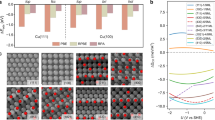
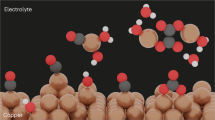
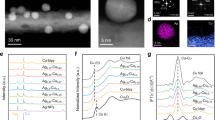
Abstract
Efficient electrocatalysts should provide optimal binding sites for intermediates under operating conditions. Atomic rearrangements in catalysts during electrochemical CO2 reduction reaction (CO2RR) alter the original structures of active sites. Here we report a general principle for understanding and predicting the reconstruction of Cu bimetallic catalysts during CO2RR in terms of selective dissolution–redeposition. We categorize the reconstruction trends of Cu bound to a secondary metal (X, where X = Ag, Fe, Zn or Pd) according to the oxophilicity and miscibility of Cu and X. Cross-sectional microscopy analysis of gas diffusion electrodes reveals that the surface states of reconstructed Cu–X are determined by atomic miscibility. We find that CO2RR intermediates alter elemental preferences for dissolution, shifting them away from oxophilicity-governed behaviour and leading to selective Cu dissolution–redeposition in Cu–X. This reconstruction affects spillover in CO2RR, controlling the selectivities of ethylene/ethanol and C1/C2 products. We also develop a methodology for the control of reconstruction dynamics. Our findings provide insights into designing catalysts that undergo reconstruction during electrolysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The optimized computational models are provided as Supplementary Data. In situ e-LCTEM recordings are provided as Supplementary Videos 1 and 2. Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding authors upon reasonable request.
References
Jordaan, S. M. & Wang, C. Electrocatalytic conversion of carbon dioxide for the Paris goals. Nat. Catal. 4, 915–920 (2021).
Masel, R. I. et al. An industrial perspective on catalysts for low-temperature CO2 electrolysis. Nat. Nanotechnol. 16, 118–128 (2021).
Lee, C. W. et al. Defining a materials database for the design of copper binary alloy catalysts for electrochemical CO2 conversion. Adv. Mater. 30, 1704717 (2018).
Kim, C. et al. Alloy nanocatalysts for the electrochemical oxygen reduction (ORR) and the direct electrochemical carbon dioxide reduction reaction (CO2RR). Adv. Mater. 31, 1805617 (2019).
Ren, B. et al. Nano-crumples induced Sn–Bi bimetallic interface pattern with moderate electron bank for highly efficient CO2 electroreduction. Nat. Commun. 13, 2486 (2022).
Lee, S., Park, G. & Lee, J. Importance of Ag–Cu biphasic boundaries for selective electrochemical reduction of CO2 to ethanol. ACS Catal. 7, 8594–8604 (2017).
Kim, D., Resasco, J., Yu, Y., Asiri, A. M. & Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 5, 4948 (2014).
Jeon, H. S. et al. Operando insight into the correlation between the structure and composition of CuZn nanoparticles and their selectivity for the electrochemical CO2 reduction. J. Am. Chem. Soc. 141, 19879–19887 (2019).
Ren, D. et al. Atomic layer deposition of ZnO on CuO enables selective and efficient electroreduction of carbon dioxide to liquid fuels. Angew. Chem. Int. Ed. 58, 15036–15040 (2019).
Ye, K. et al. In situ reconstruction of a hierarchical Sn–Cu/SnOx core/shell catalyst for high-performance CO2 electroreduction. Angew. Chem. Int. Ed. 59, 4814–4821 (2020).
Herzog, A. et al. Operando investigation of Ag-decorated Cu2O nanocube catalysts with enhanced CO2 electroreduction toward liquid products. Angew. Chem. Int. Ed. 60, 7426–7435 (2021).
Li, L. et al. Stable, active CO2 reduction to formate via redox-modulated stabilization of active sites. Nat. Commun. 12, 5223 (2021).
Zhu, C. et al. Dynamic restructuring of epitaxial Au–Cu biphasic interface for tandem CO2-to-C2+ alcohol conversion. Chem 8, 3288–3301 (2022).
Chen, P.-C. et al. Chemical and structural evolution of AgCu catalysts in electrochemical CO2 reduction. J. Am. Chem. Soc. 145, 10116–10125 (2023).
Okatenko, V. et al. Alloying as a strategy to boost the stability of copper nanocatalysts during the electrochemical CO2 reduction reaction. J. Am. Chem. Soc. 145, 5370–5383 (2023).
Li, F. et al. Interplay of electrochemical and electrical effects induces structural transformations in electrocatalysts. Nat. Catal. 4, 479–487 (2021).
Kim, T. & Palmore, G. T. R. A scalable method for preparing Cu electrocatalysts that convert CO2 into C2+ products. Nat. Commun. 11, 3622 (2020).
Amirbeigiarab, R. et al. Atomic-scale surface restructuring of copper electrodes under CO2 electroreduction conditions. Nat. Catal. 6, 837–846 (2023).
Lee, S. H. et al. Oxidation state and surface reconstruction of Cu under CO2 reduction conditions from in situ X-ray characterization. J. Am. Chem. Soc. 143, 588–592 (2021).
Wang, J., Tan, H.-Y., Zhu, Y., Chu, H. & Chen, H. M. Linking the dynamic chemical state of catalysts with the product profile of electrocatalytic CO2 reduction. Angew. Chem. Int. Ed. 60, 17254–17267 (2021).
Grosse, P. et al. Dynamic transformation of cubic copper catalysts during CO2 electroreduction and its impact on catalytic selectivity. Nat. Commun. 12, 6736 (2021).
Vavra, J., Shen, T.-H., Stoian, D., Tileli, V. & Buonsanti, R. Real-time monitoring reveals dissolution/redeposition mechanism in copper nanocatalysts during the initial stages of the CO2 reduction reaction. Angew. Chem. Int. Ed. 60, 1347–1354 (2021).
Vavra, J. et al. Solution-based Cu+ transient species mediate the reconstruction of copper electrocatalysts for CO2 reduction. Nat. Catal. 7, 89–97 (2024).
Huang, J. et al. Potential-induced nanoclustering of metallic catalysts during electrochemical CO2 reduction. Nat. Commun. 9, 3117 (2018).
Yang, Y. et al. Operando studies reveal active Cu nanograins for CO2 electroreduction. Nature 614, 262–269 (2023).
Nam, D.-H. et al. High-rate and selective CO2 electrolysis to ethylene via metal–organic-framework-augmented CO2 availability. Adv. Mater. 34, 2207088 (2022).
Choi, W. et al. Origin of hydrogen incorporated into ethylene during electrochemical CO2 reduction in membrane electrode assembly. ACS Energy Lett. 7, 939–945 (2022).
Kim, J.-Y. et al. Selective hydrocarbon or oxygenate production in CO2 electroreduction over metallurgical alloy catalysts. Nat. Synth. 3, 452–465 (2024).
Leitner, J., Sedmidubský, D., Lojka, M. & Jankovský, O. The effect of nanosizing on the oxidation of partially oxidized copper nanoparticles. Materials (Basel) 13, 2878 (2020).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Zhan, C. et al. Revealing the CO coverage-driven C–C coupling mechanism for electrochemical CO2 reduction on Cu2O nanocubes via operando Raman spectroscopy. ACS Catal. 11, 7694–7701 (2021).
An, H. et al. Sub-second time-resolved surface-enhanced Raman spectroscopy reveals dynamic CO intermediates during electrochemical CO2 reduction on copper. Angew. Chem. Int. Ed. 60, 16576–16584 (2021).
McCafferty, E. Introduction to Corrosion Science (Springer, 2010).
Li, Y. C. et al. Binding site diversity promotes CO2 electroreduction to ethanol. J. Am. Chem. Soc. 141, 8584–8591 (2019).
Iyengar, P., Kolb, M. J., Pankhurst, J. R., Calle-Vallejo, F. & Buonsanti, R. Elucidating the facet-dependent selectivity for CO2 electroreduction to ethanol of Cu–Ag tandem catalysts. ACS Catal. 11, 4456–4463 (2021).
Chen, C. et al. Exploration of the bio-analogous asymmetric C–C coupling mechanism in tandem CO2 electroreduction. Nat. Catal. 5, 878–887 (2022).
Shin, S.-J. et al. A unifying mechanism for cation effect modulating C1 and C2 productions from CO2 electroreduction. Nat. Commun. 13, 5482 (2022).
Bagger, A., Ju, W., Varela, A. S., Strasser, P. & Rossmeisl, J. Electrochemical CO2 reduction: a classification problem. ChemPhysChem 18, 3266–3273 (2017).
Kim, J. et al. Vitamin C-induced CO2 capture enables high-rate ethylene production in CO2 electroreduction. Nat. Commun. 15, 192 (2024).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Gao, J. et al. Selective C–C coupling in carbon dioxide electroreduction via efficient spillover of intermediates as supported by operando Raman spectroscopy. J. Am. Chem. Soc. 141, 18704–18714 (2019).
Li, F. et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 3, 75–82 (2020).
Wang, D. et al. Revealing the structural evolution of CuAg composites during electrochemical carbon monoxide reduction. Nat. Commun. 15, 4692 (2024).
Sprunger, P. T., Lægsgaard, E. & Besenbacher, F. Growth of Ag on Cu(100) studied by STM: from surface alloying to Ag superstructures. Phys. Rev. B 54, 8163–8171 (1996).
Gu, Z. et al. Efficient electrocatalytic CO2 reduction to C2+ alcohols at defect-site-rich Cu surface. Joule 5, 429–440 (2021).
Chang, C.-J. et al. Dynamic reoxidation/reduction-driven atomic interdiffusion for highly selective CO2 reduction toward methane. J. Am. Chem. Soc. 142, 12119–12132 (2020).
Wei, D. et al. Decrypting the controlled product selectivity over Ag–Cu bimetallic surface alloys for electrochemical CO2 reduction. Angew. Chem. Int. Ed. 62, e202217369 (2023).
Kim, C. et al. Robust Co alloy design for Co interconnects using a self-forming barrier layer. Sci. Rep. 12, 12291 (2022).
Yang, Y. et al. Elucidating cathodic corrosion mechanisms with operando electrochemical transmission electron microscopy. J. Am. Chem. Soc. 144, 15698–15708 (2022).
Kim, J. et al. Self-supervised machine learning framework for high-throughput electron microscopy. Sci. Adv. 11, eads5552 (2025).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Hao, Y.-C. et al. Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water. Nat. Catal. 2, 448–456 (2019).
Mathew, K., Kolluru, V. S. C., Mula, S., Steinmann, S. N. & Hennig, R. G. Implicit self-consistent electrolyte model in plane-wave density-functional theory. J. Chem. Phys. 151, 234101 (2019).
Hjorth Larsen, A. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter 29, 273002 (2017).
Acknowledgements
This research was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government’s Ministry of Science and ICT (MSIT) (RS-2021-NR061733 and NRF-2021M3D1A2047041 to D.-H.N.). Y.-C.J. acknowledges support from the Creative Materials Discovery Program through the NRF, funded by MSIT and Future Planning (2017M3D1A1040689), as well as from an NRF grant funded by the Korean government’s MSIT (RS-2024-00457199). J.P. acknowledges support from the NRF, funded by MSIT (RS-2024-00421181 and RS-2024-00449965). S.B. acknowledges the support from the Nano and Material Technology Development Program through the NRF funded by MSIT (RS-2024-00406517) and generous supercomputing time from Korea Institute of Science and Technology Information. We acknowledge support received from the Hanwha Solutions Chemical Division. We thank B. Jeon, G. Kim and K. Seob Song of the Catalysis Technology Center of the Hanwha Solutions Chemical Division for valuable support. Material characterization, including grazing incidence XRD, SEM and TEM analyses, was supported by the Research Institute of Advanced Materials and National Center for Inter-University Research Facilities at Seoul National University. ICP-MS, NMR analysis and FIB preparation were supported by the National Instrumentation Center for Environmental Management at Seoul National University. XPS analysis was supported by the Korea Institute of Ceramic Engineering and Technology. Experiments at Pohang Light Source II were supported in part by MSIT and Pohang University of Science and Technology.
Author information
Authors and Affiliations
Contributions
D.-H.N., Y.-C.J., J.P. and S.B. designed and supervised the overall project. I.K., G.-B.L., S.K. and H.D.J. conceived of the idea and conducted the experiments. I.K. and G.-B.L. carried out electrode fabrication, characterization and electrochemical measurements. S.K. performed the e-LCTEM experiments with assistance from H.C. under the supervision of J.P. H.D.J. conducted the DFT calculations and analyses under the supervision of S.B. J.-Y.K. contributed to CO2RR performance evaluation. T.L. carried out the real-time XAS and Raman analyses with the assistance of Y.L. J.J. performed the high-resolution TEM EDS. H.G.K. contributed to TEM measurement under the supervision of M.K. G.K. contributed to thermodynamic calculations. S.-H.O. contributed to SEM measurement and analysis. W.K. contributed to XPS analysis. D.H. and J.-Y.K. contributed to data analysis. U.K. contributed to fabrication. H.K. contributed to SEM EDS analysis of the segmented electrodes. All authors discussed the results and contributed to writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–6, Figs. 1–90 and Tables 1 and 2.
Supplementary Video 1
In situ e-LCTEM of a Cu–Ag thin film during CO2RR using a CO2-saturated 0.1 M KHCO3 electrolyte at an applied potential of −0.8 V (versus the RHE) for 160 s.
Supplementary Video 2
In situ e-LCTEM of a Cu–Zn thin film during CO2RR at an applied potential of −0.8 V (versus the RHE) for 600 s.
Supplementary Data 1
Optimized computational models.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, I., Lee, GB., Kim, S. et al. Unveiling the reconstruction of copper bimetallic catalysts during CO2 electroreduction. Nat Catal 8, 697–713 (2025). https://doi.org/10.1038/s41929-025-01368-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01368-9