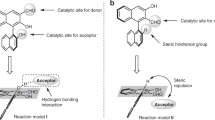
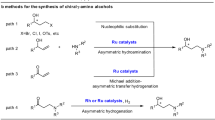
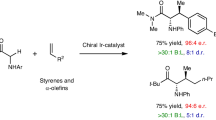
Abstract
Efficient biomimetic reactions provide a powerful platform for producing bioactive chiral compounds. Chiral β-hydroxy-α-amino acids display exceptionally high bioactivity and are key functional components of many marketed drugs. However, the efficient synthesis of chiral β-hydroxy-α-amino acids remains a long-standing challenge for both chemical and biological syntheses. Chemical synthesis is problematic due to low step- and atom-efficiencies, high costs and environmental hazards. The enzymatic aldol reaction of glycine can make β-hydroxy-α-amino acids in one step, but it is constrained by issues such as limited substrate scope, unsatisfactory stereoselectivity and low conversion. Here we have successfully realized the biomimetic aldol reaction of glycinate with aldehydes, using 0.01–1.0 mol% of a chiral pyridoxal as the catalyst to produce a remarkably broad range of chiral β-hydroxy-α-amino esters. Moreover, a high-diversity parallel asymmetric synthesis has also been tested using over 1,100 aldehydes and it yielded over 1,700 chiral β-hydroxy-α-amino acid esters, demonstrating its important potential for drug innovation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information or from the corresponding authors upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2353485 [(2R,3S)-4a in Supplementary Fig. 6) and CCDC 2353487 ((2R,3S)-4an in Supplementary Fig. 7). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/.
References
Calcaterra, A. & D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 147, 323–340 (2018).
Dückers, N., Baer, K., Simon, S., Gröger, H. & Hummel, W. Threonine aldolases—screening, properties and applications in the synthesis of non-proteinogenic β-hydroxy-α-amino acids. Appl. Microbiol. Biotechnol. 88, 409–424 (2010).
He, Y. et al. Discovery and engineering of the l-threonine aldolase from neptunomonas marine for the efficient synthesis of β-hydroxy-α-amino acids via C–C formation. ACS Catal. 13, 7210–7220 (2023).
Dowling, P. M. Chloramphenicol, Thiamphenicol, and Florfenicol. in Antimicrobial Therapy in Veterinary Medicine (eds Giguère S., Prescott J. F. & Dowling P. M.) 269–277 (Wiley-Blackwell, 2013).
Zhang, Q.-Q., Ying, G.-G., Pan, C.-G., Liu, Y.-S. & Zhao, J.-L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 49, 6772–6782 (2015).
Mathias, C. J. l-Dihydroxyphenylserine (Droxidopa) in the treatment of orthostatic hypotension. Clin. Auton. Res. 18, 25–29 (2008).
Bennett, L. & Turcotte, K. Eliglustat tartrate for the treatment of adults with type 1 Gaucher disease. Drug Des. Dev. 9, 4639–4647 (2015).
Hossion, A. G. (ed) Vancomycin: Biosynthesis, Clinical Uses and Adverse Effects (Nova Science Publishers, 2013).
Selvam, S. P. & Ogretmen, B. Sphingolipids in Disease (eds Gulbins, E. & Petrache, I.) 3–27 (Springer, 2013).
Pu, Y., Lowe, C., Sailer, M. & Vederas, J. C. Synthesis, stability, and antimicrobial activity of (+)-obafluorin and related β-lactone antibiotics. J. Org. Chem. 59, 3642–3655 (1994).
Ōmura, S. & Crump, A. Lactacystin: first-in-class proteasome inhibitor still excelling and an exemplar for future antibiotic research. J. Antibiot. 72, 189–201 (2019).
Zhang, Y., Farrants, H. & Li, X. Adding a functional handle to nature′s building blocks: the asymmetric synthesis of β‐hydroxy‐α‐amino acids. Chem. Asian J. 9, 1752–1764 (2014).
Luo, Y.-C., Zhang, H.-H., Wang, Y. & Xu, P.-F. Synthesis of α-amino acids based on chiral tricycloiminolactone derived from natural (+)-camphor. Acc. Chem. Res. 43, 1317–1330 (2010).
Ooi, T., Taniguchi, M., Kameda, M. & Maruoka, K. Direct asymmetric aldol reactions of glycine schiff base with aldehydes catalyzed by chiral quaternary ammonium salts. Angew. Chem. Int. Ed. 41, 4542–4544 (2002).
Li, L., Klauber, E. G. & Seidel, D. Catalytic enantioselective aldol additions of α-isothiocyanato imides to aldehydes. J. Am. Chem. Soc. 130, 12248–12249 (2008).
Sladojevich, F., Trabocchi, A., Guarna, A. & Dixon, D. J. A new family of cinchona-derived amino phosphine precatalysts: application to the highly enantio- and diastereoselective silver-catalyzed isocyanoacetate aldol reaction. J. Am. Chem. Soc. 133, 1710–1713 (2011).
Trost, B. M. & Miege, F. Development of prophenol ligands for the diastereo- and enantioselective synthesis of β-hydroxy-α-amino esters. J. Am. Chem. Soc. 136, 3016–3019 (2014).
Seiple, I. B., Mercer, J. A. M., Sussman, R. J., Zhang, Z. & Myers, A. G. Stereocontrolled synthesis of syn‐β‐hydroxy‐α‐amino acids by direct aldolization of pseudoephenamine glycinamide. Angew. Chem. Int. Ed. 53, 4642–4647 (2014).
Franz, S. E. & Stewart, J. D. Chapter three - threonine aldolases. Adv. Appl. Microbiol. 88, 57–101 (2014).
Kimura, T., Vassilev, V. P., Shen, G.-J. & Wong, C.-H. Enzymatic synthesis of β-hydroxy-α-amino acids based on recombinant d- and l-threonine aldolases. J. Am. Chem. Soc. 119, 11734–11742 (1997).
Steinreiber, J. et al. Synthesis of γ-halogenated and long-chain β-hydroxy-α-amino acids and 2-amino-1,3-diols using threonine aldolases. Tetrahedron 63, 8088–8093 (2007).
Goldberg, S. L. et al. Preparation of β-hydroxy-α-amino acid using recombinant d-threonine aldolase. Org. Process Res. Dev. 19, 1308–1316 (2015).
Beaudoin, S. F., Hanna, M. P., Ghiviriga, I. & Stewart, J. D. Progress in using threonine aldolases for preparative synthesis. Enzym. Microb. Technol. 119, 1–9 (2018).
Cai, B. et al. Computer-aided directed evolution of l-threonine aldolase for asymmetric biocatalytic synthesis of a chloramphenicol intermediate. Bioorg. Med. Chem. 68, 116880–116889 (2022).
Chen, Q. et al. Improving and inverting Cβ-stereoselectivity of threonine aldolase via substrate-binding-guided mutagenesis and a stepwise visual screening. ACS Catal. 9, 4462–4469 (2019).
Zheng, W. et al. Directed evolution of l-threonine aldolase for the diastereoselective synthesis of β-hydroxy-α-amino acids. ACS Catal. 11, 3198–3205 (2021).
Wu, G. Z., Schumacher, D. P., Tormos, W., Clark, J. E. & Murphy, B. L. An improved industrial synthesis of florfenicol plus an enantioselective total synthesis of thiamphenicol and florfenicol. J. Org. Chem. 62, 2996–2998 (1997).
Guan, Y.-Q., Gao, M., Deng, X., Lv, H. & Zhang, X. Rhodium-catalyzed asymmetric hydrogenation of tetrasubstituted β-acetoxy-α-enamido esters and efficient synthesis of droxidopa. Chem. Commun. 53, 8136–8139 (2017).
Sun, G. et al. Development of an efficient and scalable asymmetric synthesis of eliglustat via ruthenium(II)-catalyzed asymmetric transfer hydrogenation. Org. Process Res. Dev. 23, 1204–1212 (2019).
Akiyama, H., Tobiki, H., Mitani, T., Miura, Y. & Suzuki, H. Microbiocidal d-threo-1-(p-methylsulfonylphenyl)-2-(dichloroacetamido)propane-1,3-diol. DE patent 1,938,513 (1970).
Tobiki, H., Okamoto, T. & Akiyama, H. Process for producing β-phenylserine copper complex. US patent 3,927,054 (1975).
Pimplaskar, H., Kadam, S. V., Mallesh, V. & Kawale, P. M. Method for the synthesis of droxidopa. WO patent 2,013,142,093 (2013).
Teng, H., Chen, K., Wang, L., Wu, J. & Xu, G. Rational immobilization of 1-threonine aldolase from Bacillus nealsonii for efficiently synthesis of 1-syn-3-[4-(methylsulfonyl)] phenylserine. Process Biochem. 130, 685–694 (2023).
Zha, R. et al. Improving the Cβ stereoselectivity of l-threonine aldolase for the preparation of l-threo-3,4-dihydroxyphenylserine, a powerful anti-Parkinson’s disease drug. Biochem. Eng. J. 191, 108766–108772 (2023).
Hughes, D. L. Highlights of the recent patent literature─focus on biocatalysis innovation. Org. Process Res. Dev. 26, 1878–1899 (2022).
Kuzuhara, H., Watanabe, N. & Ando, M. Asymmetric synthesis of allo-threonine and threonine; the use of a chiral pyridoxal–like pyridinophane-zinc complex as an enzyme mimic. J. Chem. Soc. Chem. Commun. 95–96 (1987).
Koh, J. T., Delaude, L. & Breslow, R. Geometric control of a pyridoxal-catalyzed aldol condensation. J. Am. Chem. Soc. 116, 11234–11240 (1994).
Chen, J. et al. Carbonyl catalysis enables a biomimetic asymmetric Mannich reaction. Science 360, 1438–1442 (2018).
Cheng, A. et al. Efficient asymmetric biomimetic aldol reaction of glycinates and trifluoromethyl ketones by carbonyl catalysis. Angew. Chem. Int. Ed. 60, 20166–20172 (2021).
Xiao, X. & Zhao, B. Vitamin B6-based biomimetic asymmetric catalysis. Acc. Chem. Res. 56, 1097–1117 (2023).
Li, B.-J., Ei-Nachef, C. & Beauchemin, A. M. Organocatalysis using aldehydes: the development and improvement of catalytic hydroaminations, hydrations and hydrolyses. Chem. Commun. 53, 13192–13204 (2017).
Wang, Q., Gu, Q. & You, S. L. Enantioselective carbonyl catalysis enabled by chiral aldehydes. Angew. Chem. Int. Ed. 58, 6818–6825 (2019).
Wen, W. & Guo, Q.-X. Chiral aldehyde catalysis-enabled asymmetric α-functionalization of activated primary amines. Acc. Chem. Res. 57, 776–794 (2024).
Fesko, K. Comparison of l-threonine aldolase variants in the aldol and retro-aldol reactions. Front. Bioeng. Biotechnol. 7, 119–130 (2019).
Tanner, M. E. Understanding nature’s strategies for enzyme-catalyzed racemization and epimerization. Acc. Chem. Res. 35, 237–246 (2002).
Hou, C. et al. Catalytic asymmetric α C(sp3)–H addition of benzylamines to aldehydes. Nat. Catal. 5, 1061–1068 (2022).
Crugeiras, J., Rios, A., Riveiros, E. & Richard, J. P. Substituent effects on electrophilic catalysis by the carbonyl group: anatomy of the rate acceleration for PLP-catalyzed deprotonation of glycine. J. Am. Chem. Soc. 133, 3173–3183 (2011).
Alemán, J., Parra, A., Jiang, H. & Jørgensen, K. A. Squaramides: bridging from molecular recognition to bifunctional organocatalysis. Chem. Eur. J. 17, 6890–6899 (2011).
Doyle, A. G. & Jacobsen, E. N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 107, 5713–5743 (2007).
Park, S.-H. et al. Cβ-Selective aldol addition of d-threonine aldolase by spatial constraint of aldehyde binding. ACS Catal. 11, 6892–6899 (2021).
Dalko, P. I. & Moisan, L. Enantioselective organocatalysis. Angew. Chem. Int. Ed. 40, 3726–3748 (2001).
List, B. Introduction: organocatalysis. Chem. Rev. 107, 5413–5415 (2007).
MacMillan, D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008).
Meng, G. et al. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 574, 86–89 (2019).
Zhu, L. & Wang, D. Deciphering the cooperative effect of base and N-substituents on the origin of enantioselectivity switching for Mannich reactions of glycinate by carbonyl catalysts. J. Catal. 415, 1–11 (2022).
Han, L.-L. et al. The chiral pyridoxal-catalyzed biomimetic Mannich reaction: the mechanism and origin of stereoselectivity. Org. Chem. Front. 9, 4401–4410 (2022).
Acknowledgements
We are grateful for the generous financial support from the National Key R&D Program of China (2023YFA1506400, B.Z. and X.X.), the National Natural Science Foundation of China (NSFC) (22271192, B.Z.; 22471171, X.X.; 22403065, S.L.), the Shanghai Municipal Science and Technology Major Project (B.Z.) and the Shanghai Engineering Research Center of Green Energy Chemical Engineering (18DZ2254200, B.Z.).
Author information
Authors and Affiliations
Contributions
B.Z. conceived and directed the project and wrote the manuscript. X.X. co-directed the project and co-wrote the manuscript. K.D. co-directed the project. H.L. conducted most of the experiments. P.R. conducted part of the optimization of conditions. P.R. and L.W. conducted the synthesis of catalysts and racemic products. H.L., P.R., D.C. and S.G. conducted the high-diversity parallel asymmetric synthesis in Fig. 5 and prepared the Supplementary Information. S.L. conducted the DFT calculation of transition states.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Luca Bernardi, Donghui Wei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Tables 1–12, Methods and References.
Supplementary Data 1
The .cif file of compound (2R,3S)-4a (CCDC 2353485).
Supplementary Data 2
The .cif file of compound (2R,3S)-4an (CCDC 2353487).
Supplementary Data 3
The calculation data for the transition state of (2R,3S)-4a.
Supplementary Data 4
The calculation data for the transition state of (2R,3R)-4a.
Supplementary Data 5
The calculation data for the transition state of (2S,3R)-4a.
Supplementary Data 6
The calculation data for the transition state of (2S,3S)-4a.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, H., Ren, P., Wang, L. et al. Asymmetric biomimetic aldol reaction of glycinate enables highly efficient synthesis of chiral β-hydroxy-α-amino acid derivatives. Nat Catal 8, 668–677 (2025). https://doi.org/10.1038/s41929-025-01364-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01364-z
This article is cited by
-
Biomimetic aldol reaction of glycinate
Nature Catalysis (2025)