Abstract
Ceramides are lipids that play vital roles in complex lipid synthesis, membrane function, and cell signaling. Disrupted ceramide homeostasis is implicated in cell-death and several neurologic diseases. Ceramides are often analyzed in tissue, but this approach fails to resolve cell-type differences in ceramide homeostasis that are likely essential to understanding cell and non-cell autonomous contributions to neurodegeneration. We show that human iPSC-derived neurons and glia differ in their rate of ceramide synthesis, ceramide isoform composition, and responses to altered ceramide levels. RNA-sequencing of cells treated to increase or decrease ceramides revealed connections to inflammation, ER stress, and apoptosis. Moreover, introducing labeled sphinganine showed that glia readily synthesize ceramide de novo and that neurons are relatively more sensitive to ceramide toxicity. Our findings provide a framework for understanding neurologic diseases with sphingolipid alternations and insights into designing therapeutics that target ceramide for treating them.

Similar content being viewed by others
Introduction
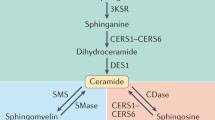
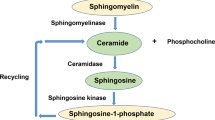
Ceramides are sphingolipids that perform both structural and signaling functions within the cell. Structurally, ceramides can regulate membrane fluidity and curvature under normal conditions and contribute to membrane pore formation during cellular stress1,2. Ceramide is generated through two different pathways: de novo synthesis, and through its salvage following the hydrolysis of complex lipids such as sphingomyelin, glucosylceramide, and galactosylceramide (Fig. 1A)3. The de novo synthesis pathway is initiated by the rate limiting enzyme complex serine palmitoyltransferase (SPT), followed by the synthesis of dihydroceramide by six ceramide synthases (CerS1-6). Dihydroceramide is then desaturated by DEGS to form ceramide4,5,6,7,8,9,10,11. Each CerS preferentially generates ceramides with specific fatty acid chain lengths, conferring different properties to each ceramide species.
A Diagram illustrating the different pathways involved in ceramide homeostasis. FB1 is an inhibitor of ceramide synthases (CerS) which generate dihydroceramide (dHCer). Compound 667161 inhibits UGCG (UDP-glucose ceramide glucosyltransferase) which generates glucosylceramide (GlcCer) from ceramide. Graphs of both cytoplasmic (B) and nuclear (C) profiles of different ceramide chain lengths (N = 8 wells per condition). Motor neurons (MNs) had significantly higher levels of cytoplasmic and nuclear C18-ceramide (****p < 0.01). Graphs comparing the cytoplasmic (D) and nuclear (E) profiles of different neuronal subtypes. Statistical comparisons made by one-way ANOVA within each ceramide chain length (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; n = 8 replicate wells).
Disrupted ceramide homeostasis (i.e., ceramide cacostasis) is implicated in the pathogenesis of both rare and common diseases of the CNS. Farber disease (FD) is a rare lysosomal storage disease caused by a complete deficiency in acid ceramidase (ASAH1), an enzyme that breaks down ceramide into sphingosine. In FD patients and FD models, the resultant accumulation of ceramide leads to inflamed joints, infiltration of macrophages into multiple tissues, and neurological dysfunction12. Mutations that result in partial loss of ASAH1 activity are associated with non-5q spinal muscular atrophy, and morpholino knockdown of ASAH1 in zebrafish leads to a loss of motor neuron axons, further bolstering evidence that ceramide accumulation triggers neurodegeneration13. Interestingly, SPTLC1 (an SPT complex member) gain of function mutations that increase ceramide synthesis also leads to motor neuron disease in children14,15,16. Moreover, mutations in SPTLC that result in deoxysphingolipid accumulation lead to Hereditary Sensory and Autonomic Neuropathy Type I (HSAN-I), a disease with sensory loss and distal muscle weakness15,17,18. On the other hand, loss of function of DEGS1 disrupts ceramide de novo synthesis and leads to hypomyelinating leukodystrophy type 18 (HLD18), illustrating the importance of precise ceramide synthesis regulation for brain development and function9,11.
Studies indicate that ceramide cacostasis also contributes to the pathogenesis of more common neurodegenerative diseases including Parkinson’s disease (PD) and Alzheimer Disease (AD). For example, ceramide is increased in PD patient plasma but decreased in cortical areas19,20,21. Knockout of PD risk genes in both flies (iPLA2-VIA knockout) and mice (Lrrk2 knockout) resulted in increased ceramides, and overexpression of cellular alpha-synuclein (a pathological hallmark of PD) caused ceramide accumulation22,23. Multiple studies point to increased ceramide levels in AD patients24,25,26. Ceramides are elevated in serum exosomes of 5xFAD model mice, toxic amyloid treatment increases astrocyte ceramide levels, and ceramide-containing exosomes activate cell death pathways27,28. Inhibition of ceramide synthesis is beneficial in mouse models of AD (TgCRND8), reducing both cortical ceramide and hyperphosphorylated tau levels29.
While ceramide dysregulation contributes to multiple CNS diseases, it is unclear how ceramide levels are regulated in different CNS cell-types14,16. Here, we utilized motor and cortical neurons, astrocytes, and microglia derived from human induced pluripotent stem cells (iPSCs), to understand cellular differences in ceramide homeostasis. Each cell type had a unique ceramide composition, expression profile of ceramide synthases and ceramidases, and ceramide synthase activity. Specifically, glia tended to have higher levels of ceramide synthesis related genes, higher de novo synthesis activity, and increased sensitivity to CerS inhibition. By RNA sequencing, we additionally show that glial, but not neuronal, RNA expression profiles are altered by CerS inhibition.
Notably, the ceramide profile of neurons appeared uniquely stable relative to microglia and astrocytes. Glia, and particularly astrocytes, have been implicated in the release of toxic lipid species, with neurons and oligodendrocytes often disproportionately affected by the toxicity. We therefore sought to understand how each cell might respond to ceramide precursors. Despite a very stable ceramide profile, neurons were relatively more susceptible to sphinganine toxicity than astrocytes. Surprisingly, microglia showed similar levels of sensitivity to sphinganine toxicity to neurons. These results were recapitulated to a certain extent by inhibition of glucosylceramide synthase inhibitor that triggered ceramide accumulation, as well as treatment of neurons with sphinganine in a liposomal formulation. This baseline understanding of how different cell types synthesize ceramide and respond to alterations in ceramide synthesis, may provide insights into the mechanisms behind multiple neurodegenerative diseases and illuminate new avenues for therapeutic intervention.
Results
Neurons and glia have distinct ceramide profiles
Previous studies have established that mouse CNS cell-types differ in their general lipidomic profiles30. However, the regulation of ceramide homeostasis in these cell types is not understood. Specifically, it is not known whether they share common ceramide species or whether ceramide generation occurs through distinct pathways (Fig. 1A). Furthermore, although sphingolipids play important roles in nuclear signaling and metabolism31, the subcellular composition of ceramides in CNS cell-types has not been investigated. Therefore, we utilized liquid chromatography/mass spectrometry (LC/MS) to profile nuclear and cytoplasmic fraction ceramides in human iPSC-derived cells (Fig. 1B–E). This allowed us to establish whether there are basal differences in human neuronal and glial ceramide profiles. Cytoplasmic C16- and C18-ceramide were highest in motor neurons (MNs), whereas astrocytes had the highest C24-ceramide and microglia the highest C24:1 (Fig. 1B). Astrocytes and microglia had significantly higher levels of nuclear fraction long-chain ceramides (C22, C24) than MNs (Fig. 1C). Consistent with the cytoplasmic fraction, MNs had significantly higher levels of nuclear C18 than glial cells (Fig. 1C). In vivo studies have shown that CerS1 is neuronally localized and generates C18-ceramide32. This illustrates that the differing ceramide profiles in our iPSC-derived neuronal cell types may match in vivo profiles.
Ceramide profiles in different neuronal subtypes also vary
As neurons are often uniquely affected in diseases with sphingolipid dysregulation, we next determined the relative levels of ceramides in glutamatergic and GABAergic iPSC derived cortical neurons. Consistent with MNs, C18-ceramide levels were high in cortical neurons relative to the levels seen in glia (Fig. 1D, E). Glutamatergic neurons had the highest levels of C16- and C18-ceramide in both nuclear and cytoplasmic fractions (Fig. 1D) and had the highest levels of all ceramide chain lengths in the nuclear fraction (Fig. 1E). MNs had uniquely low levels of C24:1 in both fractions and C14 in the cytoplasmic fraction (Fig. 1D, E). This establishes that, while neurons have some commonalities in their ceramide profiles, the neuronal-subtype ceramide signatures are significantly different in ways that could be relevant to normal physiology and disease.
Bulk RNA-Seq characterization of ceramide pathway members within MNs and glia
Our lipidomics data establish that neurons and glia have distinct ceramide profiles. To identify the underlying genetic expression driving our lipidomic profile differences, we performed RNA sequencing on iPSC-derived astrocytes, MNs, and microglia. Due to differences in cellular viability, RNA libraries from neurons and glia were prepared differently. We are not comparing cellular expression directly by RNA-sequencing, we only assessed the relative expression levels within each cell-type. These data suggest that both glia and MNs depend on SPTLC1, CERS2, DEGS1, and SPTSSA, for ceramide production and that MNs might be distinctly dependent on SPTSSB and DEGS2 (Fig. 2A). MNs, astrocytes, and microglia have relatively lower CERS3 expression relative to other ceramide synthesis pathway members (Fig. 2A). Microglia had uniquely low CerS1 expression as compared to other CerS. ORMDLs, negative regulators of SPTLC33 were expressed in all cell types with ORMDL1 and ORMDL3 levels being relatively higher than ORMDL2 in astrocytes and MNs (Fig. 2A). Expression of enzymes comprising the sphingolipid anteome, metabolic pathways (e.g., NADPH, L-serine and Acyl-CoA) that directly converge on ceramide synthesis34, showed similar patterns of expression across all cell types with a few exceptions (Supplementary Fig. 1C).
Heatmaps illustrating the expression levels of genes involved in ceramide synthesis (A), ceramide/sphingosine phosphorylation and dephosphorylation (B), glycosphingolipid utilization and generation of ceramide (C), ceramide hydrolysis (D), ceramide transport (E), and sphingomyelin homeostasis (F), in iPSC-derived cells analyzed by bulk RNA-Seq (n = 3 replicate wells for astrocytes and motor neurons (MNs), n = 4 microglia replicate wells). G–L iPSC-derived cells were lysed and analyzed by Taqman qPCR for the expression of genes involved in ceramide synthesis and ceramidases. All gene expression was normalized to GAPDH controls. (One-way ANOVA, Tukey’s multiple comparisons test; n = 3 replicate wells, 3 qPCR reactions per well).
We also found cell type differences in the expression of enzymes regulating the generation of ceramide via its salvage from the dephosphorylation or hydrolysis of more complex lipids, the use of ceramide as a substrate in lipid synthesis, ceramide hydrolysis, and ceramide transport (Fig. 2B–F). PLPP1-3 are enzymes that dephosphorylate ceramide-1P to ceramide. All cells had relatively low PLPP2 expression and microglia had low PLPP3 as compared to PLPP1 (Fig. 2B). Lysosomal enzymes (i.e., GALC, ARSA, GLB1, HEXB and SMPD1) that generate ceramide through lipid hydrolysis had high levels of expression in glia relative to other pathway members (Fig. 2C and F). SMPD3, a non-lysosomal hydrolytic enzyme of sphingomyelin, was the most highly expressed SMPD gene in MNs, while being amongst the lowest in glia (Fig. 2F). Similarly, enzymes regulating ceramide hydrolysis also varied by cell type with ASAH1 being enriched in glia and ACER1 in MNs (Fig. 2D). The expression pattern of lipid synthases that use ceramide to make more complex lipids were comparable across cell types except for UGCG, which was enriched in microglia relative to other synthases (Fig. 2C).
Because we were not able to directly compare gene expression of different cell-types by RNA-sequencing, we performed qPCRs on ceramide synthesis pathway members to perform more direct comparisons of CerS. The SPT complex is an essential rate limiting step in ceramide synthesis, so we also assessed the expression levels of complex members SPTLC1, SPTLC2, SPTSSA, and SPTSSB. Microglia had higher expression of multiple synthesis genes (CERS1, SPTLC1, SPTLC2, SPTSSA) as compared to astrocytes and MNs (Fig. 2G, Supplementary Fig. 1A and B). Microglia also expressed ceramidases (ASAH1 and ACER3) at significantly higher levels (Fig. 2K and L). Notably, SPTSSA was highest in microglia, as this preferentially uses shorter chain acyl-CoAs to produce long chain bases, whereas MNs were highest for SPTSSB (Supplementary Fig. 2B). SPTSSB prefers longer chain acyl-CoAs such as C18 to produce long chain bases35. By qPCR, MNs also expressed significantly higher levels of CerS1 than astrocytes (Fig. 2G). CerS1 generates C18-fatty acid chain length and is known to be enriched in neurons in vivo. Astrocytes were highest for expression of CerS4 and CerS5 (Fig. 2H).
Neurons and glia differ in ceramide synthesis enzyme activities
RNA expression levels do not always correlate well with protein expression or protein function in the CNS, as might be indicated by our RNA-seq, qPCR, and lipidomics results. For example, CerC18 was highest in MNs relative to glia, CERS1 expression measured by RNA-seq was low in MNs relative to other ceramide synthesis pathway members, and CERS1 expression measured by qPCR was higher than astrocytes but lower than microglia (Figs. 1 and 2). To clarify the synthesis activity of each cell type, we characterized the functional activity of the CerS enzymes (Fig. 3A) by utilizing a ceramide synthesis activity assay36. Microglia had significantly higher levels of CerS1/4 (Fig. 3B), CerS2 (Fig. 3C), and CerS5/6 activity (Fig. 3D), than did astrocytes and MNs. Astrocytes had significantly higher CerS5/6 activity than MNs (Fig. 3D), whereas MNs had significantly higher CerS1/4 activity than astrocytes (Fig. 3B). This is consistent with our data showing high CerC18 levels in MNs (Fig. 1), and in vivo reports suggesting that neurons have uniquely high CerS1 expression and CerC18 levels32. This is additional confirmation that our iPSC derived cells maintain features of in vivo cell types. Based on comparing the different activity assays, astrocytes might be uniquely dependent on CerS5/6 activity for ceramide synthesis, while neurons uniquely dependent on CerS1. All microglial CerS activity was higher than the other cell types. Additionally, the unique dependence of astrocytes on CerS5/6 does not match the relative levels of astroglial CerC14 and CerC16 compared to neurons and microglia. These discrepancies between RNA levels and CerS activity, indicate that functional outputs for ceramide synthesis are needed. The lack of alignment between lipid levels and CerS activity is also likely due to cell type dependent differences in the generation of ceramide from alternative pathways.
A Schematic of the different fatty acid carbon chain lengths produced by different CerS enzymes. B Assay of CerS1/4 (18:0 palmitoyl-CoA and NBD-sphinganine added to lysate) activity in astrocyte, microglia, and motor neuron (MN) cell lysates. MNs had significantly higher CerS1/4 than did astrocytes (One-way ANOVA, Tukey’s multiple comparisons test). C Assay of CerS2 (24:1 palmitoyl-CoA and NBD-sphinganine added to lysate) activity in astrocyte, microglial, and MN cell lysates. D Assay of CerS5/6 (16:0 palmitoyl-CoA and NBD-sphinganine added to lysate) activity in astrocyte, microglial, and MN cell lysates. B–D Microglia had the highest CerS activity for all ceramide synthases measured (One-way ANOVA, Tukey’s multiple comparisons test). D Astrocytes had significantly higher CerS5/6 activity than did MNs (One-way ANOVA, Tukey’s multiple comparisons test). All synthesis activity was normalized to cell number (activity per 200 k cells) by taking nuclear cell counts and subtracting dead cells using nuclear cell-death stains from representative culture wells (activity assay is n = 3–6 wells of plated cells as shown in graphs, with n = 3 CerS activity reactions performed per well).
Pharmacological inhibition of CerS has cell-type specific effects on ceramide profiles
While basal ceramide levels and RNA expression data are informative, it is important to determine if CerS activity drives basal ceramide levels in differentiated neurons and glia. By treating with the pan-CerS inhibitor fumonisin-B1 (FB1), we isolated the portion of the ceramide profile driven by de novo CerS synthesis, versus lipids that are generated through alternative pathways (Fig. 1A). This might clarify differences observed in CerS-activity, lipidomics, and RNA expression. A variety of ceramide chain lengths were reduced after 24 hours of FB1 treatment of glial cells (Fig. 4A, B, and C, Supplementary Fig. 4C, 4E). Notably, the magnitude of reduction in astrocyte ceramides was larger than changes in microglial ceramide in response to FB1 inhibition (Fig. 4A, B, and C, Supplementary Fig. 4B and C). This could indicate differences in the levels of ceramide synthesis between astrocytes and microglia, or a greater compensatory change in microglia to keep ceramide levels stable via other ceramide generating pathways. Microglia did have less significant ceramide changes in the cytoplasmic fraction (Fig. 4C), indicating that cellular compartments such as lysosomes might give microglia unique capacities to compensate for changes in lipid synthesis. Interestingly, some of the largest changes in astrocyte ceramides were in CerC14 and CerC16, consistent with the high levels of CerS5/6 activity in these cells (Figs. 3D, 4B, and E). Microglia had some of the largest changes in longer-chain ceramides, consistent with their high level of CerS2 activity.
Motor neuron (MN) (A, D) astrocyte (B, E) and microglial (C, F) cytoplasmic and nuclear ceramide profiles after 72 hours of CerS inhibition. A–F Ceramides analyzed by 2-way ANOVA (3 μM FB1 treatment group shown, see Supplementary Fig. 4 for complete comparisons). G–I By introducing a traceable sphinganine (d17:0) precursor, in the presence or absence of 3 μM FB1, we were able to track the active CerS-dependent ceramide synthesis in each cell type over a 2-hour period. A significant percentage of astroglial (H) and microglial (I) ceramides were labeled (bars with green outlines) after the two hours. Notably, MNs (G) had a lower percent of each ceramide labeled. Further labeling data in Supplementary fig. 3 and 4. G–I Each ceramide chain length, vehicle vs FB1 treatment, was analyzed by Student’s t-test (****P < 0.0001, ***P < 0.001, *P < 0.05; n = 4 replicates).
In MNs, there were minimal changes in ceramide in response to FB1 inhibition – even after 72 hours of treatment. MNs showed some small changes in CerC18 and CerC24:1 (Fig. 4A and Supplementary Fig. 5A). By contrast, in response to FB1 treatment, glutamatergic and GABAergic cortical neurons both showed more robust significant reductions in ceramides (Supplementary Fig. 5). This may suggest that MNs have some distinct properties in maintaining ceramide stability, though we cannot exclude that differences in ceramide signatures could be partially driven by differences in cellular sizes and morphologies.
Labeling with d17:0-sphinganine confirms glial and neuronal ceramide synthesis rates
Perturbation of CerS and measures of overall ceramide levels may fail to adequately detect small changes in synthesis or the overall de novo synthesis capacity of each cell type. For a more sensitive measure of acute ceramide synthesis by CerS, we introduced a sphinganine precursor with an unnatural 17-carbon chain length (d17:0-sphinganine). This label is then incorporated into ceramide produced through CerS/de novo ceramide synthesis pathway. Across cell types, label incorporation was higher in dihydro-ceramides (dhCer) than in ceramides, consistent with the newly synthesized lipids incorporating the label, but labeled dihydroceramide not yet converted to ceramide by DEGS activity (Fig. 4–I). This, along with the lower label incorporation in FB1 treated cells, is indicative that the d17:0-sphinganine label is labeling ceramide generated through de novo synthesis as expected. There was a greater reduction in labeled ceramides with FB1 treatment than in unlabeled ceramides, consistent with labeled ceramides acting as a metric of acute ceramide synthesis (Fig. 4G–I).
Labeled ceramide was a more significant proportion of total ceramide in glia than MNs after two hours of incubation (Fig. 4G–I, Supplementary Fig. 2). This is consistent with our observation that MNs have significantly lower activity from multiple CerS than glial cells and show a minimal change in ceramide following FB1 treatment. This may also suggest that the high level of MN CerS1/4 activity in our enzyme assay may not directly parallel CerS1 kinetics in MNs in vitro, or account for ceramide incorporation into more complex lipids or its breakdown. Notably, newly synthesized lipids tracked cellular CerS activity in most cases. MNs had high CerS1/4 activity and low activity for other CerS (Fig. 3B), and correspondingly the limited newly synthesized ceramides were mostly dhCerC18 (Fig. 4G, Supplementary Fig. 3E, and F) which results from CerS1 activity. Consistent with these findings CerS5/6 (Fig. 3D) was maximal in astrocytes, which correspondingly had higher amounts of labeled dHCer16 and Cer16 (Fig. 4H). Microglia were high for activity of all CerS enzymes (Fig. 3) and had high levels of newly synthesized Cer16 and Cer24:1 (Fig. 4I). The main inconsistency between CerS-activity and ceramide labeling is the high level of microglial CerS1/4 (Fig. 3B) activity but no detectable levels of newly synthesized Cer18 (Fig. 4I). Future experiments will need to determine the relative contribution of CerS1 and CerS4 in regulating Cer18 levels in microglia. If CerS1 plays a more limited role than Cers4, which is suggested by our RNA-seq findings, this would be consistent with the essential role for CerS4 in regulating immune cells and the context specificity of the ceramide chain lengths generated by CerS437. This discrepancy could also be due to microglia having an increased capacity for hydrolyzing CerC18 or incorporating it into more complex sphingolipids and glycosphingolipids compared to other cell types.
Inhibition of glucosylceramide synthesis alters ceramide profiles in neurons and glia
The de novo ceramide synthesis pathway is highly dependent on CerS generation of dHCer and its subsequent conversion to ceramide by DEGS. FB1 was a logical tool to reduce ceramide synthesis and the addition of sphinganine allowed us to track de novo synthesis in these cell types. To induce ceramide accumulation in cells without the addition of an exogenous lipid, we treated cells with a glucosylceramide synthase inhibitor (GCSi; 667161) to prevent ceramide from being used to synthesize glucosylceramide (Fig. 1A). Given the differences in glial and MN dependence on de novo ceramide synthesis, we predicted that cellular differences in ceramide accumulation would likely occur after GCSi treatment. In astrocytes, GCSi treatment significantly increased CerC14 and CerC16 (Fig. 5B and E), consistent with high CerS5/6 activity and the reduction in the same ceramides with FB1 treatment. Microglia also had small accumulations in CerC14 and CerC16 in response to GCSi (Fig. 5C and F). The most dramatic increase in MNs after GCSi treatment was C18-ceramide in the cytoplasmic fraction (Fig. 5A and D), the expected chain length based on MN CerS1 activity and which ceramides were reduced after FB1 treatment. We note that microglia did not significantly accumulate CerC24 or Cer24:1(although there was a trend) in response to GCSi treatment as we would have expected from CerS-activity, ceramide labeling, and FB1 treatment. This observation also supports the idea that microglia are more readily adaptive to elevated ceramide levels (e.g., by increasing hydrolysis or increasing complex lipid synthesis) compared to astrocytes and MNs.
A–F To alter ceramide production through inhibition of downstream glucosylceramide production, MNs (A, D), astrocytes (B, E) and microglia (C, F) were treated with a GCSi (Genz-667161) and ceramides were measured in nuclear and cytoplasmic fractions. Each ceramide chain length vehicle vs GCSi treatment was analyzed by Student’s t-test, (****P < 0.0001, ***P < 0.001, *P < 0.05; n = 4 wells).
Bulk RNAseq of cells treated with FB1 to reduce ceramide synthesis and GCSi to induce ceramide accumulation
While we observed different gene expression profiles across cell-types by qPCR and RNA-seq, this did not determine which of their cellular functions depend on ceramide synthesis. Ceramide labeling, FB1 treatment, and GCSi treatment all revealed cell-type differences in ceramide homeostasis. To assess how ceramide accumulation influences cellular function we treated each cell type with the pan-CerS inhibitor FB1 or GCSi (Genz-667161), then performed RNA-seq. As in previous experiments, cells were cultured for 1-week post thaw then treated with 3 μM FB1 or Genz-667161 for 72 hours before lysis. After CerS inhibition, we observed many differentially expressed genes (DEGs) in both astrocytes (1820 genes) and microglia (1734 genes) (Fig. 6B–D). Notably, there were no DEGs in MNs after FB1 treatment, consistent with our data showing minimal reliance on ceramide synthesis in MNs relative to glia (Fig. 6A). GCSi induced significant DEGs in all three cell types (astrocytes 409 DEGs, Microglia 920 DEGs, and MNs 576 DEGs) (Fig. 6F–I).
A-E Motor neurons (MNs), astrocytes, and microglia were cultured for 7-days, then treated with 3 μM FB1 for 72-hours, as with previous protocols. We then isolated RNA and samples were sent for bulk sequencing. A–C Volcano plots of RNA-seq data in MNs, astrocytes, and microglia after FB1 treatment. B Astrocytes showed 1820 significant differentially expressed genes (sDEGs) in response to FB1 treatment. C Microglia showed 1,734 sDEGs in response to FB1 treatment. A MNs had no sDEGs. D In response to FB1 treatment, astrocytes and microglia had 143 common sDEGs. E The top 10 common IPA pathway changes in both astrocytes and microglia after FB1 treatment graphed by -log(p-value). F–H Volcano plots of RNA expression in astrocytes, microglia, and MNs after 72 hours 3 μM GCSi treatment. I Venn diagram of common gene expression changes after GCSi in MNs, astrocytes, and microglia. J, K Venn diagrams of genes that both reduce expression after FB1 treatment and increase expression after GCSi treatment in astrocytes or microglia.
Ceramide is implicated in many cellular functions, including glial reactivity, so we first looked at Ingenuity Pathway Analysis (IPA) to determine if ceramides had different functions across cell types38. Our RNA-seq data indicate that ceramide levels may act as a key regulator of reactivity and inflammation. Within the top 10 differentially regulated IPA pathways in response to FB1, astrocytes had multiple pathways involved in inflammatory regulation (Supplementary Data 1: astrocyte FB1 treatment pathways). Microglial inflammatory pathways were similarly impacted by FB1 treatment (Supplementary Data 1: microglia FB1 treatment pathways). Additionally, consistent with their role in brain lipid regulation, FB1 significantly altered the Superpathway of Cholesterol Biosynthesis in astrocytes. Of interest, astrocytes also had pathway changes that could relate to axon guidance. Common pathway changes in response to FB1 treatment in both astrocytes and microglia showed themes in lipid synthesis, NAD regulation, and inflammation (Fig. 6E).
GCSi treatment altered many inflammatory pathways in astrocytes, similar to FB1 treatment (Supplementary Data 1: astrocyte pathways in GCSi treatment). Astrocytes also showed changes in lipid/cholesterol regulation after GCSi treatment (Hepatic Cholestasis, LXR/RXR Activation). GCSi induced a variety of inflammatory changes in microglia as well, many involved in inflammatory cell migration and infiltration (Supplementary Data 1: microglia pathways in GCSi treatment).
While MNs did not respond to FB1, there were a variety of pathway changes in response to GCSi. The changes centered around amino acid degradation, lipid/cholesterol regulation (LXR/RXR Activation and FXR/RXR Activation), and inflammatory responses. Consistent pathway changes across these cell-types indicate that ceramides may have key roles in regulating inflammation and cholesterol homeostasis.
To assess which pathways were commonly altered in response to ceramide changes, we analyzed the pathways that changed the most in response to FB1 and GCSi across all three cell types. Of the 20 most highly affected pathways, many were involved in inflammatory functions (including IL-33 Signaling Pathway, IL-17 Signaling). Many of these pathways were modified in opposing directions in response to FB1 and GCSi in astrocytes but changed in the same direction in microglia (Supplementary Fig. 7). Notably, IL-33 is known to be an astrocyte-derived cytokine capable of regulating microglial-synaptic pruning, and IL-17 similarly regulates synaptic activity39,40.
Of interest were common and unique gene changes between cell types. In response to FB1 treatment, there were 143 DEGs in common between astrocytes and microglia (Supplementary Data 7). Of the 143 shared DEGs, 38 genes increased expression. These related to ER stress/apoptosis (STC2, CHAC1), inflammatory regulation (AREG, BEX2, TREML3P, FOXD1), glycolysis (GCKR), antioxidant activity (CHAC1), NAD metabolism/mitochondrial function (ALDH1L2, NMNAT2), and cellular adhesion/cytoskeletal dynamics/cellular motility (PCDH19, PAPPA). There were 41 downregulated genes in astrocytes and microglia in response to FB1 treatment. These included genes involved in cholesterol and fatty acid regulation (MSMO1, HMGCS1, CYP2U1-AS1), inflammatory regulation (RNASE1, CXCL14, TGFB2-AS1, PTGES3L), oxidative stress (MAOB), axon and synaptic development (CBLN2, TMEM108), sulfatation (ARSI), and cellular adhesion/cytoskeletal dynamics/cellular motility (DNAH6, MYH2, ADAM19, ADAMTS12, CLDN5, TPM2).
In response to GCSi treatment, there were no common DEGs amongst all three cell-types. However, GCSi treatment induced 24 common DEGs between astrocytes and microglia, astrocytes and MNs shared 2 DEGs, and microglia and MNs had 16 common DEGs (Supplementary Data 8). The 16 common DEGs between microglia and MNs had functions in inflammatory regulation (IL18RAP, GIMAP4, CD1B, TAFA3) and ubiquitin degradation of proteins (ASB15). CD1B is of particular interest as it functions to present lipid and glycolipid self-antigens41. The 24 common DEGs between microglia and astrocytes had functions in the extracellular matrix and blood clotting regulation (TFIP2, SERPINE1, PCED1B, CLDN5, SPON1), inflammatory regulation (IL11, CLCF1), and apoptosis (DTHD1). It is notable that both IL11 and CLCF1 had increased expression in response to GCSi and both promote oligodendrocyte survival42,43,44. It is possible that lipid stress induced by GCSi treatment is detected by glial cells as a sign of dysmyelination or demyelination. In response to GCSi astrocytes and MNs had only 2 DEGs, PRRG and KCNE1B.
Ceramide precursors are differentially toxic between glia and neurons
We showed that ceramide synthesis is significantly different between iPSC-derived cell types. Additionally, glia and neurons incorporated sphinganine into ceramide chain lengths differently. In our RNA-seq data, CerS inhibition and GCSi-induced gene changes related to ER stress, ferroptosis, and apoptosis. Sphingolipids are dysregulated in a variety of neurological diseases, and we showed that sphinganine can be turned into ceramide when applied to glia and, to a lesser extent, neurons. We therefore treated cells to alter ceramide and/or sphinganine levels, and analyzed the resulting toxicity by Cell-Titer Glo (CTG), DAPI/cytotox staining, and neurite analysis.
We first sought to determine the toxic doses of GCSi (Genz-667161), as these may directly relate to the ceramide accumulation seen in Fig. 5. Despite having large changes in nuclear ceramides with GCSi treatment (Fig. 5E), astrocytes were more resistant to GCSi toxicity than MNs and microglia (Fig. 7A–C). We did see small decreases in astroglial viability at 72 hours (Fig. 7B), but both microglia and MNs showed more significant toxicity (Fig. 7A and C).
A–C Astrocytes, microglia, and motor neurons (MNs) were cultured for 7-days, then treated with increasing doses of GCSi. D–F Sphinganine was dosed into cells at increasing concentrations and Incucyte live imaging and analysis was performed. Representative images show MN cell-death and disintegrating neurites, microglia showed signs of cell death, while astrocytes remained largely resilient to 10 μM sphinganine treatment (scale bars 200 μm). G–I Cell titer glo (CTG) analysis of MN cell viability was consistent with neurite findings that toxicity starts at 10 μM doses with similar levels of toxicity in microglia, but resistance to 10 μM dose toxicity in astrocytes. J Live imaging of MNs and analysis of neurites by phase microscopy revealed acute toxicity at 10 μM and higher (transparent and opaque datapoints correspond to the individual replicate and mean values, respectively). Data analyze by one-way ANOVA with Bonferroni’s multiple comparisons test (n = 4 or 6 technical replicates as depicted in graphs).
To alter ceramide levels by a different mechanism, we treated cells with increasing sphinganine concentrations to determine the effects on the viability of different cell-types. Both sphinganine (Fig. 7D, G, and J, Supplementary Fig. 8A–C) and sphingosine (Supplementary Fig. 8D–G) were significantly toxic to neurons at 10 μM doses, as assessed by CTG, DAPI/cytotox staining, and neurite analysis. High doses were acutely toxic and 10 μM sphinganine treatment showed more acute toxicity than 10 μM sphingosine.
Sphinganine was toxic to astrocytes as well, but only starting at the 30 μM dose (Fig. 7E and H, Supplementary Fig. 9A, C, and D). This illustrates the potential resistance of astroglial cells to this sphingolipid driven toxicity. We therefore treated microglia to see if other glia had similar resistance to toxicity. Microglia had higher mRNA expression of ceramidases, so we anticipated that microglia might have heightened resistance to sphinganine toxicity. In contrast to our expectations, and similar to MNs, microglia underwent cell death starting at 10 μM doses (Fig. 7F and I, Supplementary Fig. 9E–G). We also observed significant acute morphological changes in microglial cells in response to sub-toxic doses (3 μM) of sphinganine (Supplementary videos 1 and 2). These morphological changes normalized over time, though there were signs of intracellular inclusion (possibly lipid droplets). It’s known that lipid accumulation can alter microglial activity45, but future studies will need to ascertain whether sphinganine similarly alters their activity.
Liposomal delivery significantly alters sphinganine delivery to neurons
Bulk application of free lipids is not the most physiological delivery and therefore may not recapitulate in vivo lipid-related toxicity. There is evidence that astrocytes can release lipoparticles and/or exosomes containing toxic lipids including ceramides28,46. Therefore, we repeated our sphingolipid treatment assay with the delivery of sphinganine, but in a lipoparticle/liposomal format. NBD-sphinganine was mixed with POPC in a 5:95 ratio and delivered to cells as liposomes or liposomes bound to APOE. We compared this delivery to liposomes lacking sphinganine (with and without APOE) as well as the delivery of free NBD-sphinganine at doses equivalent to that of NBD-sphinganine delivered by liposomes. NBD (green) allowed us to track the rate of incorporation and localization of the NBD-sphinganine within MNs, though we can’t rule out that the sphinganine could be incorporated into other lipids and/or metabolized in some way.
We observe that treatment of MNs with NBD-sphinganine (NBD-SA) in both a free and liposomal (LP) format led to NBD localization in MNs, with no green signal detected in POPC treated neurons (Fig. 8B–E and Supplementary Fig. 10A). A large portion of NBD signal in free NBD-sphinganine treatment localized to cell bodies and dead cell clumps. It is difficult to remove these clumps without negatively impacting live MN health. We can’t exclude that a large portion of free NBD-sphinganine signal in our analyses could localize to dead cells. Notably, we saw many neurites that were NBD+ in the liposomal treated cells (NBD-SA + POPC + APOE LP; Fig. 8B, C, and E).
A Diagrams illustrating the treatment of motor neurons (MNs) with liposomes with NBD-labeled (green) sphinganine and liposomes lacking sphinganine. These were delivered either bound or not bound to APOE. These treatments were compared to the delivery of free NBD-sphinganine. B, C To quantitatively assess if liposomal delivery increased NBD-label incorporation into neurites, we performed an analysis for green-labeled neurites. Graphs illustrate the delivery of 1.0 μM and 10.0 μM of NBD-sphinganine in both free and liposomal forms, with increased NBD+ neurite area in liposomal delivery regardless of APOE status (transparent and opaque data points correspond to the individual replicate and mean values, respectively). Dotted line in B is the timepoint images taken for D, E 8 h post-treatment, showing significant NBD labeling in neurites when NBD-sphinganine was delivered in liposomes (scale bars 200 μm) (E) but did not see the same extent of labeling in the free NBD-sphinganine (D) delivery condition. No green labeling was observed in neurons treated with liposomes lacking NBD-sphinganine (Supplementary Fig. 10). We noted F, G Cell titer glo (CTG) analysis showed that NBD-sphinganine had a similar toxicity curve when delivered freely or delivered in liposomes. Notably, liposomes without NBD-sphinganine showed minimal toxicity (Supplementary Fig. 10), showing that APOE and the carrier lipid POPC (19x the concentration of NBD-sphinganine in the liposome) are not contributing significantly to lipid toxicity. Diagrams generated in BioRender.
After 72 hours of treatment, CTG was performed for cell viability. POPC and APOE were kept in consistent proportion to the sphinganine. NBD-sphinganine was similarly toxic when delivered freely or in liposomal formulations, regardless of whether the liposomes had APOE bound or not (Fig. 8F and G, Supplementary Fig. 10C and D). We note that POPC delivered in the same doses, but lacking sphinganine, did not show significant toxicity (Supplementary Fig. 10A and B). As POPC was a significantly higher proportion of the liposome than sphinganine, this indicates that bulk lipid application alone does not drive toxicity.
Based on the finding that neurites were NBD+, we utilized Incucyte neurite analysis for NBD+ (green) neurites to assess lipid incorporation. At multiple doses, liposomal delivery of NBD-sphinganine induced significantly more NBD incorporation into neurites than free NBD-sphinganine delivery (Fig. 8B–E, Supplementary Fig. 10A and E). APOE did not significantly alter this labeling. This might indicate better incorporation of the lipid into the whole cell or that delivery of the lipid through a different pathway might alter neurite incorporation.
We additionally analyzed neurites by phase microscopy in cells treated with liposomal NBD-sphinganine, as a measure of neuronal health. Consistent with our previous free sphinganine treatment experiment (Fig. 7 and Supplementary Fig. 8), there was acute neurite loss in response to sphinganine treatment and then a plateau, as dead neurites were not retracted and were still picked up by the analysis software. Surprisingly, the analysis detected more neurite loss in neurons treated with NBD-sphinganine liposomes bound with APOE (Supplementary Fig. 10C and D). In both instances, the neurites appeared dead but without APOE the neurite debris stayed in place, whereas there might have been more neurite retraction with APOE. This was a subtle effect picked up by coarse phase analysis, so future studies will need to better characterize the effect and if APOE alters neurodegeneration mechanisms.
Methods
iPSC-derived cell culture protocols
6-well, 12-well, or 24-well plates were coated using 2.5 mL PDL (1 mg/mL, Sigma P7886) in 47.5 mL borate buffer (100 mM Boric Acid, 75 mM Sodium Chloride, pH 8.4, Boston Bioproducts Inc. C-8852S) overnight at room temperature. Plates were then washed with water three times and allowed to dry at room temperature before plating. PDL-coated plates were stored at 4-degrees for a maximum of one month before plating.
BrainXell differentiated microglia were thawed by water-bath and diluted 5 mL in microglial medium, centrifuged at 300 x g for 5 minutes, resuspended in 1 mL microglial media, and counted by hemocytometer or Countess. Cells were plated at specified densities for each assay in microglial media (DMEM/F-12, Gibco 1330-032; N2, Gibco 17502048; B27, Gibco #17504044; NEAA, Gibco #11140-050; Chemically Defined Lipid Mix, Gibco #11905-031; Glutamax, Gibco #35050-061; AA2P solution, Sigma #A8960; 2-Mercaptoethanol, Sigma #M3148; Pen/Strep, Gibco #15140-122; 20 ng/mL M-CSF, Peprotech #300-25; 100 ng/mL IL-34, Peprotech #200-34; 2 ng/mL TGF-β1 Peprotech #100-21 C) and only half media changes were ever performed.
Each vial of BrainXell differentiated motor neurons was thawed by water-bath, diluted to 5 mLs with media, and then counted by hemocytometer or Countess. Cells were not centrifuged and pipetting up and down was limited. Cells were then plated on day-0 at specified densities in motor neuron seeding media (DMEM/F12, Gibco 1330-032; Neurobasal Medium, Life Technologies #21103-049; B27 supplement, Life Technologies #21103-049; Gibco #17504044; N2 supplement, Gibco 17502048; GlutaMAX, Gibco #35050-061; BrainXell Motor neuron seeding supplement) on PDL coated plates. On day-1, a full media change was performed using the day-0 media, with the addition of growth factors (10 ng/mL BDNF, Peprotech #450-02; 10 ng/mL GDNF, Peprotech #450-10; 1 ng/mL TGF-β1, Peprotech #100-21 C; 1:10 of Geltrex diluted 1:10 in DMEM/F-12, Life Technologies #A1413201). On day-4, half media was changed with day-0 media containing BrainXell day-4 supplement instead of seeding supplement and with BDNF, GDNF, and TGF-β1. Day-7 and on, the same media as day-4 was used, but without the BrainXell supplement.
BrainXell astrocyte vials were thawed and diluted with media to 5 mLs and counted by hemocytometer or Countess. Cells were then plated on day-0 at specified densities on PDL-coated plates in astrocyte seeding medium (DMEM/F12, Gibco 1330-032; Neurobasal Medium, Life Technologies #21103-049; N2 supplement, Gibco 17502048; GlutaMAX, Gibco #35050-061; BrainXell Astrocyte Supplement; Fetal Bovine Serum, ThermoFisher A3840001). On day-1, media was changed in full (DMEM/F12, Gibco 1330-032; Neurobasal Medium, Life Technologies #21103-049; N2 supplement, Gibco 17502048; GlutaMAX, Gibco #35050-061; BrainXell Astrocyte Supplement), thereafter astrocytes were fed with the same media by half media changes every 3-4 days.
For all experiments, BrainXell WC30-1A iPSC-derived cells were used. This line was generated from a 46-year old healthy male donor with normal karyotype and APOE3/APOE3 genotype.
RNA sequencing
After 72 hours of cell treatment with 3 μM FB1 or Genz-667161, RNA was isolated by Qiagen RNeasy Mini Kit (Qiagen cat# 74104) and sent to Azenta for RNA sequencing. RNA quality control was performed at Azenta using Qubit system. RIN values lower than 6.0 were considered suboptimal. As neuronal samples often had RIN values of lower than 6.0 because of difficulties removing dead cells without compromising healthy neurons, rRNA depletion library preparation was used for neurons followed by Illumina 2 × 150 bp sequencing, ~30 M reads/sample. Astrocyte and microglial RNA was prepped using Poly-A library preparation by Illumina 2 × 150 bp sequencing, ~30 M reads/sample.
RNA sequencing data analysis
RNASeq pre-processing to raw counts was performed by Azenta as previously described. Analysis for differentially expressed genes (DEGs) was performed using the DESeq247 implemented within Array Studio (Version 11, Omicsoft Corporation, Research Triangle Park, NC, USA). Analyses were restricted to intra cell type comparisons. Each condition was contrasted with the DMSO control. Genes were considered statistically significantly altered if the absolute value of fold change |FC | >2 and adj.pValue < 0.05. Volcano plots were generated using the “EnhancedVolcano” package, within the R/RStudio environment (version 3.6.3/version 1.1.463). Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis).
Lipidomics Cell preparation
Cells for lipidomics other than labeling were plated at 60k/well in a 24-well dish. For all other experiments astrocytes (20k/96-well, 240k/24-well, 600k/6-well), microglia (40k/96-well, 240k/24-well, 500k/12-well), and motor neurons (40k/96-well, 240k/24-well, and 500k/12-well) were cultured according to BrainXell cell culture protocols outlined above. Cells were then lysed, scraped, and processed for lipidomics with a protocol adapted from Mitchell et al.48. Briefly, cells were lysed and scraped in lipidomics lysis buffer (10 mM HEPES, 10 mM NaCl, 1 mM KH2PO4, 5 mM NaHCO3, 5 mM EDTA, 1 mM CaCl2, and 0.5 mM MgCl2), lysed using bead tissue lyser or by 15 x shearing through a syringe, and 25ul of 20% sucrose was added per 500 μl of lysate. Lysate was then centrifuged at 6300 x g at 4-degrees for 14 minutes. Supernatant formed the cytoplasmic fraction. The pellet was washed, centrifuged, then the nuclear pellet was resuspended and stored at -80 degrees Celsius until analysis.
Ceramide and dHCer profiling
Cytoplasm or nuclear fraction (50 μL) was extracted with 1 mL of extraction solution containing internal standard (methanol/acetonitrile/water, 80/15/5%, v/v/v containing 10 ng/mL d35Cer16 as IS). The mixture was vortexed for 15 minutes in multi-tube vortex and spined down for 8 minutes at 8400 g. The supernatant (200 μL) was carefully transferred to a MS vial for analysis. Standard curves (0.03-1000 ng/mL) were prepared in the same way as samples with Ceramide standards: Ceramide_C14, Ceramide_C16, Ceramide_C18, Ceramide_C24, Ceramide_C24:1, and dH-Ceramide_C16.
LC-MS/MS analysis was conducted on a Waters Acquity UPLC system coupled with a Sciex QTRAP6500 mass spectrometer using multiple-reaction monitoring (MRM). The Ceramide profile is separated by a Waters Acquity UPLC BEH C8 (100 × 2.1 mm, 1.7um particles, cat#186002878) at a flow rate of 0.75 mL/min. The column is heated at 60 C during the run. The mobile phase consists of A) 0.2% Formic Acid; 5 mM Ammonium formate in DI water and B) 0.2% Formic Acid; 5 mM Ammonium formate in (50:50) Methanol:Acetonitrile. The injection volume was 5 μL and the total runtime was 6 min. The step gradient was as follows: 0–0.2 min, 85% solvent B; 0.2–1.8 min, 85 to 98% solvent B; 1.81-3.4 min, 100% solvent B, 3.4–3.5 min, 100 to 85% solvent B; 3.5–6 min 85% solvent B.
The ESI+ source temperature was 450 °C; the ESI needle was 4,500 V; the declustering potential was 60 V; the entrance potential was 10 V; and the collision cell exit potential was 10 V. The collision and curtain gas were set at medium and 15, respectively. GS1 and GS2 were set at 80 and 20. The collision energy was 34 eV for Ceramide and 40 eV for dHCer. For MRM, the dwell time was set at 50 ms for each of the signal from transitions.
Data were acquired and analyzed by MultiQuant 3.0 (AB Sciex). Calibration curves were constructed by plotting the corresponding peak area ratios of analyte/internal standard versus the corresponding analyte concentrations using 1/x weighing linear regression analysis.
qPCR analysis
Cells were washed two times with half media removal and the addition of PBS, then the complete volume was removed, and direct Cells-to-Ct lysis buffer (Invitrogen, A25602) was added, and lysates frozen at −80 degrees. Quantitative PCRs were performed according to kit instructions using ThermoFisher taqman gene expression assays with GAPDH probes as loading controls on a QuantStudio 7 Flex Real-Time PCR System. Data was analyzed using -deltadeltaCt relative to MN expression levels and alternatively -deltaCt to relative gene expression.
Ceramide synthesis assays
Cells were lysed and scraped on ice using HEPES buffer (20 mM HEPES, 25 mM KCl, 250 mM Sucrose, 2 mM MgCl2) made according to kit (640011, 640012, 640014, Avanti Polar Lipids Inc.) instructions36. Lysates, NBD-sphinganine (810206 P, Avanti Polar Lipids Inc.), and Acyl-CoA (18:0, 870718 P, Avanti Polar Lipids Inc.; 16:0, 870716 P, Avanti Polar Lipids Inc.; 24:1, 870725 P, Avanti Polar Lipids Inc.) for reaction were water-bath sonicated prior to the reaction. All reagents were kept warm at 37-degrees prior to the start of the reaction. Reactions were performed on a thermal cycler for temperature consistency and reactions were neutralized using methanol containing 1% formic acid. Lipid column (8E-S001-BGB, Phenomenex) washes and elutions were performed according to manufacturer instructions (640011, 640012, 640014, Avanti Polar Lipids Inc.) using a vacuum manifold (AH0-8950, Phenomenex) and NBD-ceramide levels were read by FlexStation 3 microplate reader.
d17:0-sphinganine labeling and ceramide measures
Stock d17:0-sphinganine (860654 P, Avanti Polar Lipids Inc.) was warm water-bath sonicated at 37-degrees Celsius for 15-20 minutes prior to addition to cells at 1 μM for 2 hours or 24 hours. For inhibitor conditions, FB1 (Sigma-Aldrich, F1147) was added at 3 μM and pre-incubated for 1-hour prior to d17:0-sphinganine addition.
Preparation of NBD-sphinganine/POPC liposomes
We made a mixture of POPC (16:0-18:1 PC; Avanti 850457) and NBD-sphinganine (Avanti 810206P) with 95% POPC and 5% of NBD-sphinganine in 100% ethanol. Lipids were then added to PBS at a 1:9 ratio of ethanol/lipid solution to PBS. Components were vortexed to mix components, then incubated at 37-degrees to form a mixture. Resultant mixtures were freeze/thawed 3-times, rapidly on dry ice then at room temperature to increase the uniformity of the liposomes in the mixture. We then used the lipid solution to resuspend APOE (abcam ab280330) at a ratio of 3 μg of APOE for every 10 μg of lipid in the NBD-sphinganine: POPC liposomes (equal volume of POPC liposomes were added to APOE for controls). All dosing was then performed based on the molarity of NBD-sphinganine added to cells, or equal volumes of similarly prepared control solutions.
Statistics and reproducibility
All quantification and statistics were done using Graphpad Prism (v9.1.2-v10.1.2). Statistical analysis across conditions was performed using Student’s T-test, one-way ANOVA, two-way ANOVA, with Tukey, Bonferroni, or Sidak post hoc tests. Bar graphs report data as mean ± s.e.m. For all panels where statistical significance is reported, p-values are specifically listed or *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Separate wells within a culture were considered technical replicates and a minimum of 3 technical replicates were performed for each experiment. Further information is available in the Nature Portfolio Reporting Summary linked to this article.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Discussion
Diseases of the CNS are often characterized by glial reactivity and the selective vulnerability of neuronal and oligodendroglial cells to cell-death. The nature of the cellular dysfunction and death, as well as the vulnerable cell types, underlies each disease’s symptomology. Ceramide is a sphingolipid essential to normal cellular functions but is also involved in cell-death pathways. Despite the variable dysfunctions and cell types affected in Alzheimer’s disease, multiple sclerosis, ALS, and Farber disease, CNS ceramide accumulation has been observed in each of these diseases49. Ceramide also accumulates during the process of aging, is associated with cognitive decline, and ceramide/sphingomyelin ratios could be predictive of Alzheimer’s disease progression50,51,52. Cellular stress and disease can induce ceramide accumulation in mitochondrial membranes, of interest since de novo ceramide biosynthesis occurs at the ER-mitochondria associate membranes (MAMs)11. At high concentrations, ceramides form pores in mitochondrial membranes, releasing cytochrome C, and contributing to the initiation of cell death. This and other important functions of ceramide in ER function, membrane trafficking, and rafting, as well as metabolism, likely explain why disrupted ceramide homeostasis is associated with a variety of CNS diseases.
Despite the known role of ceramide in neurodegeneration and the possibility that astroglial ceramides are markers of neuroinflammation, how different cell types of the CNS regulate ceramide has not been fully elucidated38,53. Efforts have been made to characterize mouse cell-type specific lipid regulation, but this did not focus on ceramides30. Our understanding of ceramide alterations in disease largely derives from lipidomics analysis of whole tissue lysates and CSF, or transcriptomic observations54,55,56,57. Additionally, some essential signaling mechanisms related to ceramide have been determined in cancer cells58.
We utilized healthy human iPSC-derived cells to determine how ceramides are regulated differently across CNS cell-types. There is evidence from other works that disease relevant changes in lipid profiles can be found in iPSC-derived cells59,60. We found that neurons had higher levels of C18-ceramide than other cells, consistent with in vivo studies32. In many cases, our RNA data did not match well with activity and lipidomics data. It is well known that RNA signatures don’t always match protein expression61. CerS expression may not directly correlate with CerS activity as multiple proteins are known to modulate CerS activity, some through post-translational modifications60,62. Future studies will need to determine if the discrepancies identified here are due to translational regulation, post-translational modifications, or similar.
While we could not address how cell-type specific CerS post-translational modifications altered cellular regulation of ceramides, we determined the functional lipid output of CerS in each cell-type. Upon addition of sphinganine, a precursor for ceramide synthesis, it was notable that each cell-type largely turned the sphinganine precursor into ceramide chain lengths predicted from our CerS activity assays. Astroglial CerS5/6 generating Cer16, microglia CerS2/5/6 generating Cer16 and Cer24:1, and motor neurons (MNs) CerS1/4 generating Cer18 and to a lesser extent Cer16. It’s notable that MNs treated with precursor were able to generate detectable labeled C18-ceramide, but it was a significantly smaller percentage of the overall ceramide profile than the labeled ceramides in the glial cells. Additionally, MN ceramide profile was minimally altered by FB1 treatment (pan-CerS inhibitor), which seems to indicate that MN do not rely largely on de novo ceramide synthesis to function.
Sphinganine addition allowed us to increase ceramide precursor. An alternative way to determine relative ceramide production, is to block complex glycosphingolipid production downstream of ceramide. In our experiments we used a GCSi, which blocks the generation of glucosylceramide from ceramide and also affects disease progression in preclinical models of ALS63, PD64, and AD65. Notably, after GCSi treatment iPSC-derived neurons and glia accumulated similar ceramide species that occurred in response after the addition of the sphinganine precursor. This provided further confirmation that homeostatic regulation of certain ceramide species differs amongst astrocytes, microglia, and neurons. Additionally, GCSi induction of ceramide may explain exacerbation of disease phenotypes for some neurodegenerative diseases63.
Determining the ceramides that each cell type produces in response to increased precursor and inhibited downstream synthesis was key to understanding cellular metabolic differences. We wanted to further understand how altered ceramide levels influence the functions of different cell-types by analyzing changes in RNA expression after FB1 and GCSi treatment. Surprisingly, but consistent with our other data, MNs had no statistically significant gene changes in response to FB1 treatment. Astrocytes and microglia both had >1.5k differentially expressed genes (DEGs) in response to FB1. This seems to indicate that ceramide synthesis occurs largely in glial cells over these time scales, and that MNs do not synthesize large amounts of ceramide de novo, nor show significant responses to inhibition of ceramide synthesis. In contrast to this finding, all three cell-types had many significant DEGs after GCSi treatment. This possibly indicates that while MNs do not rely heavily on ceramide synthesis, they do respond to small increases in ceramide accumulation and/or inhibition of glucosylceramide synthesis.
We additionally assessed pathways impacted by FB1 and GCSi treatment, and commonalities between cell-type response to FB1 and GCSi. Ceramide alterations resulted in changes to both lipid homeostasis and inflammatory activity. This could underlie some of the lipid, cholesterol, and inflammatory changes characteristic of neurodegenerative diseases. We note that these changes were accompanied by specific responses such as increased pro-myelination and oligodendrocyte survival signals (IL11 and CLCF1) after GCSi treatment and decreased lipid self-antigen presentation (CD1B)41,42,43,44. This could indicate that ceramide accumulation acts as a signal of myelin dysfunction leading to an adaptive response. This could extend to signals regulating axon growth and synapses as well, because FB1 treatment of astrocytes and microglia decreased expression of genes involved in axon growth and synaptic development (CBLN2 and TMEM108), and we saw alterations in axon guidance pathways in astrocytes after FB1 treatment. This latter finding also likely highlights that the homeostatic regulation of ceramide within an optimal range is critical for the normal function of the CNS – too little or too much ceramide likely leads to a cacostatic state. Indeed, LOF mutations in DEGS1 provoke lower ceramide and increased DhCer levels, leading to childhood-onset leukodystrophy9.
Notably, some of the changes in response to FB1 and GCSi treatment mirrored changes found in neurodegenerative diseases with altered ceramide homeostasis. CCL2, a cytokine that is elevated in mouse models of Farber disease66, decreased after FB1 treatment and increased after GSCi. Similar changes were observed for the biomarker MMP10, which is predictive of AD symptom progression67, and SREBF1 which has variants that are associated with PD risk68. IL-17 signaling was similarly changed by altered ceramides and is known to be dysregulated in Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis69,70,71. RNA-sequencing also confirmed the known role of ceramides in mitochondrial, ER stress, lipid dysregulation, and apoptosis axis (STC2, CHAC1, ALDH1L2, NMNAT2, PDK4, KLHDC7B, BIRC3, PLN, SREBF1)11. Thus, ceramide dysregulation likely plays an important role in the ER and mitochondrial dysfunction present in many neurodegenerative diseases. It will need to be determined if manipulating ceramides in preclinical neurodegenerative models can alter these biomarkers and inflammatory factors in ways that modify disease progression.
Given our RNA expression findings, we had significant questions surrounding how ceramide dysregulation would alter cell survival. In certain disease contexts, astrocytes can take on a reactive state that is toxic to neurons and oligodendrocytes72. The precise identity and source of the toxicity remains to be determined and is likely context dependent, but there is evidence that the ceramide synthesis pathway could be involved45,46,73,74,75. This is consistent with evidence that glial cells can produce saturated lipids that are toxic to neurons and that astrocytes may even release ceramide extracellularly in response to disease stimuli, triggering neuronal cell-death28,46. Based on our findings of ceramide generation from precursors, we treated each cell type with escalating doses of sphinganine to see if the differences in ceramide handling between cell-types also altered vulnerability to toxicity. Astrocytes synthesized the largest proportion of ceramides using labeled sphinganine precursor, so it was notable that astrocytes also showed the most resistance to sphinganine toxicity. Microglia and neurons both had decreased viability starting at 10 μM sphinganine. Of interest, our live imaging revealed that sub-toxic doses of sphinganine led to acute morphological changes in microglia that then normalized with time (Supplementary Videos 1 and 2). Microglia may respond to toxic doses of lipid with morphologic changes and resolve the stress in some cases (Supplementary Videos, Supplementary Fig. 9E and F), and in other cases the toxic dose of lipid may trigger cell-death (Fig. 7I). Future studies will need to determine whether morphological changes represent an acute inflammatory response to sphinganine and whether similar acute responses occur in neurons and astrocytes at sub-toxic doses. Notably, a similar morphological alteration is seen by others in response to oleic acid, and that this added microglial lipid burden may contribute to neuronal dysfunction45.
While addition of sphinganine is a method of increasing ceramide and assessing toxicity, we are adding significant excess lipid. We therefore used a well-characterized inhibitor of glucosylceramide synthase to see if ceramide would accumulate and if there was associated toxicity. With this inhibitor treatment, we did find accumulation of certain ceramides. Notably, the ceramide chain lengths that accumulated in each cell type tended to be those generated by each cell type’s most active CerS. We also found a significant impact on cell viability with GCSi treatment in all three cell types. Paralleling our findings with sphinganine treatment, astrocyte viability was not impacted to the same extent as microglia and MNs with GCSi treatment. RNA sequencing of cells treated with GCSi revealed a similar number of DEGs in both MNs and astrocytes, but the extent of the changes was larger in astrocytes.
Given the central role of ceramides in the synthesis of more complex lipids and the known function of astrocytes in providing lipids to neurons, we sought to deliver sphinganine to neurons in a more physiological manner in order to address how this would alter trafficking and toxicity46,76. It is known that in vivo lipids are often transferred in lipoparticle/APOE mediated form and that alterations of this through APOE variants confers Alzheimer’s disease risk. Additionally, ceramides can be found in blood-derived high-density lipoprotein (HDL), indicating that ceramides can be packaged into such apolipoprotein particles77. In a step towards mimicking this in vivo complexity, we adapted our sphinganine delivery into a liposomal formulation as a rough mimic of HDL delivery. Our liposomal preparations had minimal impact on cellular viability when just the carrier lipid (POPC) and APOE were delivered. As POPC made up ~95% of the liposomes, this indicated that simple bulk lipid addition to MNs doesn’t drive toxicity. Sphinganine drove toxicity regardless of free delivery or delivery by liposome. Interestingly, the liposomal delivery did introduce more lipid label into neuronal neurites independent of APOE status. When sphinganine liposomes were delivered bound to APOE, neurites did appear to degenerate more, despite no changes in measured toxicity. This indicates that the method of lipid delivery matters, but it will be important to understand how this alters physiology. It’s probable that other aspects of neuronal function (e.g., ER stress response) are dependent on the format of lipid exposure. Additionally, more chronic treatments might reveal differences in toxicity, whereas these could have been missed with these acute toxic doses. Future experiments examining non-cell autonomous APOE mediated lipid transfer, in vivo and in vitro, will be important to determine how these processes contribute to ceramide homeostasis.
Our findings have implications for interpreting bulk lipidomics of tissue in animal models and patients. Specially, we found that cortical neurons, MNs, astrocytes, and microglia feature unique cytoplasmic and nuclear ceramide composition. Thus, disease-reported changes in ceramide species may suggest the loss or enrichment of a particular cell type. For example, the accumulation of C24 and C24:1 ceramide isoforms in the spinal cords of ALS patients63 may be indicative of increased glial activation.
Additionally, our RNA-seq data indicate that changes in lipid signatures can alter cytokines and chemokines. CCL2 and similar factors can alter immune cell infiltration, possibly altering the cellular milieu through increased CNS immune cells. Our data show that microglia differ significantly from other CNS cell-types in ceramide signature. It is probable that sufficient immune cell infiltration could alter lipidomic signatures with consequences for interpreting tissue lipidomic signatures in stroke, multiple sclerosis, and other neurodegenerative diseases.
Based on our in vitro labeling data that revealed acute lipid changes separate from iPSC differentiation effects, it makes sense for more studies to explore in vivo labeling approaches. It is likely that lipids generated prior to disease onset or through alternative synthesis pathways often create noise when attempting to determine disease and therapeutic effects in pre-clinical models. Labeling would allow both the isolation of defined time points and pathways of lipid synthesis.
Together our data reinforce the notion that glia are the lipid-handling cells of the brain, with astrocytes having a particularly large ceramide synthesis capacity. Microglia did have significant synthesis capacity as well and uniquely synthesized longer-chain ceramides. Astrocytes were differentially resilient to ceramide/sphinganine-related toxicity, which might be associated with their function in synthesizing lipids and transferring them to other cell-types in the brain. Future studies are necessary to extend these findings in vivo. Additionally, understanding selective lipotoxicity vulnerable cell-types and cell-type-specific lipid synthesis could contribute significantly to our understanding of neurodegenerative diseases with lipid dysregulation. It may be important to measure both free and apolipoprotein-associated lipids to better understand contexts where lipids become toxic in disease.
Data availability
All data are available through the primary manuscript, Supplementary materials, or in source data files. Transcriptomic data are available at Synapse project ID syn53113026. Any additional information available upon request.
References
Pinto, S. N., Silva, L. C., Futerman, A. H. & Prieto, M. Effect of ceramide structure on membrane biophysical properties: the role of acyl chain length and unsaturation. Biochim. Biophys. Acta 1808, 2753–2760 (2011).
Kaltenegger, M. et al. Intrinsic lipid curvatures of mammalian plasma membrane outer leaflet lipids and ceramides. Biochim Biophys. Acta Biomembr. 1863, 183709 (2021).
Kitatani, K., Idkowiak-Baldys, J. & Hannun, Y. A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal 20, 1010–1018 (2008).
Merrill, A. H. Jr. Characterization of serine palmitoyltransferase activity in Chinese hamster ovary cells. Biochim. Biophys. Acta 754, 284–291 (1983).
Ruangsiriluk, W. et al. Silencing of enzymes involved in ceramide biosynthesis causes distinct global alterations of lipid homeostasis and gene expression. J. Lipid Res. 53, 1459–1471 (2012).
Venkataraman, K. et al. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J. Biol. Chem. 277, 35642–35649 (2002).
Mizutani, Y., Kihara, A. & Igarashi, Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 390, 263–271 (2005).
Pewzner-Jung, Y., Ben-Dor, S. & Futerman, A. H. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J. Biol. Chem. 281, 25001–25005 (2006).
Pant, D. C. et al. Loss of the sphingolipid desaturase DEGS1 causes hypomyelinating leukodystrophy. J. Clin. Invest. 129, 1240–1256 (2019).
Ternes, P., Franke, S., Zähringer, U., Sperling, P. & Heinz, E. Identification and characterization of a sphingolipid delta 4-desaturase family. J. Biol. Chem. 277, 25512–25518 (2002).
Planas-Serra, L. et al. Sphingolipid desaturase DEGS1 is essential for mitochondria-associated membrane integrity. J. Clin. Invest. 133, e162957 (2023).
Alayoubi, A. M. et al. Systemic ceramide accumulation leads to severe and varied pathological consequences. EMBO Mol. Med. 5, 827–842 (2013).
Zhou, J. et al. Spinal muscular atrophy associated with progressive myoclonic epilepsy is caused by mutations in ASAH1. Am. J. Hum. Genet. 91, 5–14 (2012).
Johnson, J. O. et al. Association of Variants in the SPTLC1 Gene With Juvenile Amyotrophic Lateral Sclerosis. JAMA Neurol. 78, 1236–1248 (2021).
Kölbel, H. et al. New insights into the neuromyogenic spectrum of a gain of function mutation in SPTLC1. Genes 13, 893 (2022).
Mohassel, P. et al. Childhood amyotrophic lateral sclerosis caused by excess sphingolipid synthesis. Nat. Med. 27, 1197–1204 (2021).
Dawkins, J. L., Hulme, D. J., Brahmbhatt, S. B., Auer-Grumbach, M. & Nicholson, G. A. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat. Genet 27, 309–312 (2001).
Penno, A. et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 285, 11178–11187 (2010).
Abbott, S. K. et al. Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov. Disord. 29, 518–526 (2014).
Mielke, M. M. et al. Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson’s disease and associated with cognitive impairment: a pilot study. PLoS One 8, e73094 (2013).
Guedes, L. C. et al. Serum lipid alterations in GBA-associated Parkinson’s disease. Parkinsonism Relat. Disord. 44, 58–65 (2017).
Lin, G. et al. Phospholipase PLA2G6, a Parkinsonism-associated gene, affects Vps26 and Vps35, Retromer function, and ceramide levels, similar to α-Synuclein gain. Cell Metab. 28, 605–618.e606 (2018).
Ferrazza, R. et al. LRRK2 deficiency impacts ceramide metabolism in brain. Biochem. Biophys. Res. Commun. 478, 1141–1146 (2016).
Kim, M. et al. Association between Plasma ceramides and phosphatidylcholines and hippocampal brain volume in late onset Alzheimer’s disease. J. Alzheimers Dis. 60, 809–817 (2017).
Cutler, R. G. et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl Acad. Sci. USA 101, 2070–2075 (2004).
Han, X. et al. Metabolomics in early Alzheimer’s disease: identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS One 6, e21643 (2011).
Elsherbini, A. et al. Association of Aβ with ceramide-enriched astrosomes mediates Aβ neurotoxicity. Acta Neuropathol. Commun. 8, 60 (2020).
Wang, G. et al. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD). J. Biol. Chem. 287, 21384–21395 (2012).
Geekiyanage, H., Upadhye, A. & Chan, C. Inhibition of serine palmitoyltransferase reduces Aβ and tau hyperphosphorylation in a murine model: a safe therapeutic strategy for Alzheimer’s disease. Neurobiol. Aging 34, 2037–2051 (2013).
Fitzner, D. et al. Cell-type- and brain-region-resolved mouse brain lipidome. Cell Rep. 32, 108132 (2020).
Lucki, N. C. & Sewer, M. B. Nuclear sphingolipid metabolism. Annu. Rev. Physiol. 74, 131–151 (2012).
Ginkel, C. et al. Ablation of neuronal ceramide synthase 1 in mice decreases ganglioside levels and expression of myelin-associated glycoprotein in oligodendrocytes. J. Biol. Chem. 287, 41888–41902 (2012).
Cai, L. et al. ORMDL proteins regulate ceramide levels during sterile inflammation. J. Lipid Res. 57, 1412–1422 (2016).
Santos, T. C. B., Dingjan, T. & Futerman, A. H. The sphingolipid anteome: implications for evolution of the sphingolipid metabolic pathway. FEBS Lett. 596, 2345–2363 (2022).
Han, G. et al. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc. Natl Acad. Sci. USA 106, 8186–8191 (2009).
Tidhar, R., Sims, K., Rosenfeld-Gur, E., Shaw, W. & Futerman, A. H. A rapid ceramide synthase activity using NBD-sphinganine and solid phase extraction. J. Lipid Res. 56, 193–199 (2015).
El-Hindi, K. et al. T-cell-specific CerS4 depletion prolonged inflammation and enhanced tumor burden in the AOM/DSS-induced CAC Model. Int. J. Mol. Sci. 23, 1866 (2022).
de Wit, N. M. et al. Astrocytic ceramide as possible indicator of neuroinflammation. J. Neuroinflamm. 16, 48 (2019).
Vainchtein, I. D. et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 359, 1269–1273 (2018).
Luo, H. et al. Interleukin-17 regulates neuron-glial communications, synaptic transmission, and neuropathic pain after chemotherapy. Cell Rep. 29, 2384–2397.e2385 (2019).
Shahine, A. The intricacies of self-lipid antigen presentation by CD1b. Mol. Immunol. 104, 27–36 (2018).
Ji-Wei, S. et al. CNTF induces Clcf1 in astrocytes to promote the differentiation of oligodendrocyte precursor cells. Biochem. Biophys. Res. Commun. 636, 170–177 (2022).
Zhang, Y. et al. Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. J. Neurosci. 26, 12174–12185 (2006).
Gurfein, B. T. et al. IL-11 regulates autoimmune demyelination. J. Immunol. 183, 4229–4240 (2009).
Victor, M. B. et al. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell 29, 1197–1212.e1198 (2022).
Guttenplan, K. A. et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 599, 102–107 (2021).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Mitchell, J. C. et al. Wild type human TDP-43 potentiates ALS-linked mutant TDP-43 driven progressive motor and cortical neuron degeneration with pathological features of ALS. Acta Neuropathol. Commun. 3, 36 (2015).
Han, X. et al. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J. Neurochem. 82, 809–818 (2002).
van Kruining, D. et al. Plasma ceramides relate to mild cognitive impairment in middle-aged men: The Maastricht Study. Alzheimers Dement. 15, e12459 (2023).
Chan, P. et al. Ceramide accumulation is associated with declining verbal memory in coronary artery disease patients: an observational study. J. Alzheimers Dis. 64, 1235–1246 (2018).
Mielke, M. M. et al. Plasma sphingomyelins are associated with cognitive progression in Alzheimer’s disease. J. Alzheimers Dis. 27, 259–269 (2011).
Dodge, J. C. Lipid involvement in neurodegenerative diseases of the motor system: insights from lysosomal storage diseases. Front Mol. Neurosci. 10, 356 (2017).
Barrier, L., Fauconneau, B., Noël, A. & Ingrand, S. Ceramide and related-Sphingolipid levels are not altered in disease-associated brain regions of APP and APP/PS1 Mouse Models of Alzheimer’s disease: relationship with the lack of neurodegeneration? Int. J. Alzheimers Dis. 2011, 920958 (2010).
Pousinis, P., Ramos, I. R., Woodroofe, M. N. & Cole, L. M. Lipidomic UPLC-MS/MS profiles of normal-appearing white matter differentiate primary and secondary progressive multiple sclerosis. Metabolites 10, 366 (2020).
Fernández-Beltrán, L. C. et al. A transcriptomic meta-analysis shows lipid metabolism dysregulation as an early pathological mechanism in the spinal cord of SOD1 mice. Int. J. Mol. Sci. 22, 9553 (2021).
Henriques, A. et al. Sphingolipid metabolism is dysregulated at transcriptomic and metabolic levels in the spinal cord of an animal model of amyotrophic lateral sclerosis. Front Mol. Neurosci. 10, 433 (2017).
Fekry, B. et al. C(16)-ceramide is a natural regulatory ligand of p53 in cellular stress response. Nat. Commun. 9, 4149 (2018).
Lieberman, R. et al. Human iPSC-derived astrocytes generated from donors with globoid cell leukodystrophy display phenotypes associated with disease. PLoS One 17, e0271360 (2022).
Tomasello, D. L. et al. 16pdel lipid changes in iPSC-derived neurons and function of FAM57B in lipid metabolism and synaptogenesis. iScience 25, 103551 (2022).
Tasaki, S. et al. Inferring protein expression changes from mRNA in Alzheimer’s dementia using deep neural networks. Nat. Commun. 13, 655 (2022).
Sassa, T., Hirayama, T. & Kihara, A. Enzyme activities of the ceramide synthases CERS2-6 are regulated by phosphorylation in the C-terminal region. J. Biol. Chem. 291, 7477–7487 (2016).
Dodge, J. C. et al. Glycosphingolipids are modulators of disease pathogenesis in amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 112, 8100–8105 (2015).
Sardi, S. P. et al. Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models. Proc. Natl Acad. Sci. USA 114, 2699–2704 (2017).
Dodge, J. C. et al. Glucosylceramide synthase inhibition reduces ganglioside GM3 accumulation, alleviates amyloid neuropathology, and stabilizes remote contextual memory in a mouse model of Alzheimer’s disease. Alzheimers Res. Ther. 14, 19 (2022).
Dworski, S. et al. Acid Ceramidase Deficiency is characterized by a unique plasma cytokine and ceramide profile that is altered by therapy. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 386–394 (2017).
Martino Adami, P. V. et al. Matrix metalloproteinase 10 is linked to the risk of progression to dementia of the Alzheimer’s type. Brain 145, 2507–2517 (2022).
Ivatt, R. M. et al. Genome-wide RNAi screen identifies the Parkinson disease GWAS risk locus SREBF1 as a regulator of mitophagy. Proc. Natl Acad. Sci. USA 111, 8494–8499 (2014).
Chen, J., Liu, X. & Zhong, Y. Interleukin-17A: The Key Cytokine in neurodegenerative diseases. Front. Aging Neurosci. 12, 566922 (2020).
Gautam, A. S., Pulivarthi, C. B. & Singh, R. K. Proinflammatory IL-17 levels in serum/cerebrospinal fluid of patients with neurodegenerative diseases: a meta-analysis study. Naunyn Schmiedebergs Arch. Pharm. 396, 577–588 (2023).
Brigas, H. C. et al. IL-17 triggers the onset of cognitive and synaptic deficits in early stages of Alzheimer’s disease. Cell Rep. 36, 109574 (2021).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Chung, H. L. et al. Very-long-chain fatty acids induce glial-derived sphingosine-1-phosphate synthesis, secretion, and neuroinflammation. Cell Metab. 35, 855–874.e855 (2023).
Wieder, N. et al. FALCON systematically interrogates free fatty acid biology and identifies a novel mediator of lipotoxicity. Cell Metab. 35, 887–905.e811 (2023).
Arredondo, C. et al. Excessive release of inorganic polyphosphate by ALS/FTD astrocytes causes non-cell-autonomous toxicity to motoneurons. Neuron 110, 1656–1670.e1612 (2022).
Qi, G. et al. ApoE4 impairs neuron-astrocyte coupling of fatty acid metabolism. Cell Rep. 34, 108572 (2021).
Argraves, K. M. et al. S1P, dihydro-S1P and C24:1-ceramide levels in the HDL-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis. 10, 70 (2011).
Acknowledgements
We thank Zhaohua Huang, Pablo Sardi, and Michael Hendrickson (BrainXell) for input and technical support.
Author information
Authors and Affiliations
Contributions
J.J.M., D.S., L.G., B.Z. and J.C.D conceptualized the experiments. J.J.M., D.S., and J.C.D prepared the manuscript. J.J.M., D.S., L.G., M.G., R.P., and G.G. performed the experiments and performed data analysis. M.R.D analyzed the RNA-seq data.
Corresponding authors
Ethics declarations
Competing interests
All authors were employees of Sanofi at the time of the work. The authors declare no other competing interests..
Peer review
Peer review information
Communications Biology thanks Fei Yin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Zheng-Jiang Zhu and Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
McInnis, J.J., Sood, D., Guo, L. et al. Unravelling neuronal and glial differences in ceramide composition, synthesis, and sensitivity to toxicity. Commun Biol 7, 1597 (2024). https://doi.org/10.1038/s42003-024-07231-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-07231-0