Abstract
Vibrio species are recognized for their role in food- and water-borne diseases in humans, fish, and aquatic invertebrates. We screened bacterial strains isolated from raw food shrimp for those that are bactericidal to Vibrio strains. Here we identify and characterize Aeromonas dhakensis strain A603 which shows robust bactericidal activity specifically towards Vibrio and related taxa but less potency toward other Gram-negative species. Using the A603 genome and genetic analysis, we show that two antibacterial mechanisms account for its vibriocidal activity -- a highly potent Type Six Secretion System (T6SS) and biosynthesis of a vibriocidal phenazine-like small molecule, named here as Ad-Phen. Further analysis indicates coregulation between Ad-Phen and a pore-forming T6SS effector TseC, which potentiates V. cholerae to killing by Ad-Phen.
Similar content being viewed by others
Introduction
The type VI secretion system (T6SS) is conserved across many Gram-negative bacteria and often confers competitive fitness via effector protein-based antagonism in bacterial communities1,2. The T6SS apparatus is a nanomachine assembled by proteins in the cytoplasm and membrane to secrete effector proteins into other bacterial or eukaryotic cells in a contact-dependent manner3,4,5,6,7. The T6SS assembled complex resembles a long tube composed of sheath proteins that is filled with thousands of copies of hemolysin co-regulated protein TssD (commonly named Hcp)8. This tube is attached to a membrane-associated baseplate anchor and capped with a trimeric spike composed of TssI (commonly named VgrG) proteins. Several distinct effectors assemble into the tip of the T6SS apparatus by association with TssI and other chaperones1,9,10. Once assembled, rapid conformational changes in the sheath protein interactions propel contraction of the entire tube11, driving the translocation of TssI and effector proteins into other cells or the extracellular environment12.
Because T6SS structural proteins in bacteria are highly conserved, genetic islands with genes necessary to encode the T6SS nanomachine and effector proteins are easily identified13. Genes that encode secreted effector proteins are often predictable by their genetic association or fusion with TssD and TssI genes and by conserved motifs14,15,16. Combinations of distinct effector molecules are secreted into a cell during one ejection event and these effectors may act synergistically14. Effectors often target the critical bacterial elements including cell wall components such as the murein (peptidoglycan) sacculus, membrane lipids, DNA and RNA, and other essential cytoplasmic molecules2. Bacteria that possess T6SS systems must also encode cognate immunity proteins to protect both themselves and sister cells from such effector-mediated damage17,18.
Beyond effector immunity, mechanical blockage, and the ability of exogenous attack to trigger T6SS counterattacks, little is known about how or if prey range is demonstrated by T6SS that targets Gram-negative bacteria. Furthermore, if some T6SS effectors work synergistically to damage prey cells, it is conceivable that small molecules might enhance the bactericidal activity of T6SS; to our knowledge, no small exogenous toxic molecules that potentiate T6SS killing of prey cells have been previously reported.
Here we report the characterization of an Aeromonas dhakensis strain (A603), an aquatic isolate from live shrimp that efficiently kills diverse Vibrio strains. Genetic analysis established that a secreted phenazine-like molecule and a T6SS account for the potent contact-dependent vibriocidal activity that A603 displays. We provide genetic evidence for synergy between T6SS and a phenazine biosynthetic operon that establishes prey specificity for Vibrio isolates, including significant threats to humans (e.g., V. cholerae) and aquaculture (e.g., V. parahaemolyticus). Phenazine molecules, including those similar to the one predicted to be synthesized in A603, are well-recognized for their broad range antibiotic activity though multiple mechanisms of action19,20,21,22. Thus, A603 may be used to control environmental contamination by pathogenic Vibrio species.
Results
Isolation of A603 and characterization of in vitro antibacterial activity
Using a panel of bacteria isolated from fresh white-legged table shrimp (Litopenaeus vannamei) we employed a contact-dependent killing assay to screen for strains that killed V. cholerae bacteria. We reasoned this screen would identify operative T6SS and other contact-dependent antibacterial mechanisms in environmental strains. One isolate we named A603 was the most vibriocidal of the strains tested. When V. cholerae prey bacteria are co-incubated with a comparable number of A603 predatory bacteria for 90 min on an agar medium, the number of colony-forming units (CFU) of V. cholerae is reduced >10-million-fold. Conversely, other Gram-negative bacterial strains previously demonstrated to be sensitive to other T6SS+ species were more resistant to A603 killing6,23. Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baylii CFU were only reduced 100–1000-fold within 90 min when co-incubated with A603 on agar surfaces (Fig. 1a).
Recovery of indicated prey bacteria following co-incubation with A603 and A603 T6SS/Ad-Phen mutants after 90 min (a) and with other isolates collected from shrimp (b). Killing by A603ΔtssBΔehpO was not significantly different to “no co-incubation” controls for any prey species (data not shown). All statistical comparisons are one-way ANOVA comparisons made to no co-incubation samples (*P < 0.05, **P < 0.01, *** P < 0.001, **** P < 0.0001, ns P > 0.05). All samples are n = 3 and individual values are plotted.
We sequenced the 4.8 megabase genome of A603 and analyzed its 4827 predicted genes to characterize the strain and identify potential antagonistic mechanisms (Supplemental Data S1). When compared to 67 other Aeromonas genomes, A603 is closely related to A. dhakensis, a species clade that includes both clinical and environmental isolates (Supplementary Data S2, Supplementary Fig. S1a)9,20,24,25. Notably, essential T3SS genes and the secreted virulence factor Ast enterotoxin are absent in A603, suggesting it is a likely environmental Aeromonas strain (Supplementary Fig. S1b).
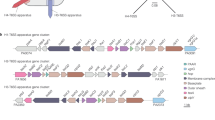
We inspected the A603 genome for candidate antibacterial products and identified T6SS genes. Similar to other aeromonads, there is a chromosomal island (T6SS-Core) that carries the core T6SS gene cluster encoding all genes needed for the T6SS apparatus and two effector-immunity pairs; a second accessory T6SS island (T6SS-Acc) was found that encoded a TssD ortholog, a TssI, and one effector-immunity pair (Fig. 2a–c)10,26,27. Accordingly, we inactivated this T6SS by the introduction of an internal deletion in the tssB gene in T6SS-Core, which encodes a structural protein that is essential for T6SS activity as it is part of the contractile sheath component11.
a Schematic showing the assembly and firing of the T6SS machinery and key structural proteins. b, c Core and accessory gene operons identified in the A603 genome. d operon encoding genes required for phenazine-based molecule biosynthesis. Transposon insertions that prevented the killing of prey bacteria are indicated by black arrows. In Ad-Phen, the R group of the amino acid moiety (R-AA) is unknown. The closest known analogous product, AGA, is depicted for comparison (R-AA = CH3). e Zone of inhibition on a lawn on V. cholerae C6706 produced by the A603ΔtssB is absent when a transposon insertion disrupts ehpO.
Selective killing of Vibrio species by the A603 T6SS in conjunction with a small molecule
We next established whether the bactericidal activity of A603 was entirely dependent on its T6SS. Remarkably, both A603 and A603ΔtssB killed a panel of unrelated clinical and laboratory bacteria, but the extent of this killing varied significantly by taxa (Fig. 1a). Similarly, both strains killed Vibrio and Shewanella strains isolated from shrimp but were less effective at eliminating other taxa (Fig. 1b). V. cholerae C6706 was most sensitive to both strains. When compared to Vibrio species, the extent of killing of non-Vibrio species was significantly reduced. The survival of Vibrio species increased slightly in the absence of A603’s T6SS, but killing activity was not eliminated (Fig. 1a, b). This result suggested an additional non-T6SS antibacterial mechanism that primarily targets Vibrio species is operative in A603.
By spotting A603ΔtssB on a soft agar overlay containing prey C6706, we discovered a halo of growth inhibition, indicating that A603 was likely secreting a toxic, diffusible small molecule (Fig. 2d, e). To determine what might be responsible for the diffusible antibacterial activity displayed by our A603ΔtssB mutant, we employed a mariner transposon mutagenesis screen using the pTnFGL3 transposon28. Mutants that no longer produced a halo of growth inhibition were isolated at a frequency of ~ 0.1% and transposon insertion junction sequencing was used to identify which genes were disrupted. Mutants that lost production of the growth inhibition halo carried insertions in a genetic operon predicted to encode functional homologs of phenazine biosynthetic genes similar to that found in Pantoea agglomerons Eh1087 (originally classified as Erwinia herbicola) but absent in nearly all Aeromonas spp (Fig. 2d). As shown, an insertion in ehpO of this biosynthetic gene cluster completely abolished the diffusible zone of inhibition on the V. cholerae C6706 indicator strain (Fig. 2e). A precise deletion of the ehpO demonstrated the same phenotype as the Tn:ehpO strain (data not shown) and was used for further testing.
The antibacterial effect of phenazine-like molecules has been ascribed to their redox activity, propensity to generate reactive oxidative products, and their interference with electron transport19. In P.agglomerons Eh1087, EhpO is required for the conversion of phenazine-1,6,-dicarboxylic acid to griseoluteic acid (GA) and then D-alanylgriseoluteic acid (AGA), a broad-spectrum antibiotic against Gram-positive pathogens20,21. In addition to EhpO, all genes identified in the AGA biosynthetic Eh1087 operon have predicted functional homologs in A603 (Supplemental Data S3). Since EhpO adds an amino acid moiety to GA, A603’s phenazine (henceforth named Ad-Phen) likely requires an amino acid adduct for full toxicity (Fig. 2). As in other works, we are yet unable to purify this molecule through organic extraction29.
Using a phenazine ehpO biosynthetic mutant (A603 ΔAd-Phen), the A603 ΔtssB mutant, and the corresponding A603ΔehpOΔtssB double mutant, we measured the contribution of T6SS and phenazine to the killing of various bacteria. For V. cholerae, the Ad-Phen-mediated killing appears to be ~ 1000-fold more significant than that for T6SS in our co-incubation assay. In contrast, E.coli, Pseudomonas, and Acinetobacter are only modestly inhibited by either one based on this mutant analysis (Fig. 1a). We then examined the activity against bacteria that were co-isolated with A603. A603 was most potent against members of the closely related orders Vibrionales, Aeromonadales, and Alteromonada (Fig. 1b). Vibrio species were most sensitive to both T6SS and the phenazine molecule. Both Aeromonas and Stenotrophomonas exhibited relatively minor sensitivity to T6SS and phenazine and the Gram-positive Paenibacillus was largely resistant to both within 90 min. In these and additional screening assays, we find Vibrio isolates are by far the most sensitive to A603 killing when compared to other prey taxa. Thus, the A603 T6SS/Ad-Phen combination displays a narrow prey range that reflects specificity for niche competitors within the aquatic environment30,31.
T6SS effectors that contribute to vibriocidal activity in combination with phenazine production
We wondered which of the putative A603 T6SS effectors may contribute to this synergistic lethality. We focused on the two T6SS islands and three predicted effectors encoded by genes adjacent to tssI (named as shown in Fig. 2b, c). We find tseA and tseB (ADS1910 and ADS1884) are well-conserved in a diverse number of Aeromonas species and strains, but that the tseC gene is restricted in a small number of environmental Aeromonas isolates. TseA is predicted to be a DNase toxin and possesses two common T6SS effector domains, the Polymorphic Nuclease Effector (PoNe) and a domain of unknown function (FIX)32; the putative immunity protein of TseA closely resembles those of other HNH/EndoVII nucleases33 (Supplemental Fig. 2a). TseB likely targets peptidoglycan as its C-terminal possesses a predicted T4 lysozyme-like muramidase domain found in the Yersinia pestis lysozyme pesticin34. The N-terminal domain of TseB shares a similar protein fold to that in a secreted peptidoglycan targeting metalloprotease in Pseudoalteromonas (Supplemental Fig. 2b). The tseC gene (ADS2781) and its flanking genes encode proteins that resemble V. cholerae T6SS effector VasX, the VasX accessory chaperone protein VasW, and the immunity protein TsiV2. VasX is a putative pore-forming membrane protein with a colicin-like domain that is demonstrated to be antibacterial and also a requirement for T6SS-dependent killing of Dictyostelium discoideum18,35,36 (Supplemental Fig. 2c). Like those in other bacteria, these A603 effectors are expected to be secreted together and thus attack multiple essential targets simultaneously in an intoxicated prey bacterium1,9,14. Sensitive prey cells seldom survive T6SS attacks and resistance is thought to be unlikely unless mediated by highly related cognate effector immunity proteins17 or other mechanical mechanisms such as extracellular capsules37.
Based on the varied differences in the magnitude of killing by T6SS and phenazine mutants in our panels, we compared the contribution of effectors in the context of Ad-Phen. We observed that V.cholerae is killed up to 8-logs within 90 min by A603 but antibacterial activity is completely abolished for V. cholerae in an A603 double mutant defective for T6SS activity and Ad-Phen production (Table 1). When killed V.cholerae by the ΔtssB mutant is compared to the single effector knockout mutants with Ad-Phen, we find that strains lacking either TseB or TseC are nearly as defective as the inactive ΔtssB mutant. However, the ΔtseA mutant that maintains both TseB and TseC is as effective as WT A603 in killing V. cholerae (Table 1). Since ectopic periplasmic expression of TseA does not inhibit growth (data not shown), we excluded TseA from further analysis. In the ΔAd-Phen mutant background, the addition of ΔtseB or ΔtseC mutations produced strains that were still more effective at killing than T6SS null mutants carrying a ΔtssB mutation (Table 1). However, in the presence of a functional Ad-Phen locus, the loss of tseC reduces killing efficacy by ~ 45-fold, while the loss of tseB alone does not significantly change killing. From these data, we conclude that Ad-Phen is the primary vibriocidal agent produced by A603, and that its killing ability is enhanced by T6SS activity, primarily (but not exclusively) by the effector TseC.
Based on the co-occurrence of the Ad-Phen regulator phzR and the T6SS-Acc operon (see below), we hypothesized that the pore-forming TseC protein may assist in sensitizing strains to Ad-Phen. To test this model, we cloned and expressed the TseC in the periplasm of V.cholerae strain C6706 and monitored the sensitivity of the strain to phenazine. The growth of C6706 is impaired by either expression of TseC or phenazine, though bacteria can adapt to these stressors. Sensitivity to phenazine is significantly enhanced when TseC is expressed, as concentrations of phenazine that slow growth in C6706 become inhibitory, and adaptation or recovery are not seen (Fig. 3). This synthetic lethality is not seen for the expression of TseB (data not shown). These data suggest that TseC intoxication directly sensitizes prey to Ad-Phen and related phenazines.
A603-induced prey membrane depolarization
We next investigated if changes in cell morphology, permeability, and membrane polarization occurred when cells were exposed to T6SS and Ad-Phen. When observed by fluorescence microscopy using the depolarization sensing membrane probe DiBac4(3) and the permeability sensing dye propidium iodide38. We observed that V. cholerae C6706 undergoes strong and irreversible membrane depolarization within 30 min of exposure to A603 (Fig. 4a, Supplemental Video 1). When co-incubated with A603ΔAd-Phen, C6706 is depolarized in a contact-dependent manner, while A603ΔtssB can depolarize prey in a contact-independent manner (Supplemental Videos 2, 3). Other A603-resistant prey strains like E. coli str. MG1655 are depolarized similarly (Fig. 4b, Supplemental Video 4) but do not suffer loss of viability. We conclude that Vibrio susceptibility to killing by A603 reflects an inability to reverse membrane polarization caused by A603.
Fluorescence microscopy of A603 co-incubated with V. cholerae C6706 a or E. coli MG1655 b after 60 min at room temperature. Cell permeabilization was detected by propidium iodide (red), and membrane depolarization was detected by DiBac4(3) (green). Full videos for a and b are available as Supplemental Videos 1, 4 respectively.
To investigate the role of T6SS effectors in this depolarization, we expressed either TseB or TseC in the periplasm of C6706 and monitored them by fluorescence microscopy as above. TseB expression induced cell rounding and lysis in minimal media, indicating degradation of the peptidoglycan layer (Supplemental Video 5). TseC expression induced membrane depolarization without strong permeabilization, indicating disruption of the inner membrane (Supplemental Video 6). Since TseC and Ad-Phen both disrupt membrane potential, they may work together to disrupt essential processes at the membrane, such as electron transport and energy production.
Prey cell transcriptional stress response when exposed to A603 bactericidal mechanisms
Because T6SS and Ad-Phen both contribute to bacterial killing, we wondered if the prey transcriptional response might inform us of which mechanisms produce stress on prey cells. We selected V. parahaemolyticus TaMai-1, a toxigenic shrimp pathogen we sequenced and annotated in this work. Strains were co-incubated with A603 or A603 single and double mutants defective for T6SS and/or Ad-Phen. RNA-seq revealed significant expression changes to nearly a quarter of Ta Mai-1 genes in both chromosomes and a toxin-encoding plasmid within 45 min of co-incubation with A603 (Supplemental Data S4). We interpreted this as a global stress response during cell death as TaMai-1 bacteria do not recover from this period of exposure to A603. Transcriptional differences appear to be due to transcription inhibition, transcript degradation, and/ or poor RNA recovery when A603 is present rather than up- or down-regulation of specific genes and operons. Only 41 of 4641 genes were predicted to be significant implementing the ‘Exact Test’ for two-group comparisons (FDR > 0.0001) (Supplemental Data S5) and no significant GO enrichment for TaMai-1 exposed to A603 is noted using GSEA. In contrast, using the same FDR cutoff as above, 933 genes were observed to respond to Ad-Phen (A603ΔtssB) in the absence of T6SS, 451 to T6SS(A603ΔephO), and only 63 to A603ΔephOΔtssB. The Ad-Phen mutant GSEA analysis revealed few enriched pathways including the downregulation of ATPase metabolism and proton motive force processes. In contrast, the T6SS mutant-producing Ad-Phen induced many pathways related to stress, SOS response, and DNA damage repair (Supplemental Data S5). We measured two small operons, one in each chromosome, with genes that were significantly induced 2000 to 3000-fold. Both operons possess genes (TaiMaiChI_orf01323 and TaiMaiChII_orf01363) that encode vicinal oxygen chelate (VOC) family proteins. These proteins are characterized through the chelation of divalent metals and redox-active molecules, including Fosfomycin and glyoxal species39. While the exact function of these VOC genes is unknown in Vibrio, the exogenous expression of TaiMaiChI_orf01323 in C6706 provides resistance to Ad-Phen (Supplemental Fig. 3a). To approximately quantitate the level of Ad-Phen secretion, we used the transcription of the two VOC genes in a TaMai-1 reporter prey strain that responds to Ad-Phen exposure. We found that these two reporter genes were significantly up-regulated within 15 min when exposed to Ad-Phen suggesting rapid uptake (Supplemental Fig. 3b).
Discussion
The T6SS of Gram-negative bacteria has been identified as a critical survival mechanism in mixed bacterial communities, both in the environment and in animal hosts. Although capable of targeting eukaryotic cells5,40,41, the T6SS is most effective at killing or inhibiting Gram-negative prey cells23. Generally, differences in Gram-negative cell prey sensitivity have been attributed to the specificity of different T6SS effectors and their corresponding immunity proteins or to mechanical blockage of effector delivery by extracellular structures such as capsules37. It has been reported that alterations of extracellular pH through the production of basic metabolites can enhance sensitivity to one T6SS effector that has a narrow pH optimum42. However, the mechanism behind T6SS prey specificity that occurs at the genus level has not been reported previously.
In this work, we identified a strain of Aeromonas dhakensis that utilizes a T6SS, transcriptionally and functionally paired with the biosynthesis of a small molecule antibiotic (Ad-Phen), to rapidly kill Vibrio with remarkable specificity. A603 is capable of killing up to 109 Vibrio cells in a co-incubation assay in just two hours. While A603’s T6SS is able to weakly kill other Gram-negative cells, we found that A603’s selective vibriocidal activity relies on the synergy between the pore-forming T6SS effector TseC and the toxic Ad-Phen molecule. Since both components induce strong and irreversible membrane depolarization, we speculate that they disrupt the inner membrane.
We propose the ancestral strain to A603 separately acquired the phenazine biosynthetic operon and the T6SS accessory island with a TseC effector, and transposition of the transcriptional regulator phzR may facilitate coordination in expression. Together, these systems increased antibacterial activity to provide a remarkable competitive advantage against Vibrio and are several log10s more lethal than any other known bacterial T6SS. Because the tseC-encoding accessory island and phenazine operon are represented in very few Aeromonads and not co-present in clinical isolates, we reason that these two systems are advantageous in an environmental niche, such as shrimp hosts, where sensitive prey taxa are encountered as competitors or host pathogens43.
It is well-known that the expression of T6SS can be transcriptionally regulated in different ways in different bacteria, especially through quorum sensing44. In A.hydrophila, N-acyl-homoserine lactone (AHL)-mediated quorum sensing (QS) is demonstrated to control the expression and activity of T6SS45. In the A603 Ad-Phen biosynthetic operon, we identify genes that encode the autoinducer synthase gene PhzI and RsaM, a protein that has been shown to regulate QS and AHL production in other bacteria46,47. The T6SS-Acc operon encodes a predicted AHL-regulated transcriptional regulator PhzR (Fig. 2). We are currently studying the regulatory crosstalk between these Ad-Phen and T6SS based on these observations. However, coordination regulation of the production of a diffusible toxic small molecule with a contact-dependent bactericidal system that acts synergistically is novel and suggests that other examples of these interesting ecological synergistic interactions likely await discovery. Thus, we propose that the identification of other regulatory genes near antibacterial systems like T6SS may provide genomic clues for coordination between antibiotic metabolites and bacterial toxins.
The rapid depolarization we observed with Ad-Phen may be of interest to antimicrobial drug discovery efforts that seek molecules that can depolarize and permeabilize cells and thus interfere with the function of energy-dependent efflux pumps. We are currently investigating stabilized phenazine-amino acid compounds based on these principles (unpublished data). A603 itself represents a promising probiotic for aquaculture and could be utilized as a possible bioremediator for Vibrio-contaminated water sources.
Methods
Bacteria and plasmids
Sources of strains and plasmids used in this work are listed in Supplemental Table S1.
Isolation of A603 and bacteria from shrimp
Uncooked white-legged shrimp imported from Asia were purchased from a seafood market on ice in Boston, MA. Shrimp were kept on ice until arrival at the laboratory and then homogenized and filtered through sterile cheesecloth. The filtrate was plated on both LB agar and Thiosulfate-citrate-bile salts-sucrose agar to select for other aerobic bacteria and Vibrio, respectively. Bacteria were colony-purified and screened using 16 S RNA primers. Predictive species identification using the sequence was performed using the Ribosomal Database Project (RDP) Classifier48.
Bacterial co-incubation competition assay
Bacterial cultures were grown to OD 0.8 and 1.0 ml was centrifuged at 5000 rpm for 2 min to pellet cells. Cells were mixed, concentrated to OD 10 by re-suspension in LB, and 5ul of each suspension was spotted on pre-dried agar plates to force cell-cell contact. Incubations varied in different screens between 1 and 3 h at 37 °C. Bacterial spots were cut from the agar plates and vortexed for 10 s in 1.0 ml LB. The suspension was serially diluted (10-fold) and each diluted suspension was spotted on antibiotic-supplemented plates to select for CFUs of prey strains. Colonies from dilution plates were enumerated after 16 h at 37 °C.
Transposon mutagenesis
The diaminopimelic acid (DAP) auxotroph mating strain MFD-λpir was used to conjugate the mariner pTNFGL3 into A603 by co-incubating bacteria on sterile filters for four hours on LB + DAP (200 ug/ml)28,49. The bacteria were resuspended and plated on LB agar without DAP (Kan 50 ug/ml). Colonies were outgrown in 96 well plates and spotted on a lawn of TaMai-1 to screen for halo formation. Transposon insertions of colonies that failed to produce halos were sequenced using two-round semi-arbitrary PCR primers for pTNFGL3 as published28.
Bacterial strains and genetic manipulations
Strains and plasmids are listed in Supplemental Data S5. Chromosomal deletions in Aeromonas A603 were constructed using sacB-mediated allelic exchange and sucrose counter-selection as published using the pDS132 suicide vector 66. 500 nucleotides flanking both the 5-prime and 3-prime constructed deletion were cloned into the pDS132 multiple cloning site, sequenced to verify, and then mated into A603 using E.coli MFD-Pir49. In A603, the tssB gene and ehpO were precisely removed to generate A603ΔephO, A603ΔtssB, and A603ΔephOΔtssB without interrupting the start or ribosome binding sites of flanking genes. The same methods were used to remove tseA, tseB, and/or tseC from A603 and A603ΔehpO.
The pSB6532 (TaiMaiChI_orf01323) vector was constructed by PCR amplification of the TaiMaiChI_orf01323 gene with an upstream RBS and cloning into pBAD33 and inducing with 0.02% l-arabinose 45 min prior to co-incubation.
Bacterial DNA preparation and sequencing
Bacteria samples were sourced from either laboratory mono-cultured strains. Bacteria were lysed and DNA was extracted using the Quick DNA Fungal/Bacterial kit (Zymo). Illumina libraries were prepared using the DNA Ultra II Library Preparation kit (NEB) and sequenced using both Miseq and HiSeq 2500 (Illumina). DNA for A603 was sequenced in parallel using the Oxford nanopore platform. The V.parahaemolyticus TaMAi-1 strain was sequenced and both chromosomes closed using Illumina and PacBio reads.
Assembly and annotation of the A603 and TaMai-1 genome
Illumina reads were used to correct PacBio and MinION reads and available reads were used to assemble the Ta Mai and A603 genomes using SPADES version (vs 3.12.0)50. The closed circular genome was annotated using OmicsBox Suite implementing both BlastP and InterPro and the NCBI non-redundant database (nr. v.5) and the current InterProScan (5.65–97.0)51,52. Predicted GO ontologies were mapped and annotated to provide Gene Set Enrichment Analysis (GSEA) and GO enrichment analysis (CLC-Bio workbench vs 8)53. Noncoding RNAs the Rfam database54. Annotations used in this paper are provided in Supplemental Data S1.
Aeromonas core genome analysis and phylogeny
The Get_Homologues software package was used to predict core genes from 58 selected Aeromonas strains using the BDBH, COG, and OMCL algorithms55. Genes in the core set were further evaluated using BLAST hits to remove genes with similarity. The amino acid sequence of 257 unique genes extracted from strains, concatenated, and aligned using CLC Bio Workbench (vs 8)53. The alignments were used to create a Maximum Likelihood Tree and evaluated by bootstrapping 1000 replicates. Aeromonas genomes were compared to strain KN-Mc-6U21 using CMG-Biotools (v3)56. BlastAtlas was used to map and visualize A.dhakensis genomes related to A60356.
RNA-Seq and transcriptome analysis
Bacteria were incubated as mono- or co-cultures as in the competition assay for 15 or 45 min at 37 °C. Bacteria spots were cut out from agar and resuspended in RNA Later (Ambion), the bacteria were pelleted by centrifugation, and the RNA was extracted and purified using Zymo/Trizol kit. qRT-PCR was performed on 15 min samples with KAPA SYBR® FAST One-Step qRT-PCR kit (Roche). Ribosomal RNA was removed from 45 min samples using the Epicentre Ribozero Bacteria Removal Kit. The NEB RNA Ultra II Kit with strand specificity was used to build Illumina libraries of 100 nt read length. Transcriptomes were analyzed by mapping reads to the corresponding genomes53. Each experiment was completed in duplicate and mapped reads to all genes were statistically analyzed using the DGE tool in CLC Genomics Workbench (v8)53. Using this, we imposed a FDR-corrected cutoff of 0.0001 and any gene. The annotated genomes include mapped GO ontologies from both BlastP (nr v.5) and InterProScan (5.65–97.0) databases through OmicsBox suite (v3.1.9)51. Genes deemed significant were analyzed using Gene Set Enrichment Analysis and GO pathway enrichment analysis using the OmicsBox suite51.
Identification and modeling of A603 T6SS effectors
The A603 T6SS effectors TseA, TseB, and TseC were analyzed using InterProScan557 for domain analysis and functional predictions. Structure prediction for each effector was performed using AlphaFold 2.2.058 and visualized using PyMol 2.359.
Phenazine and TseC exposure
The coding sequence for TseC was amplified from the A603 genome and cloned via Gibson Assembly into the expression vector pBAD33, with a ribosome binding site and a leading Sec sequence for periplasmic localization60. Expression of TseC was induced with 0.02% l-arabinose. V. cholerae str. C6706 were monitored for growth with and without expression of TseC and exposure to indicated concentrations of phenazine (Aldrich P13207). Growth curves were performed by measuring the OD600 of cultures using a BioTek Synergy H1 microplate reader when grown at 37 °C.
Fluorescence microscopy
Bacterial samples were prepared as previously done for bacterial co-incubation competition assays. Microscope slides were prepared with pads of 1% agarose in LB containing 10 ng/mL propidium iodide (Invitrogen) and 10 nM DiBac4(3) (Invitrogen). For expression of TseB and TseC, C6706 strains containing the pBadSec-TseB or pBadSec-TseC respectively were incubated on the pads described above with the addition of 0.02% L-arabinose. The expression plasmid pBadSec-TseB was constructed with the same method as pBadSec-TseC. Time-lapse videos were recorded on a Nikon Ti-E inverted motorized microscope with Perfect Focus System and Plan Apo 100× oil Ph3 DM (NA 1.4) objective lens. in 1 min intervals for 1 h. Each frame contains images from brightfield, propidium iodide (ex. 550 nm, em. 593–650 nm), and DiBac4(3) (ex. 470, em. 502–548). Videos were assembled in ImageJ61.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data used in analyses and supporting the findings of this study are available in the Article and the Supplementary Information. Raw data used in figures is provided in Supplemental Data S6. Assembled genomes for A603 and TaMai-1 have been deposited in Genbank (Accession: PRJNA1123267 and Accession: PRJNA1122179). Raw Illumina reads and data have been deposited in NCBI Gene Expression Omnibus as Accession PRJNA1124880.
References
Ho, B. T., Dong, T. G. & Mekalanos, J. J. A View to a Kill: The Bacterial Type VI Secretion System. Cell Host Microbe 15, 9–21 (2014).
Hernandez, R. E., Gallegos-Monterrosa, R. & Coulthurst, S. J. Type VI secretion system effector proteins: Effective weapons for bacterial competitiveness. Cell. Microbiol. 22, e13241 (2020).
Pukatzki, S. et al. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. 103, 1528 (2006).
Mougous, J. D. et al. A Virulence Locus of Pseudomonas aeruginosa Encodes a Protein Secretion Apparatus. Science 312, 1526–1530 (2006).
Pukatzki, S., Ma, A. T., Revel, A. T., Sturtevant, D. & Mekalanos, J. J. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. 104, 15508–15513 (2007).
Hood, R. D. et al. A Type VI Secretion System of Pseudomonas aeruginosa Targets a Toxin to Bacteria. Cell Host Microbe 7, 25–37 (2010).
Russell, A. B. et al. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475, 343–347 (2011).
Kudryashev, M. et al. Structure of the Type VI Secretion System Contractile Sheath. Cell 160, 952–962 (2015).
Shneider, M. M. et al. PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature 500, 350–353 (2013).
Liang, X. et al. Identification of divergent type VI secretion effectors using a conserved chaperone domain. Proc. Natl. Acad. Sci. USA. 112, 9106–9111 (2015).
Basler, M., Pilhofer, M., Henderson, G. P., Jensen, G. J. & Mekalanos, J. J. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483, 182–186 (2012).
Silverman, J. M. et al. Haemolysin Coregulated Protein Is an Exported Receptor and Chaperone of Type VI Secretion Substrates. Mol. Cell 51, 584–593 (2013).
Shrivastava, S. & Mande, S. S. Identification and Functional Characterization of Gene Components of Type VI Secretion System in Bacterial Genomes. PLOS ONE 3, e2955 (2008).
Hachani, A., Allsopp, L. P., Oduko, Y. & Filloux, A. The VgrG Proteins Are “à la Carte” Delivery Systems for Bacterial Type VI Effectors. J. Biol. Chem. 289, 17872–17884 (2014).
Salomon, D. et al. Marker for type VI secretion system effectors. Proc. Natl. Acad. Sci. 111, 9271–9276 (2014).
Lien, Y.-W., Lai, E.-M. & Type, V. I. Secretion Effectors: Methodologies and Biology. Front. Cell. Infect. Microbiol. 7, 254–254 (2017).
Dong, T. G., Ho, B. T., Yoder-Himes, D. R. & Mekalanos, J. J. Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc. Natl. Acad. Sci. 110, 2623–2628 (2013).
Miyata, S. T., Unterweger, D., Rudko, S. P. & Pukatzki, S. Dual Expression Profile of Type VI Secretion System Immunity Genes Protects Pandemic Vibrio cholerae. PLOS Pathog. 9, e1003752 (2013).
Laursen, J. B. & Nielsen, J. Phenazine Natural Products: Biosynthesis, Synthetic Analogues, and Biological Activity. Chem. Rev. 104, 1663–1686 (2004).
Giddens, S. R., Feng, Y. & Mahanty, H. K. Characterization of a novel phenazine antibiotic gene cluster in Erwinia herbicola Eh1087. Mol. Microbiol. 45, 769–783 (2002).
Giddens, S. R. & Bean, D. C. Investigations into the in vitro antimicrobial activity and mode of action of the phenazine antibiotic d-alanylgriseoluteic acid. Int. J. Antimicrob. Agents 29, 93–97 (2007).
Sousa, C. A., Ribeiro, M., Vale, F. & Simões, M. Phenazines: Natural products for microbial growth control. hLife 2, 100–112 (2024).
Chou, S. et al. Structure of a Peptidoglycan Amidase Effector Targeted to Gram-Negative Bacteria by the Type VI Secretion System. Cell Rep. 1, 656–664 (2012).
Beaz-Hidalgo, R., Hossain, M. J., Liles, M. R. & Figueras, M.-J. Strategies to Avoid Wrongly Labelled Genomes Using as Example the Detected Wrong Taxonomic Affiliation for Aeromonas Genomes in the GenBank Database. PLOS ONE 10, e0115813 (2015).
Colston, S. M. et al. Bioinformatic Genome Comparisons for Taxonomic and Phylogenetic Assignments Using Aeromonas as a Test Case. mBio 5, e02136-14 (2014).
Liang, Xiaoye et al. Characterization of Lysozyme-Like Effector TseP Reveals the Dependence of Type VI Secretion System (T6SS) Secretion on Effectors in Aeromonas dhakensis Strain SSU. Appl. Environ. Microbiol. 87, e00435-21 (2021).
Moriel, B. et al. In silico comparative analysis of Aeromonas Type VI Secretion System. Braz. J. Microbiol. 52, 229–243 (2021).
Cameron, D. E., Urbach, J. M. & Mekalanos, J. J. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proc. Natl. Acad. Sci. USA. 105, 8736–8741 (2008).
Shi, Y.-M. et al. Dual phenazine gene clusters enable diversification during biosynthesis. Nat. Chem. Biol. 15, 331–339 (2019).
Sana, T. G. et al. Salmonella Typhimurium utilizes a T6SS-mediated antibacterial weapon to establish in the host gut. Proc. Natl. Acad. Sci. 113, E5044–E5051 (2016).
Chatzidaki-Livanis, M., Geva-Zatorsky, N. & Comstock, L. E. Bacteroides fragilis type VI secretion systems use novel effector and immunity proteins to antagonize human gut Bacteroidales species. Proc. Natl. Acad. Sci. 113, 3627–3632 (2016).
Jana, B., Fridman, C. M., Bosis, E. & Salomon, D. A modular effector with a DNase domain and a marker for T6SS substrates. Nat. Commun. 10, 3595 (2019).
Zhang, D., de Souza, R. F., Anantharaman, V., Iyer, L. M. & Aravind, L. Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol. Direct 7, 18 (2012).
Patzer, S. I., Albrecht, R., Braun, V. & Zeth, K. Structural and Mechanistic Studies of Pesticin, a Bacterial Homolog of Phage Lysozymes. J. Biol. Chem. 287, 23381–23396 (2012).
Miyata, S. T., Kitaoka, M., Brooks, T. M., McAuley, S. B. & Pukatzki, S. V.Cholerae Requires the Type VI Secretion System Virulence Factor VasX To Kill Dictyostelium discoideum. Infect. Immun. 79, 2941 (2011).
Liang, X. et al. An onboard checking mechanism ensures effector delivery of the type VI secretion system in Vibrio cholerae. Proc. Natl. Acad. Sci. 116, 23292–23298 (2019).
Toska, J., Ho, B. T. & Mekalanos, J. J. Exopolysaccharide protects Vibrio cholerae from exogenous attacks by the type 6 secretion system. Proc. Natl. Acad. Sci. 115, 7997–8002 (2018).
Adams, D. S. & Levin, M. Measuring Resting Membrane Potential Using the Fluorescent Voltage Reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb. Protoc. 2012, pdb.prot067702 (2012).
He, P. & Moran, G. R. Structural and mechanistic comparisons of the metal-binding members of the vicinal oxygen chelate (VOC) superfamily. J. Inorg. Biochem. 105, 1259–1272 (2011).
Toesca Isabelle, J., French Christopher, T. & Miller Jeff, F. The Type VI Secretion System Spike Protein VgrG5 Mediates Membrane Fusion during Intercellular Spread by Pseudomallei Group Burkholderia Species. Infect. Immun. 82, 1436–1444 (2014).
Trunk, K. et al. The type VI secretion system deploys antifungal effectors against microbial competitors. Nat. Microbiol. 3, 920–931 (2018).
Le, N.-H., Pinedo, V., Lopez, J., Cava, F. & Feldman, M. F. Killing of Gram-negative and Gram-positive bacteria by a bifunctional cell wall-targeting T6SS effector. Proc. Natl. Acad. Sci. 118, e2106555118 (2021).
Rattanama, P. et al. Sigma E Regulators Control Hemolytic Activity and Virulence in a Shrimp Pathogenic Vibrio harveyi. PLOS ONE 7, e32523 (2012).
Jaskólska, M., Stutzmann, S., Stoudmann, C. & Blokesch, M. QstR-dependent regulation of natural competence and type VI secretion in Vibrio cholerae. Nucleic Acids Res. 46, 10619–10634 (2018).
Khajanchi, B. K. et al. N-Acylhomoserine lactones involved in quorum sensing control the type VI secretion system, biofilm formation, protease production, and in vivo virulence in a clinical isolate of Aeromonas hydrophila. Microbiology 155, 3518–3531 (2009).
Mattiuzzo, M. et al. The plant pathogen Pseudomonas fuscovaginae contains two conserved quorum sensing systems involved in virulence and negatively regulated by RsaL and the novel regulator RsaM. Environ. Microbiol. 13, 145–162 (2011).
Michalska, K. et al. RsaM: a transcriptional regulator of Burkholderia spp. with novel fold. FEBS J. 281, 4293–4306 (2014).
Wang, Q., Garrity, G. M., Tiedje, J. M. & Cole, J. R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 73, 5261 (2007).
Ferrières, L. et al. Silent mischief: bacteriophage Mu insertions contaminate products of Escherichia coli random mutagenesis performed using suicidal transposon delivery plasmids mobilized by broad-host-range RP4 conjugative machinery. J. Bacteriol. 192, 6418–6427 (2010).
Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. J. Comput. Mol. Cell Biol. 19, 455–477 (2012).
Conesa, A. & Götz, S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genomics 2008, 619832–619832 (2008).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinforma. 10, 421 (2009).
CLC Genomics Workbench 8.0 (https://www.Qiagenbioinformatics.Com/). (Qiagen).
Kalvari, I. et al. Rfam 14: expanded coverage of metagenomic, viral and microRNA families. Nucleic Acids Res. 49, D192–D200 (2021).
Contreras-Moreira, B. & Vinuesa, P. GET_HOMOLOGUES, a versatile software package for scalable and robust microbial pangenome analysis. Appl. Environ. Microbiol. 79, 7696–7701 (2013).
Hallin, P. F., Binnewies, T. T. & Ussery, D. W. The genome BLASTatlas-a GeneWiz extension for visualization of whole-genome homology. Mol. Biosyst. 4, 363–371 (2008).
Jones, P. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240 (2014).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
The PyMOL Molecular Graphics System v2.0. Schrödinger, LLC.
Freudl, R. Signal peptides for recombinant protein secretion in bacterial expression systems. Microb. Cell Factories 17, 52 (2018).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Acknowledgements
This work was financially supported by NIH/National Institute of Allergy and Infectious Diseases Grant R01AI018045 to JJM and a generous gift from the Charoen Pokphand Group Company. We thank the Aquatic Animal Health Research Center in Charoen Pokphand Group Company for providing some bacterial strains. The contents of the manuscript describing the results of the study are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health and NIAID. We appreciate supercomputing computational support from the Harvard Medical School O2, a platform for Linux-based high-performance computing at Harvard Medical School for the implementation of ColabFold in protein prediction models presented in this work.
Author information
Authors and Affiliations
Contributions
S.B.B. and W.P.R. initiated and conceived this study, designed experiments, constructed strains and vectors, performed all experiments, performed computational analysis, and wrote this manuscript. J.T. assisted with the design and execution of microscopy. W.Z., P.S., and P.P. contributed to the interpretation of results and assisted with the design of plasmid constructs and execution of experiments in bacteria and with other microbiological assays. J.J.M. contributed to the interpretation of results, the design of experiments, and the writing of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Shukria Akbar, Alain Filloux and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Tobias Goris. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bier, S.B., Toska, J., Zhao, W. et al. A coordinated attack by a bacterial secretion system and a small molecule drives prey specificity. Commun Biol 7, 958 (2024). https://doi.org/10.1038/s42003-024-06637-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-06637-0
This article is cited by
-
Antagonistic Mechanisms of Probiotic Aliivibrio sp. Strain Vl2 Against Moritella viscosa: Evidence from Co-cultivation and Targeted Transcriptomic Analysis
Probiotics and Antimicrobial Proteins (2025)