Abstract
The human brain is tightly connected to the individual’s environment and its input dynamics. How the dynamics of periodic environmental stimuli influence neural activity and subsequent behavior via neural entrainment or alignment is not fully clear yet, though. This study explores how periodic environmental stimuli influence neural activity and behavior. EEG data was collected during a Go-NoGo task with a periodic intertrial interval (ITI) of 1.3 s (0.769 Hz). Results showed that the task’s temporal structure increased power spectrum activity at 0.769 Hz, which showed high intersubject variability. Higher task-periodicity effects were linked to stronger phase-based intertrial coherence (ITC) and reduced neural complexity, as measured by lower Lempel-Ziv Complexity (LZC). Additionally, higher periodicity in the power spectrum correlated with faster reaction times and stronger response bias. We conclude that the encoding of the inputs’ dynamics into the brains power spectrum shapes subsequent behavior, e.g., RT and response bias, through reducing neural complexity.
Similar content being viewed by others
Introduction
Events in our environment enroll dynamically on different timescales. One rhythmic process which we all are subjected to is the circadian cycle operating on a timescale of 24 h. But others may occur based on our specific environment, like a train passing by every 10 min or the (ir)regular flickering of an electronic device nearby. The brain exhibits its own very complex intrinsic dynamic activity changes over time1. How the brain and its intrinsic neural dynamics encode the external inputs’ dynamics and how that impacts subsequent behavior remains to be elucidated.
Behavioral task design used in neuroscientific research significantly influences neural activity2. External stimuli are typically presented with a particular temporal structure, i.e., rhythmic or periodic, including trial durations and intertrial intervals3,4,5,6. We will refer to ‘task-periodicity’ as the temporal pattern that emerges over the whole course of a task, involving a series of trials presented at a certain more or less regular timescale. For instance, it is known that the brain processes and encodes the inputs’ regularity and frequency within a corresponding frequency range, resulting in periodic activations3,7,8,9,10. Recent studies indicate that inter-trial or inter-stimulus-intervals in the infra-slow frequency range (periods of 15.5 to 60 s) lead to power increases in the corresponding frequency ranges of the brain’s power spectrum density (PSD)8,9,10,11. Behaviorally, a recent study shows that this power increase in the sensory region V1 influences WM performance10. These findings suggest that the task periodic power peak modulate behavior. The neural mechanisms of this modulation remain unclear, though. Addressing this gap in our knowledge is the goal of our study.
The impact of the brain’s encoding of a regular task-periodic input on neural activity in the shorter timescale of single trial durations can be subsumed under the term of neural entrainment12,13. Neural entrainment can be measured via different neural indices12. For instance, intertrial phase coherence (ITC) measures the temporal recurrence in the phase angles of a signal at each time point over several trials as for instance during repeated auditory stimuli14. This makes ITC a promising candidate to measure the effects of auditory task periodicity on phase angles.
The temporal dynamics of neural activity can also be measured in terms of Information processing through its degree of complexity by the Lempel Zev complexity (LZC, Lempel & Ziv, 1976). LZC provides a tool to estimate the compressibility of a signal15 and has widespread use in EEG16,17. A lower LZC indicates higher degree of signal compression and thus reduced neural complexity. How the encoding of the task periodicity impacts information processing and its neural complexity as well as its relation to behavior remains unclear.
The goal of this study is to investigate the neural mechanism by which task periodicity impacts behavior. We assume that the neural encoding of the task’s overall periodic structure in a corresponding frequency of the power spectrum modulates behavior through the neural dynamics as manifest in its information processing, i.e., LZC, and entrainment with ITC. For that purpose, we apply an auditory Go NoGo task which, presenting a high number of repeated stimuli, is well suited for investigating task periodicity effects in the power spectrum. While this task is usually used to measure attention and inhibition when comparing Go and NoGo trials18, we here shift the focus to assess how the neural encoding of the periodic Go stimuli within the power spectrum modulates subjects’ behavior, i.e., their decision making bias in response to the Go NoGo stimuli, through neural complexity (LZC) and entrainment (ITC). Given that the LZC measures the degree of the compression of the signal, one may expect that higher encoding of the task’s periodic structure within the power spectrum allows for better signal compression resulting in less complexity, e.g., lower LZC.
Our first specific aim is to investigate whether the regularly presented input dynamics of the external stimuli, i.e., task periodicity is neuronally processed and encoded within the power spectrum. Based on previous data8,9,10,11, we hypothesize that the frequency of the task’s regular temporal structure featured by its intertrial intervals will lead to a power increase in the corresponding frequency of the PSD. Moreover, we expect considerable intersubject variability in the degree of that power peak as that may relate to intersubject variability in subsequent behavior, i.e., reaction times and decision making bias (see below for details).
Our second specific aim is to test how the neural encoding of the task-periodicity within the power spectrum peak relates to the neural dynamics of entrainment, i.e., ITC, and the complexity or information processing, i.e., LZC. Given that the ITC indicates phase synchrony across averaged trials, we expect a higher ITC in participants with a high task-periodicity power peak. In this sense, the ITC is a validation measure of the task-periodicity peak. Previous research showed that neural variability is reduced with stronger stimulus strength19. Therefore, we hypothesize that subjects with high values in both ITC and PSD peak exhibit less neural complexity reflecting higher signal compression, i.e., low LZC.
Finally, the third goal is to assess if the neural encoding of the inputs’ dynamics through the task-periodicity in the power spectrum peak and the two measures of event related dynamics, e.g., ITC and LZC, also impacts the participants’ behavior, e.g., output dynamics. Embracing specific decision biases enables organisms to customize their choices according to the environmental requirements which can be measured by Signal detection theory (SDT20, see also ref. 21). For instance, opting for a liberal response strategy proves beneficial when accurate target detection is paramount, while a conservative approach becomes optimal for minimizing the risk of false alarms20,22. We therefore expect that a highly regular task-structure favors high degrees of anticipation which would be observable in increased false alarms and a faster RT. Hence, we hypothesize that a higher neural encoding of the input dynamics into the PSD peak, a higher ITC, and lower LZC facilitates faster RT and higher false alarm rate, that is, a more liberal bias as measured by the Criterion C in SDT.
Method
Participants
Twenty-six healthy volunteers (16 males; age: m = 26.869, sd = 6.628) participated in this study. Due to technical difficulties, one subject was excluded. The subjects gave written informed consent and the study protocol was approved by the Royal Institute of Mental Health Ethics Committee (REB # 2019004). All ethical regulations relevant to human research participants were followed.
Task design
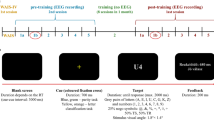
Participants completed a Go-NoGo task (Fig. 1). High pitch (1500 Hz), low pitch (1000 Hz), and ‘silent’ tones were randomly presented to participants for 150 ms. Participants were instructed to respond to each high tone as quickly as possible by pressing a button. Additionally, they were asked to abstain from responding during the low pitch and “silent” tones. High tones were presented for 70% of the trials, while low tones and ‘silent’ tones were presented for 15% of trials each. Each trial was separated by an intertrial interval of exactly 1.3 s and a total of 520 trials were presented.
A Task design. Participants are asked to give a button press to the frequent high tone and to inhibit a response after the low tone and the silence. The ITI was of 1.3 s, which corresponds to a frequency of 0.769 Hz. B Illustration of the LZC measure. The upper timeseries has a low LZC (0.648). The lower timeseries has a high LZC (1.714).
EEG data acquisition and preprocessing
EEG data was recorded during a 5-min resting state with eyes open and a task state of 11 min. To have comparable recording lengths, only the middle 5 min of the task state were considered for statistical comparisons between rest and task conditions.
EEG data was recorded using Ag/AgCl electrodes through a 64-channel Brain Vision Easycap (according to the International 10–20 System) and was sampled at 1000 Hz with DC recording. The EEG data preprocessing was performed using the EEGLAB toolbox for MATLAB (R2022a). The data was downsampled to 500 Hz and filtered with an FIR zero-phase low-pass filter at 50 Hz and a high-pass filter at 0.1 Hz23. With a custom script, noisy channels (defined as 5 interquartiles above or below each channel’s mean) were spherically interpolated (amount of channels per subject: mean = 0.444, sd = 1.086) before re-referencing to the average. Further, artifacts were identified using independent component analysis (ICA) via the EEGLAB software (infomax) creating 63 independent components. Finally, we used MARA implementation to automatically reject noisy components. We defined a threshold of artifact probability to 0.75. The number of removed components varied across subject (amount of components removed per subject: mean = 37.259, sd = 11.93).
Neurodynamic measures
Channel selection
All subsequent analyses are calculated on the channel Cz due to its implication in auditory processing24.
Task-periodicity
Neural alignment to the temporal envelope of the task is quantified with the power amplitude of the power spectrum (PSD) at exactly the stimulus presentation frequency (1.3 s/0.769 Hz). We refer to this measure hereafter with ‘peak’ or ‘peak at 0.769 Hz’. The PSD was calculated with the signal.periodogram function from scipy in python 3.10 with a hamming window and a frequency resolution of 0.00333 Hz (f2-f1).
Intertrial Phase Coherence (ITC)
The ITC indicates the phase-synchronization across trials. We computed it in mne via the tfr_morlet function from 2 to 4 Hz. Number of cycles was for half of each frequency respectively. To extract a single ITC value per subject, we averaged the ITC for each condition in the theta range from 3 to 10 Hz and from 0.2 to 0.5 s after stimulus onset (see black box in each of the subplots in Fig. 4). This window showed the strongest differentiation between conditions.
Lempel-Ziv-Complexity (LZC)
LZC is an indicator of signal compression by measuring its complexity15,25. It is based on the regularity/irregularity of the signal. LZC measures the number of distinct patterns in a binary sequence which reflects the number of bits required to reconstruct a signal15,25. Low values reflect high degrees of regularity, entailing a high degree of signal compressibility. A low LZC indicate high irregularity and low compressibility (see Fig. 1B). We computed it in python 3.10 following the description of25 for pre- and post-stim activity separately. The length of pre- and post-stimulus activity was of 0.5 s respectively which is long enough for LZC computation but short enough to avoid an overlap of pre- from post-stimulus activity.
Behavioral measures
Signal detection theory
The Go-NoGo task was analysed with SDT20 because it yields separate indicators for the accuracy in form of the cognitive bias Criterion C and sensitivity d’.
Criterion C: The criterion C of the signal detection theory20 indicates a predominance of the participant to commit rather one than the other of the two error types (false alarms or misses). It is calculated based on the Hit and False alarm rate as follows26:
Considering the facilitation of false alarms (FA) in Go-NoGo tasks, the bias is commonly negative, indicating a predominance of FA18. Still, the principle of the Criterion C remains in this task as it situates each individual on a continuum of response bias. Hence, a Criterion C close to zero indicates a rather equal number of false alarms and misses while an increase in FA is indicated by a more negative Criterion C.
Sensitivity d’
Sensitivity d’ is a measure of discriminability and indicates the ability of a participant to distinguish between two stimuli. In our case, it indicates how well the participant achieved to behaviorally distinguish Go vs NoGo trials. It is calculated based on the hit and false alarm rate (Anderson, 2015).
A hit rate that equals the false alarm rate (d’ = 0) is indicator of an inability to discriminate between the two stimuli. A higher d’ corresponds to less errors.
Statistical analysis
A Wilcoxon rank test was used to compare the task-periodicity peak during rest and task. Repeated measures ANOVA is used to compare ITC and LZC across all three conditions. Correlations were employed to assess the co-occurrence of effects. For the correlations with the ITC and LZC, we did not distinguish between conditions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Behavioral analysis: reaction time and decision making bias
To assess behavior, we computed SDT indices Criterion C (m = −0.594, SD = 0.243, max = −0.066, min = −1.124) and Sensitivity d’ (m = 3.669, SD = 0.854, max = 1.138, min = 5.213; Fig. 2A) as well as response time (m = 0.421, SD = 0.080, max = 0.335, min = 0.675; Fig. 2C). A general lower percentage of correct trials (m = 3.669, SD = 0.854, max = 1.138, min = 5.213) was found for the low NoGo condition compared to the high (Go) condition (p < 0.001) and silence (p < 0.001; high vs silent: p = 0.156). Criterion C and RT correlated (r = 0.620, p < 0.001), indicating that faster responding leads to a higher FA rate. Moreover, we observed high intersubject variability in both RT (SD = 0.080) and Criterion C (SD = 0.243) as visualized by the extension of our violin plots in Fig. 2.
A Illustration of the principle of Signal detection theory. B Sensitivity d’ and (C) Criterion C are calculated based on the Hit and Miss rate. A higher d’ indicates better performance while lower Criterion C indicates a stronger preference for false alarms compared to misses. D Percentage correct. E Response time of correct trials (high tones) and its intra-subject variability. F Correlation between response time and Criterion C.
Encoding of input dynamics into neural dynamics: task periodicity peak in the power spectrum
To respond to our first aim, we computed the PSD over 5 min resting state and 5 min task state (Fig. 3). A significant power increase was found during the task-state compared to resting state at exactly the frequency of the task’s ITI (0.769 Hz; t(25) = 5.930, p < 0.001, d = 1.163; z = 4.051, p < 0.001, matched rank biserial correlation = 0.909). We exclude the possibility that this power increase is due to motor activity related to button presses as they occur with irregular time intervals (see intra-subject variability of RT, Fig. 2C). In contrast to the power of the peak at the specific frequency of the task’s periodicity, the total power of the whole PSD was equivalent in rest and task (p > 0.8). While the ratio of the peak in relation to the total power at 0.769 Hz was significantly higher in task than in rest (t(25) = 5.677, p < 0.001, d = 1.113; z = 4.432, p < 0.001, matched rank biserial correlation = 0.994).
The top row is a schematic representation of the undelaying task structure resumed in the power peak. A Power spectrum for rest and task EEG activity. Colored lines indicate individual subjects while the thick black line indicates the group average. At the 0.769 Hz, a clear power increase is visible for the task compared to the resting state. B Quantification of the peak by extracting its amplitude in rest and task for each subject. The significant difference between rest and task comes jointly with a higher variability of the peak during task (left). C The total PSD remains stable from rest to task. (n = 25).
Interestingly, the power increase observed in task state was related to a significant increase in intersubject variability (F-test to compare two variances: F(25,25) = 0.027, p < 0.001) reflecting the neural differences in the participants’ capacity to process and encode the input dynamics, i.e., its overall temporal structure. Together, these results provide strong evidence that the periodic nature of the task design, the input dynamics, impact neural dynamics in a highly intersubjectively variable way.
Event related neural dynamics I: Intertrial coherence
Next, we analysed event-related neural dynamics. First, we computed the ITC (Fig. 4) for each condition between 0.3–0.5 sec and 3–10 Hz. A repeated measures ANOVA yielded a significant main effect for condition (F(1.62, 40.61) = 59.184, p < 0.001). Pairwise comparisons indicated a significant higher ITC for the low condition than for silent and high tones (Fig. 2B; tukey hsd: high vs. low: 0.143, p < 0.001; high vs. silence: ns, low vs. silence: −0,181, p < 0.001).
A ITC average across subjects for each condition. B Descriptive plots for the ITC. For further analyzes, the average was computed between 0.2 and 0.5 s in the frequency range from 3 to 10 Hz (black rectangle). C A significantly higher ITC is observed for incorrect trials (average ITC of all conditions). (n = 25).
Event related neural dynamics II: Lempel-Ziv complexity in pre- and post-stimulus activity
Next, we computed LZC on 500 ms long pre-and post-stimulus section of the EEG signal. Significant differences were found for the post-stimulus period (F(2,50) = 25.451, p < 0.001) in the low vs high (p < 0.001) and low vs silent (p < 0.001) conditions (high vs low: p = 0.5). Together, the event related measures were sensitive to the condition, particularly to the low tone condition which tests for response inhibition (Fig. 5A).
In general, pre-stimulus activity yielded a significantly higher LZC than during the post-stimulus interval, this indicates that the processing in the post-stimulus period exhibits higher compression of the signal (Fig. 5B; t(25) = 5.909, p < 0.001, d = 1.159; z = 4.026, p < 0.001, matched rank biserial correlation = 0.903). Finally, the pre-post-stimulus difference of LZC was significantly higher for the low tone condition compared to the high (p = 0.001) and silent (p < 0.001) condition (high vs silent: p = 0.363, Fig. 5C, see supplementary Fig S2 for pre-post differences in the PSD).
From the encoded input dynamics to the event related neural dynamics
We next investigated how the encoded input dynamics relate to our measures of the event related neural dynamics. For this, we computed the correlation between the task-periodicity power peak and both ITC and LZC (Fig. 6; see supplementary table S1 for further links between neural variables). As a reminder, for the correlations, we averaged the ITC and LZC across conditions as the peak is condition unspecific. Indeed, the peak correlated with both ITC and post-stimulus LZC as well as marginally with the LZC pre-post difference (r = −0.386, p = 0.052). A higher peak was related to a higher ITC (r = 0.409, p = 0.038), a lower pre-stimulus LZC (r = −0.413, p = 0.036), and lower post-stimulus LZC (r = −0.476, p = 0.014). This suggests that the degree of the encoded input dynamics through the power spectrum peak strongly modulate the subsequent event related neural dynamics, e.g., ITC and LZC.
Impact of task-periodicity on neuronal dynamics (averaged over conditions). A higher peak comes with an increased ITC. Further, a stronger peak is related to a higher degree of compression (pre and post stimulus LZC), indicating a reduction of complexity. The correlation between peak and LZC pre-post stimulus difference was marginally significant and showed that a higher difference might be linked to a higher peak. (n = 25).
From the encoded input dynamics to behavior
Above, we could show the strong impact of the external stimulus dynamics on the event-related dynamics. Does the input dynamics as processed by the brain modulate the subsequent behavioral responses? To answer this question, we correlated the task-periodicity peak with our behavioral measures (Fig. 7A). We observed that a higher peak comes with a faster RT (r = −0.479, p = 0.015) and a stronger Criterion C (r = −0.657, p < 001). In contrast, no relationship was found with sensitivity d’ (r = 0.078, p = 0.711). Together, this shows that the input dynamics do indeed modulate, through the encoded task-periodicity, the subsequent behavioral responses, i.e., the bias in the decision.
From the event related neural dynamics to behavior
Next, we investigated the relationship of both ITC (Fig. 7B) and the LZC (Fig. 7C, D) with the behavioral measures. The ITC correlated with RT (r = −0.66, p < 0.001) and Criterion C (r = −0.553, p = 0.004) but not with sensitivity d’ (r = 0.211, p = 0.311). Pre-stimulus LZC correlates with RT only (r = 0.465. p = 0.019; Criterion C: r = 0.320, p = 0.118; Sensitivity d’: r = −0.027, p = 0.899). Post-stimulus LZC correlated with RT only (r = 0.535, p = 0.006; Sensitivity d’: r = −0.014, p = 0.948) and marginally with Criterion C (r = 0.387, p = 0.056). The Pre-Post-Stimulus LZC difference correlated with both RT (r = −0.468, p = 0.018) and Criterion C (r = −0.438, p = 0.029) but not with accuracy (Sensitivity d’: r = −0.077, p = 0.715).
Together, we repeatedly observed a strong link of the neural measures, ITC and LZC with the behavioral measures, i.e., RT and Criterion C. Interestingly, unlike the decision making bias Criterion C, the accuracy index d’ was not related to any of the neural measures. Albeit tentatively, this suggests that the degree of the neuronally encoded input dynamics modulates the direction or type of errors (bias) but not the accuracy itself (the total number of errors).
Discussion
How does neural entrainment in form of task-periodicity impact neural activity and behavior? First, we observed a strong power increase at the frequency corresponding to the task structure (trial duration = 1.3 s). The same peak was not observed during the resting state which allows us to exclude the possibility that this strong power increase during the task might be due to noise sources external or internal to the participant. The observation of such task-periodicity effect on the PSD is not new7. Indeed, music studies and neural oscillators provide evidence of the ability of the brain to synchronize to a rhythmic external stimulation like tones3,4,27. However, our results go beyond the simple observation of such task periodicity effect by relating it to behavior.
Extending these findings, we observed high intersubject variability in the degree of the encoded power peak which is related to intersubject differences in behavior, e.g., decision making bias and reaction time. We observed that subjects with a higher power peak, reflecting better encoding of the task’s overall temporal structure, exhibit faster reaction time and a stronger liberal bias in their decision making, as measured by Criterion C. This suggests that higher degrees of neural entrainment or alignment13 to the external task’s temporal structure, i.e., its input dynamics, modulates subsequent behavior, i.e., output dynamics.
Following these findings, we asked ourselves about the neural dynamics of such effects of the encoded task periodicity on behavior. We observed increased ITC in subjects with a high peak in their PSD. That suggests that the encoded task periodicity of the input dynamics modulates event related dynamics on the phase level as measured by the ITC. Further, we also observed that the PSD peak modulated information processing as measured by LZC. LZC, an index of neural complexity15,25,28 was higher during the pre-stimulus than the post-stimulus period29. This suggests a decrease of neural complexity with higher signal compression after stimulus onset. That is in line with various findings of decreased post-stimulus trial-to-trial variability during the post-stimulus period29,30,31,32,33,34,35. Extending these findings, we show that such decrease also holds for signal complexity, e.g., LZC. Extending one recent finding about pre and post-stimulus difference in LZC29, we demonstrate that the LZC decrease is negatively related to the degree of the power peak in the PSD with a higher peak leading to lower LZC. This suggests that the better encoding of the task’s overall temporal structure, as indexed by a higher power peak, reduces neural complexity through higher degrees of signal compression, e.g., low LZC.
The analysis of behavior revealed that an increase in false alarms (FA) is associated with a faster response time (RT). In simpler terms, a faster RT indicates difficulty in inhibiting responses during rare NoGo trials and may suggest relatively stronger alignment with the temporal task structure. This alignment is substantiated by a significant correlation between the amplitude of the task periodicity peak within the power spectrum and the subjects’ RT. Intriguingly, the accuracy of subjects’ decision making measured by sensitivity d’ does not exhibit a correlation with either RT or the peak in the power spectrum, suggesting that a stronger task alignment does not impact the overall error count but rather influences the type of errors committed, that is the decision making bias as measured by Criterion C. The Inter-Trial Coherence (ITC), a neural measure indexing entrainment12 or alignment13 to the trial structure, yields similar outcomes: higher ITC, indicating greater alignment with the trial structure, correlates with faster response times and an increase in false alarms.
Together, our findings show how different measures of the processing and encoding of input dynamics relate to and shape the output, that is, behavior. The input dynamics as encoded in the PSD peak and the ITC shape both reaction time and decision bias. The same applies to the LZC. This raises the question what connects these different measures across the boundaries of input, brain, and output. Albeit tentatively, we assume that their timescales and thus, more generally their dynamics, connect and differentiate them. The peak of the PSD is measured over the whole task duration without any averaging into event-related activity, this entails a rather long timescale of the whole task duration. While the ITC is measured in the post-stimulus period over the short duration of single repeated and thereby averaged trials. Finally, the LZC takes on an intermediate timescale as measured over the continuum of 500 ms pre-stimulus and post-stimulus periods respectively. Albeit tentatively, our findings support the relevance of the brain’s multiple timescales including longer and shorter ones for yielding behavior and decision making. What we observe on the level of behavior as complex amalgam of different timescales in the output dynamics, may be the result of the brain’s neural processing of the external inputs’ timescales within the inputs, e.g., input dynamics, through its own internal neural dynamics featured by its multiple timescales25,35,36. As evidenced by our results, behavior and cognition may then be strongly shaped by the brain’s neural dynamics and its various timescales just as suggested in Spatiotemporal Neuroscience37,38 and its Dynamic Layer Model (DLB) of the brain36.
Some limitations of this study shall be mentioned. Even though we can exclude the relevance of motor activity in the task-periodicity power increase due to its variability in occurrence (RT are sometimes faster, sometimes slower, see supplementary Fig. S1), it could be of interest to add a recording of the Go-NoGo task without requiring any motor response. Further, additional studies will be necessary to estimate the impact of different forms of task-periodicity like slower or faster on subsequent behavior. The effect of task-periodicity on the behavior should also be investigated in different task-designs demanding different cognitive functions. Further, refined behavioral analysis may be warranted. For instance, in our case stronger alignment to the task-structure did not impact the number of errors but the type of error (Criterion C). Hence future studies are warranted to investigate the impact of different forms of task periodicity on distinct features of cognition and behavior.
In conclusion, we demonstrate how the neural encoding of the input dynamics like its periodicity into the brain’s power spectrum shapes subsequent behavior, e.g., decision making bias and reaction time. This is mediated by neural entrainment, e.g., ITC, and information processing, e.g., LZC, in event related dynamics. Our findings demonstrate direct relationship of input encoding with subsequent output behavior through shared neural dynamics as their “common currency”37,38. This further highlights the key role of the brain’s neural dynamics in shaping our behavior and its mental features as suggested in Spatiotemporal Neuroscience37,38 and its DLB of the brain36.
Data availability
Data is available on request. Data as plotted in this manuscript and its scripts are available at https://drive.google.com/drive/folders/1hj-LEBXQ-Qb3MSVC0XOZvr6R_kX_lDXf?usp=sharing.
Code availability
Code is available on request.
References
Buzsáki, G. Rhythms of the Brain. (Oxford University Press, 2006). https://doi.org/10.1093/acprof:oso/9780195301069.001.0001.
Mumford, J. A., Davis, T. & Poldrack, R. A. The impact of study design on pattern estimation for single-trial multivariate pattern analysis. NeuroImage 103, 130–138 (2014).
Fiveash, A. et al. A stimulus-brain coupling analysis of regular and irregular rhythms in adults with dyslexia and controls. Brain Cognit. 140, 105531 (2020).
Rhodes, D. & Di Luca, M. Temporal regularity of the environment drives time perception. PLoS ONE 11, e0159842 (2016).
Thunell, E. & Thorpe, S. J. Regularity is not a key factor for encoding repetition in rapid image streams. Sci. Rep. 9, 6872 (2019).
Zeithamova, D., De Araujo Sanchez, M.-A. & Adke, A. Trial timing and pattern-information analyses of fMRI data. NeuroImage 153, 221–231 (2017).
Huettel, S. A., Song, A. W. & McCarthy, G. Functional Magnetic Resonance Imaging (Oxford University Press, Oxford, 2014).
Klar, P., Çatal, Y., Langner, R., Huang, Z. & Northoff, G. Scale-free dynamics of core-periphery topography. Hum. Brain Mapp. 44, 1997–2017 (2023).
Klar, P., Çatal, Y., Langner, R., Huang, Z. & Northoff, G. Scale-free dynamics in the core-periphery topography and task alignment decline from conscious to unconscious states. Commun. Biol. 6, 499 (2023).
Wolman, A., Çatal, Y., Klar, P., Steffener, J. & Northoff, G. Repertoire of timescales in uni – and transmodal regions mediate working memory capacity. NeuroImage 291, 120602 (2024).
Kasagi, M. et al. Association between scale-free brain dynamics and behavioral performance: functional MRI Study in resting state and face processing task. Behav. Neurol. 2017, e2824615 (2017).
Lakatos, P., Gross, J. & Thut, G. A new unifying account of the roles of neuronal entrainment. Curr. Biol. 29, R890–R905 (2019).
Northoff, G., Klar, P., Bein, M. & Safron, A. As without, so within: how the brain’s temporo-spatial alignment to the environment shapes consciousness. Interface Focus. 13, 20220076 (2023).
Nash-Kille, A. & Sharma, A. Inter-trial coherence as a marker of cortical phase synchrony in children with sensorineural hearing loss and auditory neuropathy spectrum disorder fitted with hearing aids and cochlear implants. Clin. Neurophysiol. 125, 1459–1470 (2014).
Lempel, A. & Ziv, J. On the complexity of finite sequences. IEEE Trans. Inf. Theory 22, 75–81 (1976).
Falkenstein, M., Hoormann, J. & Hohnsbein, J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol. 101, 267–291 (1999).
Zhang, Y. et al. Neural complexity in patients with poststroke depression: a resting EEG study. J. Affect. Disord. 188, 310–318 (2015).
Young, M. E., Sutherland, S. C. & McCoy, A. W. Optimal go/no-go ratios to maximize false alarms. Behav. Res. 50, 1020–1029 (2018).
Deco, G. & Hugues, E. Neural network mechanisms underlying stimulus driven variability reduction. PLoS Comput. Biol. 8, e1002395 (2012).
Macmillan, N. A. & Creelman, C. D. Detection Theory: A User’s Guide - 2nd Editio. https://www.routledge.com/Detection-Theory-A-Users-Guide/Macmillan-Creelman/p/book/9780805842319 (2005).
Wolman, A. et al. Intrinsic neural timescales mediate the cognitive bias of self – temporal integration as key mechanism. NeuroImage 268, 119896 (2023).
Kloosterman, N. A., Kosciessa, J. Q., Lindenberger, U., Fahrenfort, J. J. & Garrett, D. D. Boosts in brain signal variability track liberal shifts in decision bias. eLife 9, e54201 (2020).
Delorme, A. EEG is better left alone. Sci. Rep. 13, 2372 (2023).
Vandewalle, G. et al. Blue light stimulates cognitive brain activity in visually blind individuals. J. Cognit. Neurosci. 25, 2072–2085 (2013).
Golesorkhi, M. et al. From temporal to spatial topography: hierarchy of neural dynamics in higher- and lower-order networks shapes their complexity. Cereb. Cortex 32, 5637–5653 (2022).
Anderson, N. D. Teaching signal detection theory with pseudoscience. Front. Psychol. 6, 762 (2015).
Doelling, K. B. & Assaneo, M. F. Neural oscillations are a start toward understanding brain activity rather than the end. PLOS Biol. 19, e3001234 (2021).
Wang, Q. et al. A model for the diagnosis of anxiety in patients with epilepsy based on phase locking value and Lempel-Ziv complexity features of the electroencephalogram. Brain Res. 1824, 148662 (2024).
Wolff, A. et al. Neural variability quenching during decision-making: neural individuality and its prestimulus complexity. Neuroimage 192, 1–14 (2019).
Churchland, M. M. et al. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat. Neurosci. 13, 369 (2010).
He, B. J. & Zempel, J. M. Average is optimal: an inverted-u relationship between trial-to-trial brain activity and behavioral performance. PLoS Comput. Biol. 9, e1003348 (2013).
Huang, Z. et al. Is there a nonadditive interaction between spontaneous and evoked activity? Phase-Dependence and Its Relation to the Temporal Structure of Scale-Free Brain Activity. Cereb. Cortex bhv288 https://doi.org/10.1093/cercor/bhv288 (2017).
Wainio-Theberge, S., Wolff, A. & Northoff, G. Dynamic relationships between spontaneous and evoked electrophysiological activity. Commun. Biol. 4, 741 (2021).
Wolff, A. et al. Atypical temporal dynamics of resting state shapes stimulus-evoked activity in depression—An EEG study on rest–stimulus interaction. Front. Psychiatry 10, 719 (2019).
Wolff, A. et al. It’s in the timing: reduced temporal precision in neural activity of schizophrenia. Cereb. Cortex 32, 3441–3456 (2022).
Northoff, G. From Brain Dynamics to the Mind (Elsevier publisher, 2024).
Northoff, G., Wainio-Theberge, S. & Evers, K. Is temporo-spatial dynamics the “common currency” of brain and mind? In Quest of “Spatiotemporal Neuroscience. Phys. Life Rev. 33, 34–54 (2020).
Northoff, G., Wainio-Theberge, S. & Evers, K. Spatiotemporal neuroscience – what is it and why we need it. Phys. Life Rev. 33, 78–87 (2020).
Author information
Authors and Affiliations
Contributions
A.W.: data recording, conception, analysis, first draft, revisions, figures; S.L.: revision; L.L.A.: data recording; J.G.: conception; G.N.: Supervision, conception, funding, first draft, revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Christian Beste and Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wolman, A., Lechner, S., Angeletti, L.L. et al. From the brain’s encoding of input dynamics to its behavior: neural dynamics shape bias in decision making. Commun Biol 7, 1538 (2024). https://doi.org/10.1038/s42003-024-07235-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-07235-w
This article is cited by
-
A division of labor in perception-action integration via hierarchical alpha-beta to beta-gamma coupling and local catecholaminergic control
Communications Biology (2026)
-
Infra-slow scale-free dynamics modulate the connection of neural and behavioral variability during attention
Communications Biology (2025)