., Steinmann, S. N. & Hennig, R. G. Implicit self-consistent electrolyte model in plane-wave density-functional theory. J. Chem. Phys. 151, 234101 (2019).
Islam, S. M. R., Khezeli, F., Ringe, S. & Plaisance, C. An implicit electrolyte model for plane wave density functional theory exhibiting nonlinear response and a nonlocal cavity definition. J. Chem. Phys. 159, 234117 (2023).
Larsen, A. H. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter. 29, 273002 (2017).
Acknowledgements
The work at Virginia Tech was supported by Department of Chemistry start-up funds and the Institute for Critical Technology and Applied Science (F.L.). F.L. also acknowledges the seedling support from the Virginia Tech College of Science Strategic Initiative in Energy (03400) and the support from the Leo and Melva Harris Faculty Fellowship. L. Liu and H.X. gratefully acknowledge the financial support from the National Science Foundation Chemical Catalysis program (CHE-2102363) (H.X.) and the computational resource provided by Advanced Research Computing at Virginia Tech. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515.
Author information
Authors and Affiliations
Contributions
F.L. and C.K. conceived the project. C.K. and F.L. designed the overall experiments. C.K., F.L. and L. Li developed the fluorescence imaging measurements. L. Liu and H.X. conducted the DFT calculations and microkinetic analysis. C.K. synthesized the materials and performed the characterization and electrochemical measurements. C.K. and Yan Zhang performed the soft-XAS and operando hXAS experiments with assistance from D.N. and D.S. The synchrotron XFM and HERFD experiments were performed by A.H., Yuxin Zhang and D.X. under the supervision of F.L. and L. Li. The hXAS measurements for key reference samples were performed by D.D. and G.D. The figures were prepared by C.K., L. Liu, H.X. and F.L., who also wrote the manuscript with assistance from all co-authors. All of the co-authors participated in the scientific discussion and approved the manuscript submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–43, Notes 1–8 and Tables 1–3.
Supplementary Data 1
Supplementary structure (CIF) files
Supplementary Data 2
Data for Supplementary Fig. 7.
Source data
Source Data Fig. 1
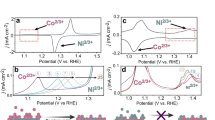
Dynamic behaviour at the electrode–electrolyte interface.
Source Data Fig. 2
Dynamic local structural change of Fe species during the OER.
Source Data Fig. 3
Interaction between the electrode and the molecular Fe species.
Source Data Fig. 4
Mechanistic understanding and schematic illustration of the SMM.
Source Data Fig. 5
OER performance of the MNF catalysts with and without the addition of iron gluconate to the electrolyte.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuai, C., Liu, L., Hu, A. et al. Dissolved Fe species enable a cooperative solid–molecular mechanism for the oxygen evolution reaction on NiFe-based catalysts. Nat Catal 8, 523–535 (2025). https://doi.org/10.1038/s41929-025-01342-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01342-5
This article is cited by
-
NiIV-enabled spontaneous water oxidation in Ni oxyhydroxides
Science China Chemistry (2026)
-
Sustainable water oxidation enabled by a complex-doped cobalt oxide electrode
Nature Communications (2025)