Abstract
Phyllosphere microorganisms execute important ecological functions including supporting host plant growth, enhancing host resistance to abiotic stresses, and promoting plant diversity. How leaf developmental stages affect plant-microbiome interactions and phyllosphere microbial community assembly and diversity is poorly understood. In this study, we utilized amplicon sequencing of 16S rRNA and ITS genes to investigate the composition and diversity of microbial communities across different leaf developmental stages of rubber trees. Our findings reveal that endophytic microbial communities, particularly bacterial communities, are more influenced by leaf senescence than by epiphytic communities. The high abundance of metabolism genes in the endosphere of yellow leaves contributes to the degradation and nutrient relocation processes. Nutrient loss leads to a higher abundance of α-Proteobacteria (r-selected microorganisms) in the yellow leaf endosphere, thereby promoting stochastic community assembly. As leaves age, the proportion of microorganisms entering the inner layer of leaves increases, consequently enhancing the diversity of microorganisms in the inner layer of leaves. These results offer insights into the mechanisms governing community assembly and diversity of leaf bacteria and fungi, thereby advancing our understanding of the evolving functions of microbial communities during leaf senescence in general, and for an important tropical crop species in particular.

Similar content being viewed by others
Introduction
The leaf surface hosts diverse microbial communities1,2,3. As integral components of the plant holobiont4, phyllosphere microbial communities have coevolved with their hosts, influencing foliar functional traits, impacting plant fitness, and contributing to essential ecosystem functions, such as supporting plant growth and survival, nutrient and water cycling, and promoting host plant diversity4,5,6,7,8,9,10. It has been found that factors such as plant compartment (leaf surface and leaf endosphere), host species identity, geographic location, and seasonal changes exert influence on phyllosphere microbial communities11,12,13,14,15,16,17,18. These mechanisms could be broadly grouped into either stochastic or deterministic processes. The former proposes that microbial community composition is shaped by limited dispersal and immigration19, while the latter occurs when species exhibit differential habitat preferences, leading to variations in species fitness and abundance within a specific environment20. Recent studies have also revealed that plant developmental stages and the secretions associated with these stages can significantly impact microbial community assembly21,22,23,24,25,26,27. However, there is limited understanding of how leaf senescence may affect phyllosphere microorganisms and consequently their assembly and functions.
Over the past two decades, rubber tree (Hevea brasiliensis) plantations have expanded across Southeast Asia28. The cultivation of this species has extended to the northern edge of the Asian tropics, where the rubber tree has become deciduous in a climate characterized by seasonal drought and cold stresses29. Leaf senescence can significantly impact a plant’s fitness and survival30. It is widely acknowledged that as leaves age, their photosynthetic ability decreases31, cell walls disassemble32, and programmed cell death (PCD) ensues30. Rubber tree leaves are crucial for rubber production and quality33. Studies have demonstrated that under seasonal drought and cold stress, stressed-induced senescent rubber tree leaves exhibit significant nutrient relocation29. During the process of senescence, whether due to aging or stress-induced factors such as cold or drought, plants reallocate nutrients from senescent leaves to young leaves and other organs34,35,36,37. This mechanism is vital for maintaining plant nutrient balance38 and for enhancing crop yield and quality39. However, there is limited understanding of the impact of leaf senescence on phyllosphere microbial community assembly and the role of microbes in nutrient relocation from senescent leaves. In this study, we hypothesized that leaf senescence would: (1) increase the abundance of metabolism genes (i.e., amino acid metabolism, carbohydrate metabolism, and lipid metabolism) due to microbes playing key roles in driving biogeochemical cycling40, thereby accelerating nutrient relocation from yellow leaves to other organs of rubber trees; (2) be accompanied by decreased nutrient content in the leaf endosphere, enriching oligotrophic (r-selected) microorganisms capable of surviving in nutrient-poor environments41,42 and promoting stochastic community assembly; and (3) increase the proportion of microorganisms entering the inner layer of leaves due to cell wall disassembly during leaf aging32, thereby enhancing microbial diversity in leaf endosphere. To test these hypotheses, we sequenced 16S rRNA and ITS genes for young (green) and old (yellow) leaves. This study provides empirical evidence of the consequences of rubber leaf aging, offering a theoretical basis for the fertilization and other management practices of rubber trees.
Results
Microbial community composition over leaf senescent stages
The PCoA ordination and PERMANOVA results demonstrated that at the genus level, significant differences existed between epiphytic (p < 0.001) and endophytic (p < 0.001) bacterial communities in green and yellow leaves (Fig. 1A, Supplementary Table 2). Moreover, in comparison to the epiphytic bacterial community (R2 = 0.03, p < 0.001), it was observed that the endophytic bacterial community (R2 = 0.07, p < 0.001) was more impacted by leaf senescence. Furthermore, the relative abundance of Actinomycetospora (p < 0.01), Methylobacterium (p < 0.001), and Bacteroidota (p < 0.01) were notably higher in the yellow leaves than in the green leaves, particularly within the endophytic community (Fig. 1B). As for fungi, significant differences between green and yellow leaves were observed in the epiphytic community (Fig. 1A). Additional PERMANOVA results (Endophytic: R2 = 0.07, p = 0.001, Epiphytic: R2 = 0.03, p < 0.001) and PERMDISP2 analysis (Endophytic: p = 0.535, Epiphytic: p = 0.531) indicated significant differences in the bacterial community between green and yellow leaves, but no significant difference was observed in the fungal endophytic community (p < 0.001 for both PERMANOVA and PERMDISP2 tests) (Supplementary Tables 2 and 4).
A Principal coordinates analysis (PCoA) of taxonomic similarity based on Bray-Curtis distances community composition. B: Bar plot of relative abundance of genera compositions. The asterisked phyla mean they are significantly different between green and yellow leaves. Significance level: *p < 0.05; **p < 0.01; ***p < 0.001.
The composition of fungal and bacterial communities is influenced not only by the leaf stage but also by the sampling season (Supplementary Fig. 3A & Supplementary Table 2) and the sampling location (Supplementary Fig. 3B & Supplementary Table 2) to a certain extent.
Diversity over leaf senescent stages
The rarefaction curves indicate a reasonable amount of sequencing data for estimating bacterial and fungal diversity in both yellow and green rubber tree leaves, as they tend to flatten off (Supplementary Fig. 4). Moreover, the upset venn maps illustrated that the total number of ASVs was higher in yellow leaves for both bacterial and fungal communities (Fig. 2B), with the exception of the endophytic fungal communities. The accumulated ASVs also indicated that yellow leaves hosted a greater number of microbes than green leaves did, except for the endophytic fungal community (Supplementary Fig. 5). In terms of α-diversity, the Chao diversity for both endophytic and epiphytic bacterial communities in green leaves was significantly higher in yellow leaves (p < 0.001; Fig. 2A). Conversely, for fungal communities, endophytic α-diversity was higher in green leaves than in yellow leaves, while the difference was not significant for epiphytic communities (Fig. 2A). Additionally, the Shannon diversity exhibited a similar trend to Chao diversity, with higher Shannon diversity observed in the yellow endophytic bacterial community, but lower Shannon diversity in the fungal community.
A Chao index and Shannon diversity (α-diversity) for bacterial and fungal communities. Significance level: *p < 0.05; **p < 0.01; ***p < 0.001. B The upset venn diagram shown the total ASVs (i.e., γ diversity, the bar plot on the left panel) for each groups, the number of unique in each group (e.g., the first red bar indicating that 4017 ASVs were unique in yellow epiphytic bacterial community), the number of ASVs shared between groups (e.g., the third green bar indicating that yellow epiphytic and green epiphytic shared 2077 ASVs).
Microbial functions over leaf senescent stages
The findings from the PCoA ordination and PERMANOVA analysis (R2 = 0.11, p < 0.001) of functional gene compositions (KEGG level 2) revealed significant differences in endophytic bacterial functional gene compositions between yellow and green leaves (Supplementary Fig. 6A and Supplementary Table 4), while no differences were observed in epiphytic compositions. The PERMDISP2 analysis for endophytic bacteria (p = 0.340) also confirmed these results (Supplementary Table 3). Additionally, a higher relative abundance of metabolism genes (KEGG level 1) was observed in yellow leaves compared to green ones (Fig. 3A). It was also observed that the abundance of amino acid metabolism (ACM), carbohydrate metabolism (CM), and lipid metabolism (LM) were higher in endophytic yellow leaves than in green ones (Fig. 3B).
The analysis of fungal functions revealed that the relative abundance of endophyte-plant pathogens and saprotroph endophyte were higher in yellow leaves than in green ones (Supplementary Fig. 7). However, no significant differences were observed in the fungal gene compositions based on Bray-Curtis distances for epiphytic fungi between green and yellow leaves (Supplementary Fig. 6B and Supplementary Table 4). Notably, the significant difference observed in endophytic fungal functional compositions between green and yellow leaves was not attributable to leaf stages (Supplementary Table 4).
Changes in leaf nutrients over leaf senescent stages
Our results indicate that total nitrogen (TN) (p < 0.001), total potassium (TK) (p < 0.001), and organic matter (OM) (p < 0.05) were significantly lower in yellow leaves than in green ones (Fig. 4A), while leaf pH, water content (WC), and total phosphorus (TP) remained unchanged (p > 0.05) (Supplementary Table 1). Additionally, the results further revealed that total nitrogen was negatively associated with the abundance of functional genes for amino acid metabolism (ACM, R2 = 0.11, p = 0.01), carbohydrate metabolism (CM, R2 = 0.12, p < 0.01), and lipid metabolism (LM, R2 = 0.09, p < 0.001). Similarly, organic matter was also negatively correlated with ACM (R2 = 0.09, p < 0.01), CM (R2 = 0.09, p < 0.001), and LM (R2 = 0.09, p < 0.001) (Fig. 4B).
Community assembly changes with leaf senescent stages
The development stage of the leaf significantly impacts community assembly. For endophytic bacterial communities, the MST of yellow leaves was significantly higher than that of green leaves (p < 0.001), suggesting that endophytic bacterial communities in yellow leaves were more influenced by stochastic processes than those in green leaves. Conversely, for epiphytic bacteria, the low MST index indicated that their assembly on yellow leaves was more controlled by deterministic processes (p = 0.001) (Fig. 5A). As for fungal communities, the MST index was higher in endophytic fungi in yellow leaves than in green ones, while it was lower in epiphytic fungi.
A MST: modified stochasticity ratio (MST < 50%: community assembly is more deterministic; MST > 50%: more stochastic) for bacterial (left panel) and fungal (right panel) communities. B Fit of the neutral community model of whole bacterial and fungal community for rubber tress. The solid black lines indicate the best fit to the model, and the dashed black lines represent 95% confidence intervals. Black solid circles show ASVs that matched model predictions. Light pink solid circles represent none core ASVs, blue, green, and dark green solid circles are core ASVs. M indicates the metacommunity size multiplied by immigration, while R2 indicates the model fit. Significant level: *p < 0.05; **p < 0.01; ***p < 0.001.
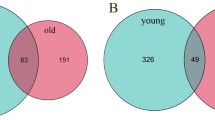
By utilizing the abundance-occupancy method, 68 amplicon sequence variants (ASVs) were identified as core ASVs in the overall bacterial communities (Supplementary Fig. 8), with a majority (over 70%) of them being members of α-Proteobacteria and Actinobacteriota (Supplementary Data 1). Additionally, 79 ASVs were identified as core ASVs within the entire fungal communities, encompassing phyla Ascomycota and Basidiomycota (Supplementary Data 2). Notably, only a limited number of ASVs were enriched in both endophytic and epiphytic fungi in yellow leaves. Further analysis revealed that the quantity of bacterial ASVs enriched in yellow leaves due to neutral processes (drift and dispersal limitation) was significantly higher than that of ASVs enriched due to deterministic processes (selection) (Fig. 5B & Supplementary Data 1). This observation helps explain why the stochastic process was more pronounced in the endophytic bacterial community of yellow leaves than that of green ones.
Microbial source tracking with leaf senescent stages
The observed significant increase in the percentage of bacteria in leaf interior originating from the surface of yellow leaves compared to green leaves (p < 0.01) (Fig. 6A) indicates a high likelihood for epiphytic bacteria entering the inner part of yellow leaves. Similarly, the presence of bacteria inside yellow leaves showed a pronounced tendency to penetrate and colonize the leaf surface compared to green leaves (p < 0.05). However, the proportion of endophytic fungi originating from leaf surface (or epiphytic fungi originating from the leaf interior) did not significantly change during leaf senescence (p > 0.05). This finding may explain the enrichment of core members of α-proteobacteria (Methylobacterium and 1174-901-12) and Actinobacteria (Actinomycetospora) in endophytic yellow leaves (Fig. 6B, Supplementary Data 1).
Discussion
Leaves serve as the site for plant’s photosynthesis and are crucial for plant’s vigor and growth. While the physiological and molecular processes involved in leaf aging are well-documented43, there is poor understanding of how leaf ontogeny influences the composition, diversity, and function of phyllosphere microbial communities. In this study, we aimed to investigate the changes in microbial communities between young (green) and old (yellow) leaves of the rubber tree. Our findings revealed that bacterial and fungal communities displayed distinct responses to plant leaf development, with bacterial communities showing greater sensitivity to leaf senescence than fungal communities, particularly in the case of endophytic bacteria.
Leaf senescence involves the mechanism of the programmed death of leaf cells43, or the so-called developmentally controlled PCD (dPCD)44. Leaf senescence is also a complex and highly regulated process that involves the degradation of macromolecules, including proteins, lipids, and nucleic acids45. Nutrients, especially nitrogen, are remobilized from old leaves to newly leaves or reserve organs during leaf senescence45. Our results revealed that leaf aging increases the abundance of metabolic genes, especial for amino acid metabolism, lipid metabolism and carbohydrate metabolism, which is crucial for macromolecules degradation of aging (yellow) leaves. Through this process, especially amino acid degradation, plants circulate nutrients from dying leaves to other plant organs to maximize nutrient retention38. α-Proteobacteria46 and Actinobacteria47 have always been categorized as oligotrophic bacteria48. It is thus not surprising to find that they were enriched in leaf endosphere as leaf nutrient concentration decreased. This explains that TN, TK and OM were significantly lower in yellow leaves than in green leaves because those nutrients have already been relocated to other parts of the plant. In addition, Actinobacteria are a group of saprophytic bacteria widely distributed in soil, water and plants, and play an important ecological role in recycling substances in nature49. A higher relative abundance of saprotroph endophyte were observed in yellow leaves, can be possibly explained by the fact that dPCD-accelerated plants enhance resistance to biotrophic and hemibiotrophic pathogens but impair resistance to necrotrophic pathogens30, which promotes invasion by saprophytic bacteria.
The observed paradox, wherein the relative abundance of endophyte pathogens in yellow leaves is higher than in green leaves, while the overall relative abundance of plant pathogens is lower in yellow leaves, could possibly be attributed to the classification scheme within FUNGuild. The “plant pathogens” category may encompass a wider range of pathogens than solely epiphytic and endophytic ones. It is plausible that other pathogen groups, not categorized as epiphytic or endophytic, exhibit reduced representation in yellow leaves, thereby causing a decrease in the overall relative abundance of plant pathogens despite an increase in endophyte pathogens. Overall, our results further indicate that bacteria dominates nutrient relocation during leaf senescence, and confirm the hypothesis that leaf senescence increases the abundance of metabolism genes, which would accelerate nutrient relocation from yellow leaves to other organs of rubber trees.
Our study showed that leaf senescence enriched oligotrophic (r-selected) microorganisms and promoted greater stochasticity during community assembly. We found that the nutrient concentration of yellow leaves was significantly lower than that of green leaves (Supplementary Table 2), especially for TN and TK. Moreover, the α-Proteobacteria and Actinobacteria were enriched in yellow leaf endosphere compared to green leaves. This may be caused by shifting microbial life strategies triggered by leaf nutrient decrease50. α-Proteobacteria (Supplementary Data 1)46 and Actinobacteria47 have always been categorized as oligotrophic bacteria48. It is thus not surprising to see that they increase in abundance as leaf nutrient concentration decreases. Oligotrophic bacteria, such as members of α-Proteobacteria, can only survive on a minimum amount of nutrients, which means they need a lot of organic nutrients51. Our results confirm that the change of the bacterial community is most likely caused by the change in substrate availability52. Based on microbial r-K strategy classification41,42, bacteria are generally classified as r-strategist, and fungi are typically slower growing than bacteria and are usually described as K-strategist53. This explains that most core fungi ASVs were depleted as leaf aged.
Our study also revealed that deterministic processes played a dominant role in the assembly of epiphytic bacterial and fungal communities in yellow leaves (Fig. 6A). This finding is consistent with previous studies showing that microbial community assembly is more governed by stochastic processes at early stages of their assembly but deterministic processes become more important at later stages54,55. For epiphytic bacteria of yellow leaves, MST value near -1 suggests less dissimilarity between the observed communities than their similarity to the expected. This observation could result from strong environmental filtering leading to community convergence under stressed conditions. Conversely, MST value of green leaf close to 1 indicates greater differences between communities, which may signal the importance of competition or specific environmental gradients in otherwise favorable conditions. It was worth noting that the MST for leaf endophytic bacteria and fungi show a trend from 0 to 1with leaf senescence, while leaf surface shows a trend from 1 to 0, these may imply that as the leaves senescence, epiphytic and endophytic microbial communities undergo a different assembly process. Some studies suggest that the importance of stochastic processes is correlated with high nutrient content and deterministic processes tend to be associated with low nutrient conditions56,57,58. If this is the case, deterministic processes should be more important in yellow leaf endosphere communities than in green leaves. However, the opposite was found in our study for bacterial community assembly in the endosphere of yellow leaves (Fig. 6A). This might help explain the opposite results of deterministic processes being more important in microbial communities at later stages than stochastic processes54,55. In line with a previous study59, we suggest that organism types (e.g., bacteria or fungi, r-selected or k-selected) should be considered when evaluating the mechanisms of microbial community assembly. We also showed that deterministic processes mostly regulated fungal community assembly, while stochastic processes dominated assembly for bacterial communities in the leaves of rubber trees. This could arise from the body size effect, which postulates that smaller organisms (bacteria) experience less environment filtering than larger organisms (fungi), suggesting the importance of deterministic processes increases with organism size60,61,62. α-Proteobacteria and Actinobacteria (belonging to oligotrophic or r-selected bacteria) are of small size63, which may contribute the stochastic processes for endophytic bacterial community. The ecological processes of most enriched endophytic core ASVs (derived from Sloan’s model) belong to limited dispersal and drift (Supplementary Data 1), also explains why stochastic processes become more important in endophytic bacterial community assembly in senescent leaves. In addition, our results revealed that most core bacterial ASVs are members of Methylobacterium, which is a prevalent bacterial genus of the phyllosphere64. A previous study reports that members of Methylorubrum grow rapidly and dominate the phyllosphere community before leaves fully senescent64. Our finding reinforces the hypothesis that oligotrophic (r-selected) microorganisms promote stochastic community assembly as leaf ages.
Our study also showed leaf senescence accompanied by cell wall disassembly increased the probability of microbial invasion and subsequently increased microbial diversity in leaf endosphere32. Previous work finds that bacterial populations are exposed to increasingly selective pressures as leaves age65 caused by the weakened immune system or protective mechanisms of senescent leaves11,17,66. Cell wall disassembly also occurs during leaf senescence32. The loss of protective mechanisms results in the rapid proliferation of microbiomes67,68. This explains the more endophytic bacterial ASVs were originated from surface of yellow leaves than green ones (Fig. 6). The higher diversity in endophytic and epiphytic yellow leaves (Fig. 3A, B) further confirms that yellow leaves are more susceptible to bacterial invasion with leaf senescence. In addition, the cells that die as a consequence of PCD in yellow leaves provide substrate for leaf microbiome, further increasing the diversity of microbial communities43. However, we found no significant difference in selective filtration effect between yellow and green leaves for fungal communities. Our work provides evidence for our hypothesis that leaf senescence leads to enhanced microbial community diversity. In addition, the accumulated ASVs curves do not reach an asymptote, indicating a rich and potentially unexplored microbial diversity within the leaf samples, highlighting the complexity of phyllosphere ecosystems and the necessity to gain further understanding of its biodiversity.
Methods
Study site and sampling
Rubber plantation is the most economically significant agroecosystem in tropical China69. Our study sites were selected on Hainan Island and in the Xishuangbanna region, where rubber plantations cover nearly one fourth and one third of the vegetated area, respectively70. A total of six study sites, encompassing major plantations, were chosen from Danzhou, Wanning, and Ledong Districts on Hainan Island, and from Jinghong, Menglun, and Mengpeng Districts in Xishuangbanna (Supplementary Fig. 1A-C). At each site, three plots separated by 5–15 km in distance were selected for sampling. We recorded the latitude, longitude, and elevation for each site, and obtained data for mean annual precipitation and mean annual temperature from the National Meteorological Information Center (https://www.data.cma.cn).
In each sampling plot, leaf samples were collected from three trees separated from one another by ~100 m. We collected leaves from three rubber trees to ensure their representativeness. Both green and yellow leaves were collected simultaneously from branches located 2 meters away from the center of the trunk and 12 meters above the ground (see Supplementary Fig. 1D). Leaves were selected from each of the four cardinal directions (i.e., north, south, east, and west), and all sampled leaves from the three trees were respectively mixed for green or yellow leaves to form a single composite sample. A total of 72 leaf samples were collected during the two seasons (i.e., rainy season and dry season), resulting in a total of 144 samples collected over the course of the experiment (September 2019 for the rainy season and December 2020 for the dry season). The leaf mass of each sample was ~200 g. The collected leaves were then placed in sterilized, sealed bags and stored in a dry ice box. After completing the field sampling, the samples were promptly transported to a nearby research station for processing within 2 h.
Approximately 185–190 g was used for nutrient measurement, and the rest was used for microbial community analysis. We randomly selected several leaves from each sample for quantifying water content (WC), total nitrogen (TN), total phosphorus (TP), total potassium (TK) and organic matter (OM). WC and OM was measured gravimetrically. TN was determined using micro-Kjeldahl digestion followed by steam distillation. TP and TK were assessed using NaOH digestion. Leaf pH was measured in a leaf/water suspension (1:2.5, w/w) using a pH meter. Detailed information about leaf physicochemical properties are presented in Supplementary Table 1. The remainder was stored at -80 °C for DNA extraction.
To collect the epiphytic suspensions, intact leaves (to minimize host DNA contamination) weighing 10–15 g were individually placed into centrifuge tubes containing 1 ml of PBS and shaken at 300 rpm for 15 min at 4 °C. Subsequently, the same leaves were washed three times in fresh PBS, transferred to new microcentrifuge tubes containing 1 ml of PBS, and then subjected to sonication in a sonication bath for 1 min at 4 °C to collect the phyllosphere suspensions. The phyllosphere suspensions were then filtered through a 0.2 μm filter membrane to isolate epiphytic microbes71. Following surface sterilization72, the same leaves were placed in sealed plastic bags for endophytic microbes. The filter membrane and the sterilized leaves were temporarily stored at -80 °C to prevent external microbial DNA degradation or contamination. Within no more than 2 days, the epiphytic and endophytic samples were sent to Majorbio Bio-pharm Technology Co., Ltd for sequencing.
DNA extraction and sequencing
Samples underwent DNA extraction using the FastDNA® Spin Kit for Soil (MP Biomedicals) following the manufacturer’s instructions. The DNA extracts were assessed on a 1% agarose gel, and DNA concentration and purity were determined using a NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, USA). The hypervariable region V4 of the bacterial 16S rRNA gene was amplified with primer pairs 515FmodF (5’-GTGYCAGCMGCCGCGGTAA-3’) and 806RmodR (5’-GGACTACNVGGGTWTCTAAT-3’)73,74. The fungal ITS1 hypervariable region was amplified using the PCR primers ITS1F (5’-CTTGGTCATTTAGAGGAAGTAA-3’) and ITS2R (5’-GCTGCGTTCTTCATCGATGC-3’)75. Polymerase chain reaction (PCR) amplification was performed as follows: initial denaturation at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 45 s, with a final extension at 72 °C for 10 min, followed by a cooling step to 10 °C. The PCR mixtures contained 5 × TransStart FastPfu buffer 4 μL, 2.5 mM dNTPs 2 μL, forward primer (5 μM) 0.8 μL, reverse primer (5 μM) 0.8 μL, TransStart FastPfu DNA Polymerase 0.4 μL, template DNA 10 ng, and ddH2O up to 20 μL76. PCR products were purified using the QIAquick gel extraction kit (Qiagen). Additionally, a negative control was performed, where no DNA samples were added, and only primers and reaction solutions were used to detect the presence of contamination in the reaction system. The results of the negative control are shown in Supplementary Fig. 2. The denoising approach of DADA2 was employed in the Amplicon Sequence Variant (ASV) analysis, which seamlessly combines the denoising procedure with chimera removal. Primer sequences and low-quality read ends with a quality score (Q) below 20 were trimmed. Paired-end sequences were merged to a single sequence of length ~300 bp, and the resultant sequences were quality-filtered (maximum expected error = 0.2), and singletons were removed using USEARCH v.1077. Subsequently, all samples were pooled in equimolar concentrations and then sequenced on the Illumina MiSeq platform with a paired-end protocol. The sequencing coverage (sequencing depth) ranged from 0.9888–0.9998. Raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: SRP342019).
Data analysis
The raw fastq files were demultiplexed and quality-filtered using QIIME278. The DADA2 package was utilized in the R environment to identify the amplicon sequence variants (ASVs) in each sample79. The taxonomy of each ASV was analyzed by RDP Classifier version 11.5 against the 16S rRNA database SILVA (version 138) and ITS database UNITE (version 8.0) using a confidence threshold of 0.780. Nonbacterial ASVs (1719 ASVs out of 70693 reads were assigned to Mitochondria or Chloroplast) were then removed15. Overall, paired-end sequencing resulted in 7,491,619 high-quality reads, which could be assembled into 13,911 bacterial ASVs at 100% identity. For fungi, 8,056,178 high-quality reads were assembled into 22,817 fungal ASVs at 100% identity.
Diversity indices (Chao and Shannon) based on identified ASVs were calculated for each sample using the R vegan package81. Additionally, the UpSet venn diagram was generated using the Majorbio cloud tool82. Principal Coordinates Analysis (PCoA) was conducted using Bray-Curtis distances for the 144 samples (72 endophytic and 72 epiphytic) to explore bacterial and fungal community compositional differences at the genus level between the green and yellow leaves. Permutational multivariate analysis of variance (PERMANOVA) was performed using the vegan package with 999 permutations to evaluate the effect of leaf development stages, seasonal changes, and sampling locations on the taxonomical composition of the phyllosphere bacterial community. Subsequently, PERMDISP2 was used to evaluate the multivariate homogeneity of group dispersions using the vegan package.
To model leaf nutrients, linear models were used to represent the relationship between leaf nutrients and functional gene abundance. A generalized linear model (GLM) based on negative binomial distribution was used to test if microbial ASVs on yellow leaves were significantly enriched or depleted compared to those on green leaves14,83. The GLMs was parameterized using the edgeR package in R environment84. Fold change (FC) was defined as the ratio of microbial abundance in yellow leaves to that in green leaves. Those with FC >1 and p < 0.05 (Bonferroni’s test) were considered as enriched ASVs, while those with FC <1 at p < 0.05 were considered as depleted ASVs.
PICRUSt2 (version 2.2.0) was used to predict bacterial functions85. Fungal functional classification in the samples and the abundance of each functional type at different stages of rubber tree leaves were obtained using FUNGuild86.
Utilizing the Sloan model is instrumental in distinguishing genuinely pivotal taxa from those that are common and less crucial, thereby enhancing our comprehension of microbial community structures and functions. Core bacterial and fungal ASVs were identified using the abundance-occupancy method87. Following this, the Sloan model was applied to fit the abundance-occupancy distributions. Core members that exceed the neutral model prediction are present in higher occupancy than expected based on their mean abundance, indicating that the plant environment selects for these taxa, while taxa that fall below the prediction are present in higher abundance than expected, suggesting that these taxa are limited by dispersal.
To elucidate the dynamic change in leaf microbial communities during leaf aging, it is essential to explore the processes governing community assembly. The modified stochasticity ratio (MST), based on Jaccard’s dissimilarity, was utilized to quantitatively assess whether community assembly was more deterministic (MST < 50%) or stochastic (MST > 50%)88. The MST was calculated using the NST package in the R environment, and the function nst.panova was applied to test the significance of the differences between green and yellow leaves. Subsequently, MST indices for the microbial communities were visualized using ggplot289.
In order to uncover the underlying mechanisms driving the differences in composition and diversity between yellow and green leaves, the fast expectation-maximization microbial source tracking (FEAST) method was employed to estimate the percentage of microorganisms in the leaf interior originating from the leaf surface. The FEAST analysis was conducted using the R package FEAST 90.
Statistics and reproducibility
The bacterial and fungal dataset analyzed in this study comprised 144 samples with 72 for green samples and 72 for yellow samples. Statistical analyses were conducted using vegan, FEAST, NST package in the R environment (https://www.R-project.org/). All statistical tests were two-tailed. The results were visualized using ggplot2.
Data availability
Raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: SRP342019). The source data for the relevant figures are available on Zenodo (https://doi.org/10.5281/zenodo.13295002).
Code availability
R code used in this study were available on Zenodo (https://doi.org/10.5281/zenodo.13295002).
References
Baldotto, L. E. & Olivares, F. L. Phylloepiphytic interaction between bacteria and different plant species in a tropical agricultural system. Can. J. Microbiol. 54, 918–931 (2008).
Lindow, S. E. & Brandl, M. T. Microbiology of the phyllosphere. Appl. Environ. Microbiol 69, 1875–1883 (2003).
Woodward, F. I. & Lomas, M. R. Vegetation dynamics–simulating responses to climatic change. Biol. Rev. Camb. Philos. Soc. 79, 643–670 (2004).
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A. & Dufresne, A. The importance of the microbiome of the plant holobiont. N. Phytol. 206, 1196–1206 (2015).
Fitzpatrick, C. R. et al. The plant microbiome: from ecology to reductionism and beyond. Annu. Rev. Microbiol. 74, 81–100 (2020).
Friesen, M. L. et al. Microbially mediated plant functional traits. Annurevecolevolsyst 42, 23–46 (2011).
Martin, F. M., Uroz, S., Barker, D. G. Ancestral alliances: plant mutualistic symbioses with fungi and bacteria. Science 356, eaad4501 (2017).
Müller, D. B., Vogel, C., Bai, Y. & Vorholt, J. A. The plant mnicrobiota: systems-level insights and perspectives. Annu. Rev. Genet. 50, 211–234 (2016).
Rosado, B. H. P., Almeida, L. C., Alves, L. F., Lambais, M. R. & Oliveira, R. S. The importance of phyllosphere on plant functional ecology: a phyllo trait manifesto. N. Phytol. 219, 1145–1149 (2018).
Preininger, C., Sauer, U., Bejarano, A. & Teresa, B. Concepts and applications of foliar spray for microbial inoculants. Appl. Microbiol. Biot. 102, 7265–7282 (2018).
Beckers, B., Op De Beeck, M., Weyens, N., Boerjan, W. & Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 5, 25 (2017).
Coleman-Derr, D. et al. Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. N. Phytol. 209, 798–811 (2016).
DeAngelis, K. M. et al. Selective progressive response of soil microbial community to wild oat roots. ISME J. 3, 168–178 (2009).
Edwards, J. et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl Acad. Sci. USA 112, E911–920 (2015).
Laforest-Lapointe, I., Messier, C. & Kembel, S. W. Host species identity, site and time drive temperate tree phyllosphere bacterial community structure. Microbiome 4, 27 (2016).
Wei, Y. et al. Phyllosphere fungal communities of rubber trees exhibited biogeographical patterns, but not bacteria. Environ. Microbiol 24, 3777–3790 (2022).
Xiong, C. et al. Plant developmental stage drives the differentiation in ecological role of the maize microbiome. Microbiome 9, 171 (2021a).
Yao, H. et al. Phyllosphere epiphytic and endophytic fungal community and network structures differ in a tropical mangrove ecosystem. Microbiome 7, 57 (2019).
Bahram, M. et al. Stochastic distribution of small soil eukaryotes resulting from high dispersal and drift in a local environment. ISME J. 10, 885–896 (2015).
Lan, G. et al. Driving factors for soil fungal and bacterial community assembly in topical forest of China. Appl. Soil. Ecol. 177, 104520 (2022).
Chen, S. et al. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 7, 136 (2019).
Cordovez, V., Dini-Andreote, F., Carrión, V. J. & Raaijmakers, J. M. Ecology and evolution of plant microbiomes. Annu. Rev. Microbiol. 73, 69–88 (2019).
Gao, C. et al. Fungal community assembly in drought-stressed sorghum shows stochasticity, selection, and universal ecological dynamics. Nat. Commun. 11, 34 (2020).
Grady, K. L., Sorensen, J. W., Stopnisek, N., Guittar, J. & Shade, A. Assembly and seasonality of core phyllosphere microbiota on perennial biofuel crops. Nat. Commun. 10, 4135 (2019).
Trivedi, P., Leach, J. E., Tringe, S. G., Sa, T. & Singh, B. K. Plant-microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol 18, 607–621 (2020).
Zhang, J. et al. Root microbiota shift in rice correlates with resident time in the field and developmental stage. Sci. China Life Sci. 61, 613–621 (2018).
Zhao, M. et al. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 44, 613–628 (2021).
Li, Y. et al. Estimating changes in soil organic carbon storage due to land use changes using a modified calculation method. iForest 8, 45–52 (2015).
Li, Y., Lan, G. & Xia, Y. Rubber trees demonstrate a clear retranslocation under seasonal drought and cold stresses. Front. Plant Sci. 7, 1907 (2016).
Zhang, Y., Wang, H.-L., Li, Z. & Guo, H. Genetic network between leaf senescence and plant immunity: crucial regulatory nodes and new insights. Plants 9, 495 (2020).
Woo, H. R., Kim, H. J., Lim, P. O. & Nam, H. G. Leaf senescence: systems and dynamics aspects. Annu. Rev. Plant Biol. 70, 347–376 (2019).
Lim, P. O., Kim, H. J. & Gil Nam, H. Leaf senescence. Annu. Rev. Plant Biol. 58, 115–136 (2007).
Yang, H. Z., Wang, L. H., Bei, M. R. & Lin, Z. M. Differences in microelement content of the leaves in fifferent varieties of young rubber tree. Chin. J. Tropical Crops 37, 2043–2047 (2016).
Fife, D. N., Nambiar, E. K. & Saur, E. Retranslocation of foliar nutrients in evergreen tree species planted in a Mediterranean environment. Tree Physiol. 28, 187–196 (2008).
Masclaux, C., Valadier, M. H., Brugière, N., Morot-Gaudry, J. F. & Hirel, B. Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211, 510–518 (2000).
Millard, P. & Grelet, G. A. Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiol. 30, 1083–1095 (2010).
Watanabe, M. et al. Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis. Plant Physiol. 162, 1290–1310 (2013).
Huang, X. Z. et al. Global distributions of foliar nitrogen and phosphorus resorption in forest ecosystems. Sci. Total Environ. 871, 162075 (2023).
An, J. P. et al. Mdbhlh93, an apple activator regulating leaf senescence, is regulated by aba and mdbt2 in antagonistic ways. N. Phytol. 222, 735–751 (2019).
van der Heijden, M. G. A., Bardgett, R. D. & van Straalen, N. M. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296–310 (2008).
Fierer, N., Bradford, M. A. & Jackson, R. B. Toward an ecological classification of soil bacteria. Ecology 88, 1354–1364 (2007).
Wang, Z. H. et al. N and P fertilization enhanced carbon decomposition function by shifting microbes towards an r-selected community in meadow grassland soils. Ecol. Indic. 132, 108306 (2021).
Quirino, B., Noh, Y. S., Himelblau, E. & Amasino, R. Molecular aspect of leaf senescence. Trend Plant Sci. 5, 278–282 (2000).
Daneva, A., Gao, Z., Van Durme, M. & Nowack, M. K. Functions and regulation of programmed cell death in plant development. Annu. Rev. Cell Dev. Biol. 32, 441–468 (2016).
Liu, J., Wu, Y. H., Yang, J. J., Liu, Y. D. & Shen, F. F. Protein degradation and nitrogen remobilization during leaf senescence. J. Plant Biol. 51, 11–19 (2008).
Männisto, M. et al. Do shifts in life strategies explain microbial community responses to increasing nitrogen in tundra soil? Soil Biol. Biochem 96, 216–28 (2016).
Morrissey, E. M. et al. Phylogenetic organization of bacterial activity. ISME J. 10, 2336–2340 (2016).
Ho, A., Di Lonardo, D. P. & Bodelier, P. L. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 93, fix006 (2017).
Khucharoen, K., Puangpetch, U., Puttaraksa, K. & Sinma, K. Grouping of actinomycetes isolated from termites using biochemical character. J. Biol. Sci. 11, 314–319 (2011).
Barder, M. J. & Crawford, D. L. Effects of carbon and nitrogen supplementation on lignin and cellulose decomposition by a Streptomyces. Can. J. Microbiol. 27, 859–863 (1981).
Li, H., Yang, S., Semenov, M. V., Yao, F. & Kuzyakov, Y. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Change Biol. 27, 2763–2779 (2021).
Tlaskal, V., Voriskova, J. & Baldrian, P. Bacterial succession on decomposing leaf litter exhibits a specific occurrence pattern of cellulolytic taxa and potential decomposers of fungal mycelia. Fems Microbiol. Ecol. 11, fiw177 (2016).
Six, J., Frey, S. D., Thiet, R. K. & Batten, K. M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 70, 555–569 (2006).
Dini-Andreote, F., Stegen, J. C., van Elsas, J. D. & Salles, J. F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl Acad. Sci. USA 112, E1326–1332 (2015).
Ferrenberg, S. et al. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. ISME J. 7, 1102–1111 (2013).
Chase, J. M. Stochastic community assembly causes higher biodiversity in more productive environments. Science 328, 1388–1391 (2010).
Feng, Y. et al. Two key features influencing community assembly processes at regional scale: initial state and degree of change in environmental conditions. Mol. Ecol. 27, 5238–5251 (2018).
Zhou, J. & Ning, D. Stochastic community assembly: does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 81, 32 (2017).
Liu, K. et al. Different community assembly mechanisms underlie similar biogeography of bacteria and microeukaryotes in Tibetan lakes. FEMS Microbiol. Ecol. 96, fiaa071 (2020).
De Bie, T. et al. Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecol. Lett. 15, 740–747 (2012).
Farjalla, V. F. et al. Ecological determinism increases with organism size. Ecology 93, 1752–1759 (2012).
Liu, J. et al. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 83, 29–39 (2015).
Luan, L. et al. Organism body size structures the soil microbial and nematode community assembly at a continental and global scale. Nat. Commun. 11, 6406 (2020).
Leducq, J. B. et al. Fine-scale adaptations to environmental variation and growth strategies drive phyllosphere methylobacterium diversity. mBio 13, e03175-21 (2022).
Goerke, C. & Wolz, C. Adaptation of staphylococcus aureus to the cystic fibrosis lung. Int. J. Med. Microbiol. 300, 520–525 (2010).
Bernard, J. et al. Plant part and a steep environmental gradient predict plant microbial composition in a tropical watershed. ISME J. 15, 999–1009 (2021).
Pang, Z. et al. Linking plant secondary metabolites and plant microbiomes: a review. Front. Plant Sci. 12, 621276 (2021).
Voříšková, J. & Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 7, 477–486 (2013).
Lan, G., Wu, Z., Chen, B. & Xie, G. Species diversity in a naturally managed rubber plantation in Hainan island, South China. Trop. Conserv Sci. 10, 194008291771242 (2017).
Zhai, D. L., Cannon, C. H., Slik, J. W., Zhang, C. P. & Dai, Z. C. Rubber and pulp plantations represent a double threat to Hainan’s natural tropical forests. J. Environ. Manag. 96, 64–73 (2012).
Lan, G. Y., Wei, Y. Q., Li, Y. W. & Wu, Z. X. Diversity and assembly of root-associated microbiomes of rubber trees. Front. Plant Sci. 14, 1136418 (2023).
Xiong, C. et al. Host selection shapes crop microbiome assembly and network complexity. N. Phytol. 229, 1091–1104 (2021b).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell 167, 1469–1480.e1412 (2016).
Walters, W. et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 1, e00009–15 (2015).
Adams, R. I., Miletto, M., Taylor, J. W. & Bruns, T. D. Dispersal in microbes: fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 7, 1262–1273 (2013).
He, R. et al. Contrasting patterns in diversity and community assembly of Phragmites australis root- associated bacterial communities from different seasons. Appl. Environ. Micro. 86, e00379–e420 (2020).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Abdelfattah, A., Wisniewski, M., Schena, L. & Tack, A. J. M. Experimental evidence of microbial inheritance in plants and transmission routes from seed to phyllosphere and root. Environ. Microbiol. 23, 2199–2214 (2021).
Oksanen, J., et al. Vegan: Community Ecology Package. R package version 2.6-4. https://CRAN.R-project.org/package=vegan (2022).
Ren, Y. et al. Majorbio cloud: a one-stop, comprehensive bioinformatic platform for multi-omics analyses. iMeta 1, e12 (2022).
He, R., Zeng, J., Zhao, D., Wang, S. & Wu, Q. L. Decreased spatial variation and deterministic processes of bacterial community assembly in the rhizosphere of Phragmites australis across the middle-lower Yangtze plain. Mol. Ecol. 31, 1180–1195 (2022).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. EDGER: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Douglas, G. M. et al. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 38, 685–688 (2020).
Nguyen, N. H. et al. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248 (2016).
Shade, A. & Stopnisek, N. Abundance-occupancy distributions to prioritize plant core microbiome membership. Curr. Opin. Microbiol. 49, 50–58 (2019).
Ning, D., Deng, Y., Tiedje, J. M. & Zhou, J. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl Acad. Sci. USA 116, 16892–16898 (2019).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis. 1st edn, Vol. 213 (Springer, 2016).
Shenhav, L. et al. FEAST: fast expectation-maximization for microbial source tracking. Nat. Methods 16, 627–632 (2019).
Acknowledgements
We would like to thank Dr. Unruh Micah Leigh at the University of Kansas for his assistance with English language and grammatical editing of the manuscript. This work was supported by the National Natural Science Foundation of China (32271603), the Central Public-interest Scientific Institution Basal Research Fund (1630022024013) and the Earmarked Fund for Chinese Agricultural Research Systems (CARS-33-ZP3).
Author information
Authors and Affiliations
Contributions
G.Y.L. and F.H. planned and designed research; Y.Q.W. and X.C.Z. performed experiments; G.Y.L., Z.X.W., Y.Q.W., B.Q.C., H.X. K.P.J. conducted fieldwork; G.Y.L. analyzed the data; G.Y.L. and F.H. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study did not include the use of any animals, human or otherwise, so did not require ethical approval.
Consent for publication
All the authors read the manuscript carefully and gave their consent for publication.
Peer review
Peer review information
Communications Biology thanks Petr Pyszko and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editors: Anna Heintz-Buschart and David Favero. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lan, G., Wei, Y., Zhang, X. et al. Assembly and maintenance of phyllosphere microbial diversity during rubber tree leaf senescence. Commun Biol 7, 1192 (2024). https://doi.org/10.1038/s42003-024-06907-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-06907-x
This article is cited by
-
Nutrient content of grass influences microbial communities in the phyllosphere more than climate change
Applied Microbiology and Biotechnology (2026)
-
Phyllosphere synthetic microbial communities: a new frontier in plant protection
BMC Plant Biology (2025)