Abstract
Aminoacyl-tRNA synthetases (aaRSs) play a central role in the translation of genetic code, serving as attractive drug targets. Within this family, the lysyl-tRNA synthetase (LysRS) constitutes a promising antimalarial target. ASP3026, an anaplastic lymphoma kinase (ALK) inhibitor was recently identified as a novel Plasmodium falciparum LysRS (PfLysRS) inhibitor. Here, based on cocrystal structures and biochemical experiments, we developed a series of ASP3026 analogues to improve the selectivity and potency of LysRS inhibition. The leading compound 36 showed a dissociation constant of 15.9 nM with PfLysRS. The inhibitory efficacy on PfLysRS and parasites has been enhanced. Covalent attachment of l-lysine to compound 36 resulted in compound 36K3, which exhibited further increased inhibitory activity against PfLysRS but significantly decreased activity against ALK. However, its inhibitory activity against parasites did not improve, suggesting potential future optimization directions. This study presents a new example of derivatization of kinase inhibitors repurposed to inhibit aaRS.
Similar content being viewed by others
Introduction
Antimicrobial resistance, encompassing both bacteria and malaria, has become a major public health threat worldwide. In recent decades, the number of new antibiotics entering the market has decreased significantly due to scientific, regulatory, and economic barriers1. This necessitates the development of novel antimicrobial compounds with innovative chemical scaffolds and mechanisms of action to conquer resistant pathogens.
Aminoacyl-tRNA synthetases (aaRSs) catalyze the synthesis of aminoacyl-tRNAs, which are crucial building blocks for protein biosynthesis. These enzymes are essential for all cellular life, playing a central role in the translation of the genetic code2,3. As the indispensable components of the protein translation apparatus, aaRSs have been considered as attractive targets for the development of antiparasitic, antifungal, antibacterial agents and for the treatment of other human diseases4,5,6,7,8,9,10.
The aminoacylation reactions catalyzed by different aaRSs follow a similar two-step process, involving the binding and activation of the cognate amino acid with ATP, followed by transfer of the activated amino acid to the 3′-end of tRNA. Correspondingly, the active center of aaRSs has three adjacent subsites that bind to ATP, amino acid, and tRNA, respectively11. These subsites are considered as hot spots in the development of aaRS inhibitors. Mupirocin, an isoleucyl-tRNA synthetase (IleRS) inhibitor, occupies the isoleucine and ATP binding sites in the IleRS active center to inhibit Gram-positive aerobic bacteria and a few Gram-negative strains4,12. It is bacteriostatic against Staphylococcus aureus at concentrations ranging from 0.05 to 0.5 μg·mL−1 (MIC is 0.05 μg·mL−1) and it is bactericidal at higher concentrations12,13,14. The febrifugine derivative Halofuginone (HF), a prolyl-tRNA synthetase (ProRS) inhibitor, binds to the proline and tRNA A76 sites8. It has been used as a veterinary drug against coccidiosis and received an orphan drug designation for the treatment of human scleroderma from the Food and Drug Administration (FDA) in 200015. HF inhibits P. falciparum proliferation in cellulo, selectively binds and inhibits recombinant PfProRS within the low nanomolar range16,17. Additionally, HF has potential therapeutic applications in cancer and fibrotic diseases which are being tested in clinical trials18. It can inhibit the proliferation and metastasis of tumor cells in several cancer models19,20,21. It was also reported to limit SARS-CoV-2 entry by reducing surface expression of TMPRSS222. In short, the development of aaRS inhibitors have been widely explored in recent years as potential therapeutic agents.
Lysyl-tRNA synthetase (LysRS) has been validated as a promising target for antimalarial, anti-Cryptosporidium, anti-Pseudomonas aeruginosa, and anti-Mycobacterium tuberculosis therapies23,24,25. The ATP-binding pocket is the most promising area for the development of LysRS inhibitors. Cladosporin, a potent LysRS inhibitor, effectively inhibits P. falciparum LysRS (PfLysRS) activity by mimicking the interaction of ATP26. Further research is underway to develop more ATP-competitive inhibitors of PfLysRS to create new chemical scaffolds with improved druggability25,27,28,29,30,31.
ASP3026, an anaplastic lymphoma kinase (ALK) inhibitor, was initially used in clinical trials for the treatment of B-cell lymphoma and solid tumors32. It has ideal metabolic stability and excellent oral absorption in the recently completed phase I clinical trial32. Recently, we found that ASP3026 is also a potent PfLysRS inhibitor with an IC50 in the submicromolar range33. ASP3026 occupies the ATP binding site of ALK or PfLysRS to prevent their binding with ATP. Normally, ALK is an important kinase in the development of the central nervous system34,35,36. ASP3026 exhibits strong inhibition activity against ALK with an IC50 of 3.5 nM33,37. To develop anti-infective molecules targeting LysRS, we aim to decrease their inhibitory activity against ALK to avoid potential adverse side effects.
Here, we develop a series of ASP3026 analogues that switch the targeting specificity from ALK to LysRS. Based on the analysis of cocrystal structures, we reconstructed the compounds to fit the ATP binding site of LysRS instead of ALK. These compounds were evaluated by thermal shift, enzyme activity assays and Plasmodium growth inhibition experiment. We determined five cocrystal structures of LysRS in complex with the compounds in order to provide a basis for further optimization. We observed that the substitution of 4-(4-methylpiperazinyl)piperidinyl moiety can decrease the inhibition against ALK. The 5-methoxy-substituted compound 36 showed stronger inhibition against PfLysRS in the ATP-hydrolysis assay and the erythrocytic-stage Plasmodium growth inhibition experiment. We linked l-lysine to compound 36 and obtained compounds 36K2–36K5, which further enhances the LysRS inhibitory activity while removing the ALK inhibitory activity. Overall, our studys provide insights into converting kinase ATP site inhibitors to aaRS inhibitors.
Results
Analysis of action modes of ASP3026 on PfLysRS and ALK
Binding of small molecules to the ATP site of PfLysRS can significantly enhance the protein’s thermal stability25,30,31,38. Based on this property, we designed a high-throughput screening experiment and found that compound ASP3026 significantly improves the thermal stability of PfLysRS. Furthermore, it was verified to be an ATP competitive inhibitor of PfLysRS33. ASP3026 was initially developed to inhibit ALK by competing with ATP. It has a strong inhibitory activity against ALK with an IC50 of 3.5 nM37, while the IC50 for inhibiting PfLysRS enzyme activity is in the submicromolar range (Supplementary Fig. 1)33. Efforts were made to modify its selectivity for LysRS inhibition.
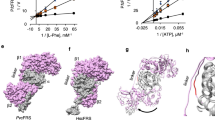
NVP-TAE684, a type 1 inhibitor of ALK, is another ATP competitive inhibitor with an inhibition constant (Ki) of 0.65 nM39, and it is very similar in structure to ASP3026 (Fig. 1a). Because the ALK/ASP3026 complex structure has not been reported, we compared the ALK/NVP-TAE684 complex structure (PDB: 2XB7) with the PfLysRS/ASP3026 complex structure (PDB: 7BT5) to identify potential compound optimization strategies33,40.
a Chemical structures of ASP3026 and NVP-TAE684. b Zoom-in view of ASP3026 localization in the conserved ATP binding site of PfLysRS (PDB: 7BT5). ASP3026 and ATP are depicted as sticks. c Zoom-in view of NVP-TAE684 localization in the conserved ATP binding site of ALK (PDB: 2XB7). ASP3026 and ADP are depicted as sticks. d ASP3026 in PfLysRS and NVP-TAE684 in ALK are superimposed using the adenine ring of ATP as a reference. e The triazine of ASP3026 replaces adenine of ATP to form stacking interaction with Phe342, and forms two H-bonds with the main chain atoms of Asn339 in PfLysRS. f The chloropyrimidine of NVP-TAE684 formed hydrophobic interactions with Leu1122, Ala1148, Leu1198, Met1199, and Leu1256, and forms two H-bonds with the main chain atoms of Met1199. g The isopropyl sulfonate moiety of ASP3026 mimics the ribose of ATP to form van der Waals interactions with Val500, Glu501, and Gly556 of PfLysRS. h The isopropyl sulfonate moiety of NVP-TAE684 is located near the position of ADP β-phosphate and forms two H-bonds with Lys1150 of ALK. i In PfLysRS, the 4-(4-methylpiperazine) piperidine group protrudes from the pocket of PfLysRS and forms van der Waals interactions with Glu332, Asp335, and His338. j In ALK, the 4-(4-methylpiperazine) piperidine group sticks out of the pocket of ALK in a more extended conformation, and forms van der Waals interactions with Asp1203, Ser1206, and Glu1210.
First, the triazine (corresponds to pyrimidine in NVP-TAE684) appears to be essential for ASP3026 to interact with both proteins. It occupies the adenine site in the complex structure of both proteins (Fig. 1b–d). In PfLysRS, the triazine of ASP3026 replaces adenine of ATP to form stacking interaction with Phe342, and two nitrogen atoms of the azine and the imine forms two H-bonds with the main chain atoms of Asn339 (Fig. 1e). In ALK, the pyrimidine of NVP-TAE684 formed hydrophobic interactions with Leu1122, Ala1148, Leu1198, Met1199, and Leu1256, and two nitrogen atoms of the pyrimidine and the imine forms two H-bonds with the main chain atoms of Met1199 (Fig. 1f). Second, the isopropyl sulfonate moiety binds to the two proteins by mimicking different parts of ATP. In PfLysRS, it mimics the ribose of ATP to form van der Waals interactions with Val500, Glu501, and Gly556 (Fig. 1g). In ALK, it is located near the position of ADP β-phosphate and forms two H-bonds with Lys1150 (Fig. 1h). The 4-(4-methylpiperazine) piperidine group binds to the enzyme in two completely different conformations, using ATP as a reference (Fig. 1d). In PfLysRS, it protrudes from the pocket of PfLysRS and forms van der Waals interactions with Glu332, Asp335, and His338 (Fig. 1i). In ALK, this part of the compound also sticks out of the pocket of ALK, but in a more extended conformation, and forms van der Waals interactions with Asp1203, Ser1206, and Glu1210 (Fig. 1j).
With these analyses, we first optimized the 4-(4-methylpiperazine) piperidine group, which has the most distinct effect on the two proteins, then modified and optimized the triazine and sulfonyl aniline moieties.
Substitution of the 4-(4-methylpiperazinyl)piperidinyl moiety significantly improved the selectivity against PfLysRS in vitro
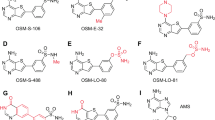
Compound 33–42 were synthesized by replacing of the original 2-methoxy-4-(4-methylpiperazinyl)piperidinyl aniline with the commercially available corresponding anilines (Supplementary Fig. 2, Supplementary Table 2). Compared with ASP3026, compound 38 has removed the 4-(4-methylpiperazine) piperidine, which is probably less useful for binding LysRS, and left the rest unchanged (Fig. 2a). In the thermal shift experiments, 38 increased the Tm value of PfLysRS by approximately 9.0 °C in the presence of l-lysine (Fig. 2b). This similar result to ASP3026 (Supplementary Table 2) indicates that the binding ability of the compound to PfLysRS remained unchanged after the removal of this group. Consistently, the IC50 of 38 for inhibiting PfLysRS activity was 910 nM (Fig. 2c), slightly higher than ASP3026 whose IC50 was 965 nM in this assay (Supplementary Fig. 1). We also performed surface plasmon resonance (SPR) experiments and sensorgram analysis for the interactions of 38 with immobilized PfLysRS resulted in a dissociation constant (Kd) of 62.8 nM (Supplementary Fig. 3a). It confirmed that the 4-(4-methylpiperazinyl)piperidinyl moiety is not essential for PfLysRS binding. However, the activity of compound 38 against ALK with the IC50 value of 532 nM (Fig. 2d) was significantly reduced compared with ASP302633,37. Compound 38 maintains a high species selectivity in vitro. Its selectivity of PfLysRS over human LysRS (HsLysRS) reached 328 times (Fig. 2c, e). Therefore, consistent with crystal structure analysis, this modification reduces the ability of the compound to inhibit ALK while maintaining its PfLysRS inhibitory activity.
a Chemical structure of compound 38. b Diagram of the Tms of PfLysRS in the presence of l-lysine and/or compound 38. Error bars represent standard deviations (SD) of four technical repeats. c The potency of compound 38 against PfLysRS is measured using the ATP hydrolysis assay. d The potency of compound 38 against ALK is measured using the ATP hydrolysis assay. e The potency of compound 38 against HsLysRS is measured using the ATP hydrolysis assay. Error bars in c–e represent SD of three technical repeats.
In comparison to compound 38, compound 36 has one more methoxy group substitution in the para position of the original 2-methoxy group (Fig. 3a). Compound 36 exhibited the highest PfLysRS binding affinity among these ASP3026 analogues (Supplementary Table 2). The ΔTm of PfLysRS induced by compound 36 was approximately 11.8 °C in the presence of l-lysine. It also improved the Tm of PfLysRS by 11.7 °C in the absence of l-lysine (Fig. 3b), indicating that the binding of compound 36 to PfLysRS is independent of the presence of l-lysine. This property is similar to ASP3026, but different from cladosporin41. The Kd value of compound 36 was 15.9 nM in SPR experiments (Supplementary Fig. 3b), indicating stronger binding affinity than compound 38. Compound 36 also showed strong inhibition of PfLysRS enzyme activity in the ATP hydrolysis assay, with an IC50 of 198 nM (Fig. 3c), which was also stronger than ASP3026 in vitro. The ALK inhibitory ability of 36 was significantly reduced, and the IC50 was greater than 19.1 μM (Fig. 3d). This result indicated that the increase of methoxide at this location may bring repulsion with the carboxyl group of Asp1203 (Fig. 1j), which is not favorable for the interaction of the compound and ALK. At the same time, the inhibition of the compound on HsLysRS was also significantly weakened (Fig. 3e).
a Chemical structure of compound 36. b Diagram of the Tms of PfLysRS in the presence of l-lysine and/or compound 36. Error bars represent SD of four technical repeats. c The potency of compound 36 against PfLysRS is measured using the ATP hydrolysis assay. d The potency of compound 36 against ALK is measured using the ATP hydrolysis assay. e The potency of compound 36 against HsLysRS is measured using the ATP hydrolysis assay. Error bars in c–e represent SD of three technical repeats.
Compared to compound 36, the methoxy group in the amino meta position of compound 34 is replaced by a methoxycarbonyl group, and the substitution position of methoxy group in compound 35 is different (Supplementary Fig. 4a). As a result, the ΔTm values of PfLysRS caused by compound 34 and 35 were both lower than that caused by compound 36 (Supplementary Fig. 4b, c), and the potency of compound 34 against PfLysRS was weaker than that of compound 36 (Supplementary Fig. 4d).
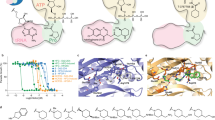
To understand how these compounds are recognized by PfLysRS, we determined the cocrystal structures of PfLysRS with the compounds 34, 35, 36, and 38 (Supplementary Table 1). Most of the interactions between the compounds and the protein were preserved compared to PfLysRS-ASP3026 (Figs. 1, 4). The methoxy group inherited from ASP3026 in these compounds were all bound to the hydrophobic cavity formed by Arg330, Glu332, His338, Asn339, and Pro340 like ASP3026. The additional methoxy group of compound 36 formed a new H-bond with Arg330 (Fig. 4a). Similarly, the carbonyl oxygen of compound 34 also formed a new H-bond with Arg330 (Fig. 4b). Without these substituent groups or with a different substitution position, compound 38 and 35 lacked this H-bond (Fig. 4c, d), consistent with their weaker activity compared to compound 36 (Figs. 2, 3, Supplementary Fig. 4, Supplementary Table 2). These compounds occupy a space in the ATP binding pocket of LysRS and overlap mostly with cladosporin (Supplementary Fig. 5). Most of the strongly interacting residues are conserved between PfLysRS and HsLysRS (Supplementary Fig. 5). It has been reported that three different residues (Thr307, Val328, and Ser344) and the unique skeleton dynamics make PfLysRS more sensitive to cladosporin than HsLysRS26,42. The selectivity of these Asp3026 derivatives to PfLysRS may be for the same reason.
a One methoxy group of compound 36 binds to the hydrophobic cavity formed by Arg330, Glu332, His338, Asn339, and Pro340, while the other methoxy group form an H-bond with Arg330. b Compound 34 also formed a new H-bond with Arg330. c Without the substituent groups, compound 38 lacked the H-bond with Arg330. d With a different substitution position, compound 35 lacked the H-bond with Arg330. Compound 34, 35, 36, 38, Arg330, Glu332, His338, Asn339, Pro340 and ASP3026 are depicted as sticks.
Replacement of aromatic ring did not show significant positive effects
Then we replaced the aromatic rings in the structure of the compound and evaluated their activity and selectivity. First, the methoxyaniline group of compound 38 were replaced with pyridine ring substrates to obtain compounds 38a and 38b, differing from compound 38 by only one atom (Fig. 5a). Surprisingly, there was a significant difference in the ΔTm value of PfLysRS between the two compounds, solely due to the different position of the N atom in the pyridine ring. The ΔTm of 38a was 7.9 °C while the ΔTm of 38b was only 2.0 °C (Fig. 5b, Supplementary Table 3). It indicates that compound 38a has a similar binding ability to PfLysRS as 38, while 38b can only weakly bind to PfLysRS. In the ATP hydrolysis assay, 38a showed slightly stronger inhibition of PfLysRS than 38, with an IC50 of 568 nM, while its inhibition ability to ALK was weakened compared to 38 (Fig. 5c, d). The IC50 of 38a against HsLysRS was 84 μM, indicating a significant enhancement in its inhibitory activity compared to 38 (Fig. 5e). The only structural difference between these two compounds is that the benzene ring in methoxyaniline block is replaced by pyridine ring. This change enhanced the HsLysRS inhibition activity of 38a to a certain extent. However, compound 38b, which also uses a pyridine ring instead of a benzene ring as a substrate, significantly decreased the ability to stabilize PfLysRS in the thermal shift assay. We performed DFT calculations for the local minimum energy conformations of compounds 38a and 38b based on the binding conformation of compound 38 in crystal structure to rationalize the activity difference (Supplementary Fig. 6a, b). The local minimum energy conformation of compound 38a is almost the same as the PfLysRS binding conformation. However, in the local minimum energy conformation of 38b (Supplementary Fig. 6b), since the C-H becomes N and reduces the repulsion with C-H in the other benzene ring, the three aromatic rings converge to the same plane due to conjugation. There is a significant difference between its minimum energy conformation and the protein binding conformation (ΔGpro-ΔGmin = 6.1 kcal/mol). Therefore, the minimum energy conformation of 38b is not conducive to binding PfLysRS.
a Chemical structures of compound 38a and 38b. b Diagram of the Tms of PfLysRS in the presence of l-lysine and compound 38a or 38b. Error bars represent SD of four technical repeats. c The potency of compound 38a against PfLysRS is measured using the ATP hydrolysis assay. d The potency of compound 38a against ALK is measured using the ATP hydrolysis assay. e The potency of compound 38a against HsLysRS is measured using the ATP hydrolysis assay. Error bars in c–e represent SD of three technical repeats.
To assess the impact of replacing the methoxy-linked benzene ring with pyridine, this modification of 38a was applied to 36, previously identified as having the highest PfLysRS inhibitory activity. Compound 36a was then synthesized (Supplementary Fig. 7a, Supplementary Fig. 2). Compound 36a increased the Tm value of PfLysRS by 10.7 °C in the presence of l-lysine (Supplementary Table 3, Supplementary Fig. 7b), which was comparable to that of 36. Compared to compound 36, the PfLysRS inhibitory activity of 36a was slightly decreased, but the HsLysRS inhibitory activity was significantly enhanced, with an IC50 of 44 μM (Supplementary Fig. 7c, e). Therefore, these results suggest that the replacement of the benzene ring with pyridine has little effect on the inhibitory activity of PfLysRS or ALK (Fig. 5d, Supplementary Fig. 7d), but significantly decreases the species selectivity of the compounds (Fig. 5e, Supplementary Fig. 7e).
Subsequently, attempts were made to replace the triazine in ASP3026 structure with other aromatic rings. We previously found that NVP-TAE684, whose triazine part is replaced, showed a significant reduction in LysRS binding33. In this study, compounds 36b–f were synthesized using Buchwald–Hartwig reaction (Supplementary Table 4). Surprisingly, these modifications almost completely abolished the binding affinity of the compounds to PfLysRS (Supplementary Table 4). Based on the crystal structure, these changes may weaken or even lose hydrogen bonding interactions with Asn339 residues and stacking interactions with Phe342 residues. Some changes such as changes in 36e will also increase the repulsion between aromatic rings (Supplementary Fig. 6c), affect the conformation between the three aromatic rings, resulting in the reduced binding. Thus, the chemical synthesis and biochemical results confirmed that the triazine part is essential for this series of compounds to bind LysRS.
Substitution of isopropylsulfonyl moiety to create synergistic binding
In the thermal shift assays, we did not observe a significant synergistic effect between l-lysine and ASP3026 or ASP3026 analogues (Figs. 2b, 3b and 5b)33. The previously reported synergistic effect between cladosporin and l-lysine might depend not only on the co-occupation of ATP and l-lysine pockets (Supplementary Fig. 8a), but also on the van der Waals interaction between the inhibitor and l-lysine41,43. Therefore, a potential strategy to enhance the effectiveness of ASP3026 against PfLysRS is to replace the isopropyl sulfonyl part with a larger moiety to mimic the van der Waals interaction between cladosporin and l-lysine, enabling the new compound to bind PfLysRS synergistically with l-lysine (Supplementary Fig. 8b). We synthesized compound 32 by replacing the isopropylsulfonyl group with a cyclohexyl group. The o-cyclohexylaniline substrate was prepared with a Cope rearrangement and hydrogenation reaction after a simple amination. Similar to the synthesis of ASP302637, the o-cyclohexylaniline reacted with 2,4-dichloro-1,3,5-triazine using N,N-diisopropylethylamine in tetrahydrofuran. The aniline derivative was then introduced using methanesulfonic acid in ethanol to yield compound 32 (Supplementary Fig. 8c, Supplementary Fig. 9). Meanwhile, taking the intermediate compound 4 as a substrate, we obtained another analogue compound 31 (Supplementary Fig. 8c, Supplementary Fig. 9).
It turned out that the substitution of an isopropylsulfonyl group for a cyclohexyl group in compound 32 or a cyclohex-2-en-1-yl group in compound 31 was not able to improve the binding of the compounds to PfLysRS. Compounds 31 and 32 increased the Tm of PfLysRS by only 2.0 °C and 4.1 °C, respectively, in the presence of l-lysine (Supplementary Fig. 8d). These results suggest that the cyclohexyl or cyclohexene segments in compounds 31 and 32 may be too large. Therefore, we considered to covalently link l-lysine to ASP3026 analogues instead of creating synergic binding between l-lysine and the inhibitors.
Covalently linking l-lysine to the inhibitor can enhance its inhibition of PfLysRS enzymatic activity
One significant distinction between the binding pockets of LysRS and ALK is that the LysRS’s active pocket includes an l-lysine binding site adjacent to the ATP binding site. Therefore, by covalently linking l-lysine to ASP3026 analogues, it is possible to obtain compounds with enhanced LysRS inhibitory activity and reduced ALK inhibitory activity. Considering that 36 has a relatively high PfLysRS inhibitory activity, l-lysine is connected to the sulfonyl group of 36 through varying lengths of carbon chains. The compounds 36K2–36K5 with a connection chain length of 2 to 5 carbons or a four-membered ring was synthesized using Buchwald–Hartwig reaction, followed by hydrogenation to remove the Cbz protection (Fig. 6a, Supplementary Fig. 10).
a Chemical structures of compound 36K2–36K5. b Diagram of the Tms of PfLysRS in the presence of l-lysine and compounds 36K2–36K5. Error bars represent SD of four technical repeats. c The potency of compound 36K3 against PfLysRS is measured using the ATP hydrolysis assay. Error bars represent SD of three technical repeats. d Cocrystal structures of PfLysRS with the compound 36K3 aligned with 36 and l-lysine. Compound 36, 36K3 and l-lysine are depicted as sticks.
As expected, the ability of the compounds to bind PfLysRS was significantly enhanced with covalently linked l-lysine. In the thermal shift assay, the ΔTm values of four new compounds were higher than that of 36, especially 36K3 and 36K4 with an alkyl chain of 3 or 4 carbons (Fig. 6b). Their ΔTm values were 12.8 °C and 12.9 °C, respectively, comparable to cladosporin41. The Kd value of 36K3 reached 12.4 nM (Supplementary Fig. 11). Meanwhile, the selectivity of these compounds for LysRS and ALK inhibition was further enhanced by l-lysine connection (Supplementary Table 5). Compounds 36K2–36K4 and 36K4’ showed strong inhibition on the activity of PfLysRS. In particular, the IC50 of 36K3 against PfLysRS inhibition reached 59.2 nM (Fig. 6c), displaying an apparent selectivity index of 1250 for ALK and 200 for HsLysRS (Supplementary Table 5).
We also determined the mode of action of 36K3 through crystal structure (Supplementary Table 1). Consistent with our design, compound 36K3 occupies both the ATP-binding site and l-lysine binding site of PfLysRS. The conformation of 36K3 at the ATP binding site is similar to that of 36, and its l-lysine part generally matches the l-lysine binding mode. Due to its covalent connection to compound 36, the l-lysine moiety is unable to adjust its conformation freely, so it is pulled out of its original binding site a little bit (Fig. 6d). Therefore, further enhancement of the compound’s activity is possible through a better linkage between 36 and l-lysine.
Some ASP3026 analogues improve the inhibition against the blood stage of P. falciparum
ASP3026 inhibited the ATP-hydrolysis activity of PfLysRS at the submicromolar level and consistently suppressed the growth of erythrocytic-stage P. falciparum 3D7 parasites with an EC50 of 5.61 μM33. To determine the inhibitory effect of ASP3026 analogues on Plasmodium growth, we further evaluated the inhibitory potency of these compounds on P. falciparum strain 3D7 in the erythrocytic-stage (Fig. 7a, b, and Supplementary Fig. 12). Among these compounds, compounds 34, 36 and 38 demonstrated greater inhibitory activity on parasite growth compared to ASP3026 (Fig. 7c–e). Compound 36, in particular, exhibited the most potent inhibition with an EC50 value of 736 nM, surpassing the potency of ASP3026 by over sevenfold (Fig. 7d). Meanwhile, compounds 36a and 38a have weaker inhibitory effects on the growth of Plasmodium falciparum compared to counterparts compounds 36 and 38 (Fig. 7f, Supplementary Fig. 12d, e). It indicates that replacing the benzene ring with the pyridine ring is not conducive to the inhibition of compound growth on P. falciparum. Furthermore, compounds 36K3, 36K4 and 36K4’ showed weaker inhibitory activity in this experiment (Supplementary Fig. 12f, g, h), which was much different from the results of enzymatic activity inhibition experiment.
a Chemical structures of compounds 34, 36, 38 and 36a. b Schematic diagrams of erythrocytic-stage parasites (strain 3D7) growth inhibition experiment. The parasites were allowed to grow with the incubation of DMSO or different concentrations of ASP3026 derived compounds for 72 h. c The potency of compound 34 against the growth of erythrocytic-stage P. falciparum 3D7 parasites. d The potency of compound 36 against the growth of erythrocytic-stage P. falciparum 3D7 parasites. e The potency of compound 38 against the growth of erythrocytic-stage P. falciparum 3D7 parasites. f The potency of compound 36a against the growth of erythrocytic-stage P. falciparum 3D7 parasites. Error bars in c–f represent SD of two or three technical repeats.
We estimated the cytotoxicity of the compounds 34, 36 38, 38a, 36a and 36K3 in human hepatocyte carcinoma HepG2 cells (Supplementary Fig. 13). Compound 38 exhibits an CC50 of 7.7 μM (Supplementary Fig. 13c), which may due to its inhibition of ALK (IC50 = 532 nM). Consistent with the weak inhibition of compound 36 on ALK and HsLysRS, it was weakly toxic and showed no significant toxicity to HepG2 (Supplementary Fig. 13b). The cytotoxicity of 34, 38a, 36a, and 36K3 was lower than that of compound 38 but higher than that of compound 36, which may be related to their mild inhibition of ALK or HsLysRS (Supplementary Fig. 13d, e). Together with the cellular antiparasitic activity, 36 is the most promising lead compound.
The conjugation of l-lysine to a compound elevates its molecular weight and enhances its polarity, potentially impeding its transmembrane permeability. The increased competition of endogenous l-lysine may also be the reason for the decreased inhibition of 36K3 on P. falciparum growth. Other strategies using the l-lysine binding sites need to be further developed. Together, the modifications of compound ASP3026 have led to the development of new compounds with improved inhibitory effects against P. falciparum.
Discussion
In this study, we designed and synthesized a series of ASP3026 derivatives. Among them, compound 36 demonstrated over sevenfold enhanced activity in inhibiting Plasmodium growth compared to ASP3026, suggesting a potential advancement in antiplasmodial efficacy through structural optimization.
The binding of amino acids to their corresponding subpockets is the most distinctive feature of the aaRSs family, and the utilization of this pocket will significantly enhance the binding affinity and selectivity of lead drug molecules. For instance, mupirocin occupies the Ile binding pocket of IleRS4, while halofuginone binds to the Pro binding pocket in ProRS8. Recently reported adenosine amino acid compounds also occupy amino acid binding sites through a reactive hijacking mechanism and amino acid formation conjugates44. Obafluorin uses o-diphenol to simulate the side hydroxyl group and main amino group of threonine to bind to a conserved zinc ion at the threonine binding site45. In this work, the enzyme inhibitory activity of l-lysine linked compound 36K3 was increased by 10 times and the selectivity was increased to 1250:1, indicating the value of this site in LysRS inhibitor development.
However, stronger in vitro PfLysRS inhibition of compound 36K2-36K5 did not translate into better inhibition of parasite growth. There could be several reasons for this. First, previous studies have shown that the addition of an amino acid moiety has adverse effects on the membrane permeability of inhibitors46. The CLogP value decreased from 3.39 to 1.77 and the molecular weight increased from 429 to 572 after l-lysine was linked to compound 36. Reducing CLogP on the one hand may help to improve solubility and bioavailability, but on the other hand may also result in a reduced ability to penetrate the cell membrane. Therefore, in future work, it will be valuable to modify the amino acid part to improve bioavailability, such as modifications in the form of amino protected prodrugs. Second, as two-site inhibitors that binds to ATP and amino acid sites, these compounds have to compete with both endogenous ATP and lysine. The binding benefit of introducing the native lysine moiety may be not sufficient to offset the disadvantage of endogenous lysine competition. The use of amino acid binding sites may require the introduction of pharmacophore that binds PfLysRS at least more strongly than lysine. Third, it has been reported that there are some important kinases in P. falciparum that can be used as targets for antimalarial drugs47. Although the receptor tyrosine kinase family to which ALK belongs does not exist in malaria, we cannot grossly rule out the possibility that well-active molecules such as 36 also inhibit the function of other P. falciparum kinases or even other ATP-binding proteins. In this sense, developing molecules that inhibit both P. falciparum aaRS and P. falciparum kinases may have advantages over molecules such as 36K3 that are highly selective to aaRS alone.
Both aaRSs and kinases are important drug targets, and both use ATP as a substrate. Kinase inhibitors have been intensely developed and derivatized in the last decades to potently inhibit kinase targets48,49. This work provides a new example of kinase inhibitor repurposing to inhibit a non-kinase target, in this case aaRS, which could help to promote the development of new aaRS inhibitors.
Methods
Compound synthesis
All reagents were purchased from commercial sources, and were used without further purification. Reaction products were purified by normal phase silica-gel flash column chromatography (300–400 mesh). Structure characterization was based on NMR spectroscopy, recorded on a 500 MHz Agilent system, a Bruker 400 MHz or a Bruker 300 MHz, as well as mass spectrometry, measured using maXis 4 G or Shimadzu LCMS-2010EV. Spectroscopic data was analyzed using MestReNova 11.0.1 (Mestrelab Research). The synthesis paths are shown in Supplementary Figs. 2, 9 and 10. The detailed methods for synthesis of the compounds are included in Supplementary Note 1.
Protein expression
PfLysRS (77–583) was constructed in the vector pET20b, with a C-terminal 6×His tag. The protein was expressed in BL21 (DE3) strain with 0.2 mM IPTG for 20 h at 16 °C. The cell pellet (from 4 to 8 L) was lysed in the wash buffer containing 500 mM NaCl, 20 mM Tris pH 8.0, and 15 mM imidazole, loaded onto a Ni-HiTrap column and washed with wash buffer. The protein was eluted with the elution buffer containing 500 mM NaCl, 20 mM Tris pH 8.0, and 250 mM imidazole. Then the protein was concentrated and further purified by gel filtration Superdex 200 column with the running buffer containing 20 mM Tris pH 8.0 and 150 mM NaCl. The peak fractions were then concentrated for activity assays or crystallization experiments. HsLysRS (70–581) was constructed in the vector pET20b with a C-terminal 6×His tag, and purified similarly.
The plasmid pCDNA3.1_8×His-tev-ALK (kinase domain, 1068–1410) was constructed50 and extracted from E. coli DH5α (DE3) strain by alkaline solution method. The plasmid was transiently transfected into 3 L Expi293 cells at a density of 3 × 106 cells/mL using transfection reagent PEI (25,000 Da). The cells were collected by centrifugation on the 5th day after transfection, and lysed at 600 bar at 4 °C. The centrifugation supernatant of cell lysate was loaded to a pre-balanced Ni-NTA column and washed with 100 mL solution containing 25 mM Tris pH 8.0, 150 mM NaCl, and 25 mM imidazole. The ALK protein was then eluted with 40 mL solution containing 25 mM Tris pH 8.0, 150 mM NaCl, and 250 mM imidazole. The eluent was concentrated to 500 μL and further purified by a Superdex 200 column with the running buffer containing 25 mM Hepes pH 7.4 and 250 mM NaCl. The peak fractions were used for enzymatic assays.
Thermal shift assay
PfLysRS protein was prepared at 10 μM concentration in a buffer containing 20 mM Tris pH 8.0, 200 mM NaCl, 500 μM l-lysine, and 200 μM Compound. Aliquots (18 µL) were added to a 96-well PCR plate and incubated at ambient temperature for 10 min. SYPRO Orange dye (Sigma) was diluted in the assay buffer containing 20 mM Tris pH 8.0 and 200 mM NaCl to a 40× concentration, and 2 µL of the 40× dye solution was added to the PCR plate to bring the final assay volume to 20 µL. After complete mixing, the final solutions were heated from 25 to 90 °C at a rate of 0.015 °C per sec, and fluorescence signals were monitored by QuantStudio 3 (Applied Biosystems by Thermo Fisher Scientific).
Surface Plasmon Resonance (SPR) assay
PfLysRS was chemically immobilized on a Biacore CM5 sensorchip (immobilization level ~20000 resonance unit/RU) at pH 5.0 according to the immobilization kit (GE Healthcare). PfLysRS (1.7 nM) was captured for 90 s at a flow of 30 µL·min−1. Affnity between PfLysRS and compounds was measured by SPR on a Biacore 8 K (GE Healthcare) at 25 °C with a running buffer (0.02 M phosphate buffer pH 7.4, 2.7 mM KCl, 0.137 M NaCl, 0.05% Tween 20, with 1 mM l-lysine). The association time was 90 s, the dissociation time was 120 s, and the flowrate was 30 µL·min−1. Kinetic evaluation of the interaction between LysRS and compounds was performed by global fitting of the data to a 1:1 interaction model using Biacore Evaluation Software 3.1 (GE Healthcare).
ATP hydrolysis assays
The ATP hydrolysis assays were based on Kinase-Glo luminescent Kit (Promega). The experiment was carried out in Corning 384-well white flat bottom microplates. The buffer in the reaction contains 25 mM Hepes pH 7.4, 140 mM NaCl, 40 mM MgCl2, 30 mM KCl, 1 mM TCEP, 0.1 mg·mL−1 BSA, and 0.004% Tween-20. The compounds were diluted to different concentrations with a 5-fold gradient.
For LysRS assays, DMSO was added into negative control wells and l-lysine was removed in positive control wells. 100 nM LysRS in 2.5 μL Hepes buffer was mixed with compounds and incubated for 10 min. Then 5 μL substrate mixture (2 μM ATP, 10 μM l-lysine and 31 nM pyrophosphatase) was added to start the reaction at 37 °C and incubated for 6 h.
For ALK assays, DMSO was added into negative control wells. 100 nM ALK in 2.5 μL Hepes buffer was mixed with compounds and incubated for 10 min. Then 5 μL substrate (2 μM ATP) was added to start the reaction at 37 °C and incubated for 6 h.
When the reaction reaches the planned time, 10 μL diluted Kinase-Glo reagent (1/50 diluted with a buffer containing 50 mM Tris pH 7.5 and 5% glycerol) was added and incubated for 15 min. Luminescence was measured on the Magellan plate reader (Tecan) and nonlinear regression was performed with Prism (GraphPad).
In vitro Plasmodium growth assay
Parasites were cultured in human O+ erythrocytes according to standard procedures. To prepare the >80% ring stage parasites, asynchronous cultures of parasites were pretreated with 5% sorbitol, and P. falciparum strain 3D7 at the mid-ring stage (6–10 h post-invasion) was used to test antimalarial effects in 96-well plates. Parasites were incubated in a 96-well plate with compound containing 1% parasitemia and, 2% hematocrit for a total volume of 200 μL. The compounds were diluted from the maximum concentration of 10 μM with a 2-fold gradient dilution. A relevant DMSO concentration was used as a negative control, and cultured erythrocytes without Plasmodium served as a positive control. The parasites were allowed to grow for 72 h at 37 °C with 5% CO2, 5% O2, and 90% N2. After 72 h, 100 μL of lysis buffer (0.12 mg·mL−1 Saponin, 0.12% Triton X-100, 30 mM Tris-HCl pH 8.0, and 7.5 mM EDTA) with 5× SYBR Green I (Invitrogen; supplied in 10000× concentration) was added to each well of the plate. The plates were then incubated for 2 h in the dark prior to reading the fluorescence signal at 485 nm excitation and 535 nm emission. The percentage of inhibition was calculated by (NC-fluorescence) × 100/(NC-PC).
Cytotoxicity assay
HepG2 cells (National Collection of Authenticated Cell Cultures) were cultured in Minimum Essential Medium (Gibco) supplemented with 10% of fetal bovine serum, 100 U·mL−1 penicillin, 100 U·mL−1 streptomycin (Gibco), 2 mM l-Glutamine (Gibco), 1× NEAA (Gibco) and 1 mM Sodium Pyruvate (Gibco). The cells were hemi-depleted each week with fresh medium and maintained at 1 ~ 2 × 106 cells/mL in 6-cm dishes at 37 °C and 5% CO2. Tested with MycoBlue Mycoplasma Detector D101 (Vazyme), the cells were free from mycoplasma contamination.
Cell viability was analyzed by Cell Counting Kit-8 (CCK8, ApexBio) according to the manufacturer’s protocols. Cells were seeded and cultured at a density of 2.5 × 103 cells per well in 100 μL medium in 96-well microplates (Corning). Then, the cells were treated with different concentrations of compound (0, 1 μM, 3 μM, 10 μM, 100 μM, 300 μM). For 36, 38 and 36K3, the highest concentration was 100 μM due to poor solubility. After treatment for 72 h, 10 μL of CCK-8 reagent was added to each well and then cultured for 4 h. All experiments were performed in pentaplicate. The absorbance was analyzed at 450 nm using a microplate reader (Tecan) using medium without cells as blanks. Data was processed using GraphPad Prism 9.
Protein crystallization
All crystallizations were done by the sitting drop method. PfLysRS protein (30 mg·mL−1) was premixed with 1 mM of compound and 1 mM of l-lysine at 4 °C and was crystallized by mixing 0.4 μL of protein solution with 0.4 μL of precipitant solution, containing 0.1 M MES pH 6.0, and 15% PEG4000. After incubation at 18 °C for 3 to 7 days, crystals were flash-frozen in liquid nitrogen for data collection with the cryo solution containing 0.08 MES pH 6.0, 12% PEG4000, 20% glycerol, and 1 mM compound.
Structure determination
The PfLysRS-compound complex crystal diffraction datasets were obtained from beamlines BL02U1 and BL19U1 at Shanghai Synchrotron Radiation Facility (SSRF)51. All datasets were processed with Xia252. The structures were solved by molecular replacement using PfLysRS structure (PDB code: 7BT5) with the program Molrep33,53. Iterative model building and refinement were performed using Coot and Phenix54,55. Data collection and refinement statistics are given in Supplementary Table 1.
Statistics and reproducibility
Thermal shift measurements were conducted in four repeats. Enzymatic assay and Plasmodium growth assay were conducted in three repeats. Cytotoxicity assay were conducted in five repeats. Acquired data are presented as the mean values ± standard deviation (SD).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
Reygaert, W. C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 4, 482–501 (2018).
Ibba, M. & Söll, D. J. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69, 617–650 (2000). A. R. o. B.
Carter, C. W. Jr Cognition, mechanism, and evolutionary relationships in aminoacyl-tRNA synthetases. Annu. Rev. Biochem. 62, 715–748 (1993).
Silvian, L. F., Wang, J. & Steitz, T. A. Insights into editing from an ile-tRNA synthetase structure with tRNAile and mupirocin. Science 285, 1074 (1999).
Rock, F. L. et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science 316, 1759–1761 (2007).
Sundrud, M. S. et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science 324, 1334–1338 (2009).
Gadakh, B. & Van Aerschot, A. Aminoacyl-tRNA synthetase inhibitors as antimicrobial agents: a patent review from 2006 till present. Expert Opin. Ther. Pat. 22, 1453–1465 (2012).
Zhou, H., Sun, L., Yang, X. L. & Schimmel, P. ATP-directed capture of bioactive herbal-based medicine on human tRNA synthetase. Nature 494, 121–124 (2013).
Kim, D. G. et al. Chemical inhibition of prometastatic lysyl-tRNA synthetase-laminin receptor interaction. Nat. Chem. Biol. 10, 29–34 (2014).
Kwon, N. H., Fox, P. L. & Kim, S. Aminoacyl-tRNA synthetases as therapeutic targets. Nat. Rev. Drug Discov. 18, 629–650 (2019).
Fang, P. & Guo, M. Evolutionary limitation and opportunities for developing tRNA synthetase inhibitors with 5-binding-mode classification. Life 5, 1703–1725 (2015).
Hughes, J. & Mellows, G. Interaction of pseudomonic acid A with Escherichia coli B isoleucyl-tRNA synthetase. Biochem. J. 191, 209–219 (1980).
Hughes, J. & Mellows, G. On the mode of action of pseudomonic acid: inhibition of protein synthesis in Staphylococcus aureus. J. Antibiot. 31, 330–335 (1978).
Sutherland, R. et al. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob. Agents Chemother. 27, 495–498 (1985).
Pines, M. & Spector, I. Halofuginone - the multifaceted molecule. Molecules (Basel, Switz.) 20, 573–594 (2015).
Derbyshire, E. R., Mazitschek, R. & Clardy, J. Characterization of Plasmodium liver stage inhibition by halofuginone. ChemMedChem 7, 844–849 (2012).
Jain, V. et al. Structure of Prolyl-tRNA synthetase-halofuginone complex provides basis for development of drugs against malaria and toxoplasmosis. Structure (Lond., Engl. 1993) 23, 819–829 (2015).
Elkin, M. et al. Inhibition of bladder carcinoma angiogenesis, stromal support, and tumor growth by halofuginone. Cancer Res. 59, 4111–4118 (1999).
Jin, M. L., Park, S. Y., Kim, Y. H., Park, G. & Lee, S. J. Halofuginone induces the apoptosis of breast cancer cells and inhibits migration via downregulation of matrix metalloproteinase-9. Int. J. Oncol. 44, 309–318 (2014).
Demiroglu-Zergeroglu, A. et al. Anticarcinogenic effects of halofuginone on lung-derived cancer cells. Cell Biol. Int. 44, 1934–1944 (2020).
Wang, C. et al. Halofuginone inhibits tumorigenic progression of 5-FU-resistant human colorectal cancer HCT-15/FU cells by targeting miR-132-3p in vitro. Oncol. Lett. 20, 385 (2020).
Chen, Y. et al. A high-throughput screen for TMPRSS2 expression identifies FDA-approved compounds that can limit SARS-CoV-2 entry. Nat. Commun. 12, 3907 (2021).
Balboa, S., Hu, Y., Dean, F. B. & Bullard, J. M. Lysyl-tRNA synthetase from pseudomonas aeruginosa: characterization and identification of inhibitory compounds. SLAS Discov. 25, 57–69 (2020).
Green, S. R. et al. Lysyl-tRNA synthetase, a target for urgently needed M. tuberculosis drugs. Nat. Commun. 13, 5992 (2022).
Baragaña, B. et al. Lysyl-tRNA synthetase as a drug target in malaria and cryptosporidiosis. Proc. Natl Acad. Sci. USA 116, 7015–7020 (2019).
Hoepfner, D. et al. Selective and specific inhibition of the plasmodium falciparum lysyl-tRNA synthetase by the fungal secondary metabolite cladosporin. Cell Host Microbe 11, 654–663 (2012).
Das, P. et al. Specific stereoisomeric conformations determine the drug potency of cladosporin scaffold against malarial parasite. J. Med. Chem. 61, 5664–5678 (2018).
Rusch, M. et al. Design and synthesis of metabolically stable tRNA synthetase inhibitors derived from cladosporin. Chembiochem 20, 644–649 (2019).
Fredenhagen, A. et al. Cladosporin derivatives obtained by biotransformation provide guidance for the focused derivatization of this antimalarial lead compound. Chembiochem 20, 650–654 (2019).
Babbar, P. et al. Design, synthesis, and structural analysis of cladosporin-based inhibitors of malaria parasites. ACS Infect. Dis. 7, 1777–1794 (2021).
Babbar, P. et al. Inhibition of plasmodium falciparum Lysyl-tRNA synthetase via a piperidine-ring scaffold inspired cladosporin analogues. Chembiochem 22, 2468–2477 (2021).
Li, T. et al. First-in-human, open-label dose-escalation and dose-expansion study of the safety, pharmacokinetics, and antitumor effects of an oral ALK inhibitor ASP3026 in patients with advanced solid tumors. J. Hematol. Oncol. 9, 23 (2016).
Zhou, J. et al. Inhibition of Plasmodium falciparum Lysyl-tRNA synthetase via an anaplastic lymphoma kinase inhibitor. Nucleic acids Res. 48, 11566–11576 (2020).
Morris, S. W. et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 263, 1281–1284 (1994).
Orthofer, M. et al. Identification of ALK in Thinness. Cell 181, 1246–1262.e1222 (2020).
Reshetnyak, A. V. et al. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature 600, 153–157 (2021).
Iikubo, K. et al. Discovery of N-{2-Methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]phenyl}-N’-[2-(propane-2-sulfonyl)phenyl]-1,3,5-triazine-2,4-diamine (ASP3026), a potent and selective anaplastic lymphoma kinase (ALK) inhibitor. Chem. Pharm. Bull. 66, 251–262 (2018).
Cai, Z. et al. Design, synthesis, and proof-of-concept of triple-site inhibitors against Aminoacyl-tRNA synthetases. J. Med Chem. 65, 5800–5820 (2022).
Galkin, A. V. et al. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proc. Natl. Acad. Sci. USA 104, 270–275 (2007).
Bossi, R. T. et al. Crystal structures of anaplastic lymphoma kinase in complex with ATP competitive inhibitors. Biochemistry 49, 6813–6825 (2010).
Fang, P. et al. Structural basis for specific inhibition of tRNA synthetase by an ATP competitive inhibitor. Chem. Biol. 22, 734–744 (2015).
Chhibber-Goel, J. & Sharma, A. Side chain rotameric changes and backbone dynamics enable specific cladosporin binding in Plasmodium falciparum lysyl-tRNA synthetase. Proteins 87, 730–737 (2019).
Khan, S., Sharma, A., Belrhali, H., Yogavel, M. & Sharma, A. Structural basis of malaria parasite lysyl-tRNA synthetase inhibition by cladosporin. J. Struct. Funct. Genomics 15, 63–71 (2014).
Xie, S. C. et al. Reaction hijacking of tyrosine tRNA synthetase as a new whole-of-life-cycle antimalarial strategy. Science 376, 1074–1079 (2022).
Qiao, H. et al. Tyrosine-targeted covalent inhibition of a tRNA synthetase aided by zinc ion. Commun. Biol. 6, 107 (2023).
Forrest, A. K. et al. Aminoalkyl adenylate and aminoacyl sulfamate intermediate analogues differing greatly in affinity for their cognate Staphylococcus aureus aminoacyl tRNA synthetases. Bioorg. Med Chem. Lett. 10, 1871–1874 (2000).
Arendse, L. B., Wyllie, S., Chibale, K. & Gilbert, I. H. Plasmodium kinases as potential drug targets for malaria: challenges and opportunities. ACS Infect. Dis. 7, 518–534 (2021).
Wu, P., Nielsen, T. E. & Clausen, M. H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol. Sci. 36, 422–439 (2015).
Roskoski, R. Jr Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 144, 19–50 (2019).
Lee, C. C. et al. Crystal structure of the ALK (anaplastic lymphoma kinase) catalytic domain. Biochem. J. 430, 425–437 (2010).
Wang, Q. S. et al. Upgrade of macromolecular crystallography beamline BL17U1 at SSRF. Nucl. Sci. Tech. 29, 68 (2018).
Winter, G., Lobley, C. M. & Prince, S. M. Decision making in xia2. Acta Crystallogr. D Biol. Crystallogr. 69, 1260–1273 (2013).
Vagin, A. & Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 66, 22–25 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr D Biol. Crystallogr 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Acknowledgements
We gratefully acknowledge the help from the staff of beamlines BL02U1 and BL19U1 at Shanghai Synchrotron Radiation Facility. This work is supported by the National Key Research and Development Program of China grant 2022YFC2303100, National Natural Science Foundation of China grants 22277132, 22277134, 21977107, 21977108, and 21977115, Shanghai Science and Technology Committee grant 22ZR1475000, Dr. Deli Lin (SJTU) and Dr. Jingli Hou (SJTU) for assistance in SPR testing, and the State Key Laboratory of Chemical Biology.
Author information
Authors and Affiliations
Contributions
Conceptualization: J.Z. and P.F. Methodology: J.Z., M.X., Z.H., H.Q., G.Y., Y.Q., P.L., Z.Z., J.W., W.L., and P.F. Investigation: J.Z., M.X., Z.H., H.Q., G.Y., Y.Q., P.L., X.G., L.J., J.W., W.L., and P.F. Visualization: J.Z., M.X., and P.F. Funding acquisition: L.J., J.W., W.L., and P.F. Project administration: L.J., J.W., W.L., and P.F. Supervision: L.J., J.W., W.L., and P.F. Writing – original draft: J.Z. and P.F. Writing – review & editing: J.W., W.L., and P.F.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Lianyun Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Joanna Timmins and Joao Valente. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, J., Xia, M., Huang, Z. et al. Structure-guided conversion from an anaplastic lymphoma kinase inhibitor into Plasmodium lysyl-tRNA synthetase selective inhibitors. Commun Biol 7, 742 (2024). https://doi.org/10.1038/s42003-024-06455-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06455-4