Abstract
Mountain forests are biodiversity hotspots with competing hypotheses proposed to explain elevational trends in habitat specialization and species richness. The altitudinal-niche-breadth hypothesis suggests decreasing specialization with elevation, which could lead to decreasing species richness and weaker differences in species richness and beta diversity among habitat types with increasing elevation. Testing these predictions for bacteria, fungi, plants, arthropods, and vertebrates, we found decreasing habitat specialization (represented by forest developmental stages) with elevation in mountain forests of the Northern Alps – supporting the altitudinal-niche-breadth hypothesis. Species richness decreased with elevation only for arthropods, whereas changes in beta diversity varied among taxa. Along the forest developmental gradient, species richness mainly followed a U-shaped pattern which remained stable along elevation. This highlights the importance of early and late developmental stages for biodiversity and indicates that climate change may alter community composition not only through distributional shifts along elevation but also across forest developmental stages.
Similar content being viewed by others
Introduction
Mountain regions are hotspots of biodiversity globally and characterized by strong changes in biodiversity along elevational gradients. Various hypotheses have been proposed to describe why species richness commonly decreases with increasing elevation1,2,3. Environmental filtering theory, for example, predicts that only species with certain adaptations to harsh climatic conditions are able to persist at high elevations4,5,6. The altitudinal-niche-breadth hypothesis provides more detail by suggesting that due to increasing spatial and temporal environmental variability with increasing elevation, species at high elevation exhibit stronger population fluctuations and are less specialized with regard to, for example, the available resources or the prevailing biotic interactions7. Lower specialization allows species to persist under more variable environmental conditions but restricts resource partitioning, with the result that fewer species are able to coexist8,9,10,11. Some studies provide evidence supporting the altitudinal-niche-breadth hypothesis7,12,13 and a recent meta-analysis confirmed a positive correlation between community-level specialization and species richness14, yet the generality of these mechanisms across trophic levels and taxonomic groups remains inconclusive.
In forest ecosystems, pulses of tree mortality are a major driver of ecosystem dynamics. Tree senescence and disturbance open the forest canopy, reset forest development, and create temporal and spatial variability in abiotic and biotic conditions15,16. After the disturbance, forests typically undergo a sequence of developmental stages characterized by distinct differences in forest structure17. Many forest species are specialized in habitat conditions provided by different developmental stages18. Thus, forest-dwelling species diversity and composition vary along forest development19, with high diversity in early and late developmental stages, and low diversity in intermediate developmental stages20. Yet, it remains unclear whether diversity patterns along forest development are modulated by climatic conditions, for example along elevational gradients. Specifically, the interactive effects of disturbance and the processes driving the altitudinal-niche-breadth hypothesis have not been investigated to date. Given that climate is warming more strongly in mountain areas21 and disturbance regimes are changing rapidly in many mountain landscapes22,23, understanding how these changes – and the resultant prevalence of different developmental stages in the landscape – affect forest-dwelling species is crucial to better predict the impacts of climate change on biodiversity.
To test the interactive effect of elevation and forest development after disturbance on habitat specialization and biodiversity, we set up a network of 150 study plots covering the full gradient of forest development replicated ten times each across three elevational zones (submontane, montane, subalpine) in the Northern Alps (Fig. 1). At each plot, we sampled multi-trophic diversity – covering bacteria, fungi, plants, arthropods, and vertebrates – and assessed habitat specialization (i.e., a species´ proportional use of different forest developmental stages), species richness, and beta diversity along forest developmental stages and elevation, respectively. Based on the processes underlying the altitudinal-niche-breadth hypothesis and considering a positive specialization-species richness correlation, we predict that both (H I) habitat specialization and (H II) species richness decrease with increasing elevation (Fig. 2 I & II). Given decreasing specialization with elevation, we further predict that (H III) community dissimilarity across developmental stages is highest at the submontane and lowest at the subalpine zone (Fig. 2 III). Combining both the elevational and the forest developmental gradient, we finally predict that (H IV) differences in species richness among forest developmental stages are strongest at lower elevations and decrease towards the tree line (Fig. 2 IV).
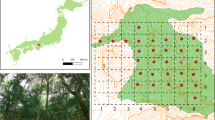
Berchtesgaden National Park and its location in Germany. The map shows the main habitats (forest, open habitats, rock, water/ice) based on data provided by the National Park administration. Plot coordinates were measured using a Trimble r12i GNSS receiver during field sampling. Point shapes illustrate the forest developmental stage and point colours the elevational zone.
Based on the altitudinal-niche-breadth hypothesis and a positive specialization-species richness correlation, we expect both decreasing (H I) habitat specialization and (H II) species richness with elevation. Given decreasing specialization with elevation, we expect (H III) decreasing community dissimilarity across developmental stages with elevation. Given decreasing community dissimilarity with elevation, we finally predict that (H IV) differences in species richness among forest developmental stages are strongest at lower elevations and decrease towards the tree line.
Results
In total, we recorded 12,687 and 9,173 Operational Taxonomic Units (OTUs, see methods) of bacteria and fungi, respectively, 443 plant species, 958 arthropod species identified by taxonomists (termed arthropodsTAX), 8,335 Barcode Index Numbers (BINs, see methods) of insects identified by DNA-metabarcoding (termed insectsBIN), and 105 vertebrate species (Supplementary Table S3). On average across all plots, we found a very broad habitat use ranging between 3.6 developmental stages used by fungi and 4.5 stages used by vertebrates. Vertebrates showed the lowest species richness (28 species) and lowest Jaccard dissimilarity (0.54), whereas bacteria showed the highest species richness (1382 OTUs) and fungi the highest Jaccard dissimilarity (0.84). All descriptive statistics are shown in the Supplementary Table S3.
Patterns of habitat specialization across elevational zones
Using the standardized effect size (SES) from a null model that allows to account for the increasing probability of using more forest developmental stages with more occurrences (supplementary Fig. S8), we found strong evidence of higher habitat specialization in the submontane compared to the montane and subalpine zone for all taxa (Fig. 3), as indicated by the probability of direction (pd ≥ 0.99, a Bayesian measure of effect existence) and the ROPE percentage (0% in ROPE, a Bayesian measure of effect importance, Supplementary Table S5; both measures described in more detail in the methods). Between the montane and subalpine zones, patterns of specialization differed among taxa. ArthropodsTAX showed a lower specialization in the subalpine compared to the montane zone (pd = 1.00, 0% in ROPE). Bacteria, fungi, and plants showed a higher specialization in the subalpine compared to the montane zone (pd = 1.00). This difference was only of substantial magnitude for bacteria and plants (0% in ROPE) and not for fungi (4% in ROPE). We found no evidence for a difference in habitat specialization between the montane and subalpine zones for insectsBIN and vertebrates (pd ≤ 0.69, ≥31% in ROPE).
Standardized effect size (SES) of habitat specialization and normalized species richness along the elevational gradient. We calculated habitat specialization using the reciprocal of the Simpson index, based on the number of different forest developmental stages used by a species within each elevational zone. We then used a null model which allows to account for the increasing probability of using more developmental stages with increasing occupancies (Supplementary Fig. S7), calculated an SES, and averaged across all species per plot for each elevational zone (see methods for more details). Predictions are from individual Bayesian multilevel models for each measure and taxon. We averaged the predictions across forest developmental stages and summarized by means of the MAP and 95% HDI (explanation in methods). To show all taxa in a joint figure for species richness, we normalized the predictions of species richness to a range between zero and one based on each taxon´s minimum and maximum values (see Table S3 and Fig. S9 in the supplement for values and figures including data points).
Patterns of species richness along elevation
ArthropodTAX and insectBIN richness decreased by 16.1% [95% HDIlow = 11.7, 95% HDIhigh = 20.4] and 10.2% [4.1, 14.9], respectively, with one unit of SD of elevation (=359 m) (Fig. 3, Supplementary Table S7). The decrease is well supported for both taxa (pd = 1.00) and was substantial for arthropodsTAX (0% in ROPE) but small for insectsBIN (43% in ROPE). Species richness of bacteria and fungi decreased with one unit SD of elevation by 0.7% [−5.2, 4.3] and 1.4% [−7.4, 5.8], and species richness of plants and vertebrates increased by 4.3% [−1.4, 12.8] and 3.0% [−1.8, 9.3], respectively. These changes can be seen as uncertain and negligible (pd < 0.94, >94% in ROPE).
Elevational patterns of beta diversity among developmental stages
All taxa except arthropodsTAX showed the highest Jaccard dissimilarity among forest developmental stages either in the submontane zone (insectsBIN and vertebrates) or Jaccard dissimilarity was equally high in both the submontane and subalpine zone (Fig. 4). For insectsBIN and vertebrates, Jaccard dissimilarity was lower in the montane and subalpine compared to the submontane zone, well supported for vertebrates (pd = 1.00, 0% in ROPE) but not for insectsBIN (pd ≤ 0.95, ≥3% in ROPE) (Supplementary Table S11 & Fig. S13). For bacteria and plants, Jaccard dissimilarity was lowest in the montane (pd = 1.00, 0% in ROPE) but did not differ between the submontane and subalpine zone (pd < 0.66, 25% in ROPE). Jaccard dissimilarity of fungi did not change across the elevational zones (pd < 0.73, >7% in ROPE). Jaccard dissimilarity of arthropodsTAX was lowest in the submontane (pd = 1.00, 0% in ROPE), but did not differ between the montane and subalpine zone (pd = 0.82, 11% in ROPE).
Jaccard dissimilarity for each taxonomic group across the three elevational zones. Jaccard dissimilarity was calculated between each plot pair of a different developmental stage within each elevational zone, modelled and predicted using individual Bayesian multilevel models with a beta probability distribution, separately for each taxonomic group. Posterior distributions were summarized by means of the MAP and 95% and 50% (thick bars) HDI (explanation in methods). Different point shapes indicate substantial differences in Jaccard dissimilarity between elevational zones. Supplementary Fig. S13 includes data points.
Interactive effects of forest development and elevation on species richness
Species richness of all taxa except bacteria followed a U-shaped pattern along the forest developmental gradient (Fig. 5). Fungi, plant and vertebrate species richness was about 23% [7, 32], 18% [6, 32] and 8% [-4, 17] lower in intermediate stages compared to gaps, on average across all elevations (Supplementary Tables S8a–S8c). While for both fungi and plants lower species richness in intermediate stages was strongly supported (pd = 1.00), the decrease was substantial only for fungi (<3% in ROPE) and of moderate magnitude for plants (<7% in ROPE). Lower vertebrate species richness in intermediate developmental stages was uncertain and negligible (pd = 0.89, >72% in ROPE). InsectBIN species richness was substantially higher (between 19% [10, 27] and 28% [21, 36]) in gaps compared to all other stages (pd = 1.00, 0% in ROPE). Species richness of arthropodsTAX was 9.9% [1, 17] higher in gaps and 12.8% [4, 20] higher in the terminal stage compared to the establishment stage. Bacteria showed no notable differences (between 1.2% [−8, 12] and 4.9% [−5, 16]) in species richness between gaps and all other developmental stages (pd < 0.81, >87% in ROPE).
Patterns of species richness along forest development (sensu Zenner et al.63) for each taxonomic group from individual Bayesian multilevel models with a negative binomial error distribution. We predicted 50 times along the elevational gradient, averaged them by developmental stage, summarized the means using the MAP (explanation in methods), normalized them between the minimum and maximum predicted value of each taxon, and fitted a loess curve for better visualization.
We found no substantial differences in species richness patterns among developmental stages in lower compared to higher elevations for any taxonomic group (all pd ≤ 0.91, always >3% in ROPE; Supplementary Table S9 & Fig. S11). Patterns in Fig. 6 suggest substantial differences between individual developmental stages, but credible intervals were wide and largely overlapped (Fig. S12). While patterns of all taxa varied little across elevational zones, bacteria showed opposing patterns in the submontane and subalpine zone (Fig. 6).
Patterns of species richness along forest development (sensu Zenner et al.63) across elevational zones for each taxonomic group. We predicted species richness for each developmental stage at 719 m, 1154 m, and 1565 m, which are approximately the central elevations of each elevational zone, using Bayesian multilevel models with a negative binomial error distribution. We summarized the predictions using the MAP (explanation in methods) and fitted a loess curve for better visualization. The patterns suggest substantial differences among elevational zones, but high data variability resulted in wide and overlapping credible intervals (Supplementary Fig. S11 includes data points and credible intervals), leading to only marginal differences (Supplementary Table S9 & Fig. S10).
Discussion
Our study provides strong evidence for the altitudinal-niche-breadth hypothesis. In particular, we found decreasing habitat specialization with elevation for all six taxa investigated. Yet, species richness decreased with elevation only for two taxa. Species richness differed among forest developmental stages in all taxa and showed mainly a U-shaped pattern along the stages, which remained stable along elevation. Beta diversity among the stages, however, varied across elevational zones for most taxa but aligned with the elevational patterns of habitat specialization. This suggests that climate and forest disturbances (i.e., the causal driver of forest developmental stages) interactively affect species communities in Central European mountain forests.
Confirming our hypothesis (H I) and in line with the altitudinal-niche-breadth hypothesis, habitat specialization decreased with elevation for all six taxa. As a mechanism behind this decrease, the altitudinal-niche-breadth hypothesis proposes decreasing specialization with elevation due to harsher and more variable environmental conditions7,24. This mechanism might explain the observed patterns in our study, since both decreasing temperature and increasing microclimatic variability are in line with this prediction (Supplementary Fig. S2c). The increase in microclimatic variability with elevation in mountain forests is partly driven by decreasing and more variable canopy cover, resulting in reduced thermal buffering and more heterogenous and extreme microclimates25,26 (Supplementary Fig. S2a & b). While specialists may struggle to survive under such fluctuating conditions, generalists might be able to make use of a wider range of microclimatic conditions or resources7. Moreover, although microclimatic variability was higher in all forest developmental stages in high-elevation forests, differences in canopy cover and microclimatic variability among developmental stages were less pronounced in the subalpine than in lower elevational zones (Supplementary Fig. S2). This could also allow more species of high-elevation forests to occur in different developmental stages. However, we observed for all taxa except arthropodsTAX that specialization did not change or even increased from the montane to the subalpine zone. One possible explanation is that topographic complexity and variability in soils under a more open and variable canopy cover increases habitat heterogeneity at higher elevations, which might promote habitat specialists by weakening microclimatic variability through a fine-scaled mosaic of microhabitats27,28,29,30,31,32. Moreover, it is important to note that specialization is not only affected by abiotic drivers, but also by associations between taxa7. This may explain similar patterns in habitat specialization among bacteria, fungi, and plants in our study since there are various associations between species of these groups33,34,35,36,37. Finally, specialization may not only drive species richness, but species richness could also affect specialization, as suggested by a recent meta-analysis on latitudinal specialization-richness patterns14, which could be a further explanation for patterns (partly) inconsistent with the altitudinal-niche-breadth hypothesis7.
Decreases in species richness along elevational gradients have been previously reported1,2,3,38,39 and can be caused by different mechanisms2. Considering a positive correlation between specialization and species richness14 in combination with the assumptions made by the altitudinal-niche-breadth hypothesis7, we expected decreasing species richness with elevation (H II). We found patterns consistent with our hypothesis for arthropodsTAX and insectsBIN. This suggests that at least for arthropodsTAX and insectsBIN, lower specialization at higher elevations could be one mechanism behind decreasing species richness with increasing elevation. As a further mechanism, environmental filtering theory suggests that harsh climatic conditions beyond the thermal limits of many species can restrict the number of species able to persist at higher elevations40,41,42, and thus lower temperatures are often associated with lower species richness39,43. These mechanisms could have contributed to the elevational richness patterns of insects and other arthropods since these are ectothermic species4,40,44,45. Environmental filtering could also be the mechanism behind the slight increase in plant species richness with elevation, but with light availability being more important than temperature. Due to overall lower canopy cover, light (energy) availability in the understory is higher in high- than low-elevation forests26 (Supplementary Fig. S2a). High light availability promotes a higher plant cover, consequently allowing for a greater number of species with viable populations – in line with the more-individuals hypothesis46,47,48. This implies that for plants, environmental filtering may be stronger at lower elevations, limiting the number of species living beneath closed canopies and leading to higher specialization. Species richness of soil bacteria and fungi declined weakly with elevation, despite a clear decrease in specialization. This weak link between specialization and species richness of soil bacteria and fungi could be explained by the generally strong dependence of these taxa on soil pH, nutrient availability, and plant species33,34,35, interfering with general mechanisms shaping species richness along elevational gradients. The marginally increasing species richness of vertebrates with elevation was mainly driven by bats and may also be explained by canopy openness of high-elevation forests. More open forests allow more bat species to forage in more developmental stages at higher elevations49,50. Our findings emphasize the crucial role of canopy cover in driving habitat specialization and species richness patterns in Central European mountain forests. Yet, taxa are affected differently by factors that do not change uniformly along the elevational gradient, leading to deviations from the expected positive specialization–richness relationship.
Considering that specialization was predicted to decrease with elevation, we hypothesized that beta diversity between forest developmental stages also decreases with elevation (H III). This hypothesis was supported for the majority of taxa (bacteria, plants, insectsBIN, and vertebrates), showing congruent patterns with specialization and strongest changes from the submontane to the montane zone. This indicates that decreasing habitat specialization with increasing elevation leads to taxonomic homogenization across forest developmental stages for these taxa51. However, higher similarity in forest structure and microclimate among developmental stages (despite increasing overall variability in microclimate with elevation; Supplementary Fig. S2) may have contributed to the observed pattern. Stabilizing or increasing beta diversity from the montane to the subalpine zone, similar to habitat specialization, may result from greater within-stage habitat heterogeneity, promoting species turnover and admixture of open habitat species27,28,29,30,31,32. Patterns of fungi and arthropodsTAX were inconsistent with our hypothesis. Even though specialization decreased for both, beta diversity patterns remained constant or increased, indicating stronger clustering with elevation. This suggests that developmental stages determine habitat conditions at lower elevations while other factors, such as topographic complexity or resource availability, may become more important at higher elevations. Furthermore, decreasing occurrences and a higher proportion of rare arthropodTAX species with increasing elevation may have caused greater differences in community composition among developmental stages (Supplementary Table S4). Overall, differences of beta diversity among developmental stages at different elevational zones suggest that forest development and climate interactively shape forest community compositions.
Species richness changes after disturbances52,53,54 and many taxa show U-shaped species richness patterns along forest developmental gradients20. We found support for a U-shaped pattern of species richness for all taxa except bacteria – highlighting the importance of early and late developmental stages for forest biodiversity15,55,56,57. However, contrary to our expectations (H IV), differences in species richness among developmental stages were rather stable and not generally weaker at higher elevations. We assumed the opposite due to lower canopy cover and higher microclimatic similarity among developmental stages at high elevations (Supplementary Fig. S2). However, high topographic complexity in mountain landscapes probably weakens or masks the macroclimatic effects of elevation27,28,29,30,31,32. Furthermore, high habitat heterogeneity provides habitats for a large number of species including many rare species58,59,60, which are, however, more frequently subject to stochastic processes61,62. This may explain why differences in species richness among forest developmental stages did not differ clearly between elevational zones as indicated by the wide credible intervals observed.
Our study shows that elevation and post-disturbance forest development are major drivers of biodiversity across a wide range of forest-dwelling taxa in a mountain forest of the European Alps. Observed patterns of habitat specialization aligned with the altitudinal-niche-breadth hypothesis for all taxa and were largely congruent with beta diversity. Yet, the expected decline in species richness with elevation was observed only in insects and other arthropods. This indicates that the mechanisms behind the altitudinal-niche-breadth hypothesis do play a role across all trophic levels in our study system, but species richness patterns are not generally explained by positive specialization-richness relationships. In contrast to species richness patterns along forest development, beta diversity among developmental stages varied with increasing elevation for most taxa. This suggests that climate (represented by the elevational gradient) and disturbance (represented by the forest developmental gradient) independently drive patterns of species richness, but interactively shape community composition.
We assessed habitat specialization based on forest developmental stages63, which integrate multiple environmental conditions instead of measuring them individually. Since it is impractical to measure all relevant environmental variables across a wide range of taxa, using forest developmental stages as a proxy offers a more general picture of habitat differences. However, it does not allow to link patterns of species diversity to individual environmental drivers. Nevertheless, forest developmental stages are a widely applied concept in forest ecology and help guide management decisions17,63,64,65,66. For testing hypotheses related to elevational patterns in ecology, ideally the full elevational gradient is covered. In this study, the elevational gradient reached the tree line but was truncated at the lower end at approximately 600 m asl. Extending the gradient to lower elevations, however, would have introduced considerable bias due to differences in forest management and fragmentation67,68,69, as well as by orders of magnitude larger spatial distances between study plots. We are confident that the observed elevational patterns would be similar if elevations below 600 m had been included since the submontane zone has a similar tree species composition and forest development regime as forests below 600 m70.
Climate change is leading to altered climatic conditions and forest dynamics71,72,73, which will have far-reaching effects on biodiversity74. If low elevations are a proxy for future climate at higher elevations, our results suggest that in our study system species richness of insects and other arthropods will increase, whereas species richness of plants and vertebrates will decrease as a result of a warming climate. Our results also suggest that differences in species richness among forest developmental stages will remain stable, but differences in community composition will be more pronounced under a warming climate. If increasing forest disturbances result in a greater proportion of the landscape at early developmental stages73, our results suggest positive effects on species richness for most taxonomic groups. This underscores the potential of early developmental stages without human intervention, such as salvage logging, for promoting species richness.
Methods
Study area
This study was conducted at Berchtesgaden National Park located in the northern limestone Alps of south-east Germany (Fig. 1). The study area is characterized by high topographic complexity and environmental heterogeneity due to the steep terrain, with elevation ranging from 603 m (lake Königssee) to 2713 m asl (Mt. Watzmann). Forests cover approximately 54% of the national park’s area of roughly 21,000 ha, with the tree line at approximately 1700 m asl75. The natural tree species composition differs between elevational zones: European beech (Fagus sylvatica L.) dominates the submontane zone (<850 m asl). Mixed forests consisting of European beech, Norway spruce (Picea abies (L.) Karst.) and Silver fir (Abies alba Mill.) are common in the montane zone (850–1400 m asl), and conifer forests of Norway spruce, European larch (Larix decidua) and Swiss stone pine (Pinus cembra) dominate the subalpine zone (1400–1700 m asl)70,76. The region has a long history of timber extraction for salt mining, which has led to increased shares of Norway spruce in the submontane and montane zone77. Canopy cover decreases and variation in canopy cover increases with elevation26 (Supplementary Figs. S2a & S2b), lowering the thermal buffering capacity25,78 and leading to relatively higher microclimatic variability at intermediate to late developmental stages but lower variation across stages in the subalpine zone (Supplementary Fig. S2c). Conventional forest management ceased when the national park was founded in 1978, and is today restricted to restoration management carried out on 25% of the park, restoring the natural species composition by planting European beech and Silver fir79 and preventing the spread of bark beetle outbreaks to neighbouring commercial forests.
Classification of forest developmental stages and plot selection
We modified a classification protocol63 to select plots covering the full gradient of forest development (Supplementary Fig. S3), differentiating five forest developmental stages: gap, establishment, optimum, plenter, terminal. We first applied the protocol to data of the last available forest inventory (2010-2012) to pre-select approximately 300 candidate plots. The candidate plots covered all major forested areas of the national park, except areas where bark-beetle trees are felled and those that were too steep to be accessed. We visited all candidate plots in the field in 2020 to verify and adjust the pre-classified developmental stage, and to exclude plots that intersected with hiking trails. We finally selected ten replicates per forest developmental stage and elevational zone (for ranges see study area), resulting in 150 plots in total (Fig. 1). Submontane plots were more clustered compared to plots in the other elevational zones, as only a few areas in the park are below 850 m asl and thus in the submontane zone (Fig. 1). The plots were circular and covered an area of 500 m2 (r = 12.62 m) with a minimum distance between plot centres of 125 m. The lowest plot was located at 605 m and the highest at 1725 m asl. Figure S4 in the supplementary material shows a compilation of selected plots.
Field sampling and species identification
We collected data from 14 taxonomic groups across all 150 plots (Table 1). Field sampling was conducted in 2021 except light trapping, which was conducted in 2022. Bacterial and fungal communities in the soil were identified through DNA metabarcoding of four soil samples from each plot, consisting of mineral and organic soil samples taken at approximately 3 m distance from the plot centre in each of the four cardinal directions. Plant species were recorded on a 200 m2 quadratic area, distinguishing the herb (<1 m height) and shrub layer (>1–5 m height). We sampled arthropods with one Malaise trap, three pitfall traps, and one light trap per plot to cover different microhabitats and taxonomic groups. Arthropods from two out of three pitfall samples and moths from light traps were identified by taxonomists (see Table 1 for a list of identified taxonomic groups). Two pitfall traps adequately reflect forest plots similar to ours80, but since single traps are sometimes disturbed (e.g., by wildlife), we placed three pitfall traps per plot. We then used only two out of the three pitfall samples to maintain comparability across plots per census. While beetles were identified for the entire sampling period, the other taxa from pitfall traps were only identified for the census around August (Table 1, Supplementary Fig. S5). Species from Malaise trap samples were identified through DNA metabarcoding for the entire sampling period. DNA metabarcoding amplifies DNA-fragments found in our samples – viable or not. However, this bias can be considered equal among all samples and does not affect the overall outcome. For an overview of the numbers of BINs/species of different taxonomic groups from DNA-metabarcoding and taxonomists, see Supplementary Fig. S7. Due to the different underlying species concepts, we analysed arthropods identified by taxonomists (“arthropodsTAX”) and those identified by metabarcoding separately and refer to the latter as “insectsBIN”, since we only analysed insects as they made up 96% of all arthropod BINs (Barcode Index Numbers) in Malaise traps. We recorded birds through passive acoustic recorders in the morning hours around sunrise and experts identified species based on these recordings. Bats were recorded using ultrasonic recorders, identified to species level through automated software (batIdent, ecoObs, Nuremberg, Germany), and subsequently evaluated by an expert. Small mammals (i.e., mice, voles, dormice, and shrews) were caught in pitfall traps, and large mammals were recorded through wildlife cameras. Further details on the field sampling, DNA metabarcoding, and data preparation is provided in the supplementary methods section.
Specialization measure
All calculations and analyses were conducted in R81 (version 4.1.1). We used the reciprocal of the Simpson index to determine habitat diversity based on a species´ proportional use of forest developmental stages:
where \(p\) represents the number of occupied plots in stage \(i\) relative to the total occupancies (proportional use) of a species. We calculated \(B\) separately for each elevational zone to capture changes in a species´ habitat use with increasing elevation. We then used a null model approach which allows to account for the increasing probability of using more developmental stages with more occupancies (Supplementary Fig. S6). To simulate 500 random communities, we applied a non-sequential algorithm for presence/absence matrices which keeps matrix, row and column sums constant (function permatfull from vegan package82). We calculated a standardized effect size (SES) for each species and elevational zone by computing \(B\) for each simulated community, subtracting the mean simulated from the observed \(B\), and dividing by the standard deviation of the simulated \(B\). As a result, we lost between four and 19% of species, depending on taxon and elevational zone, due to little variation and thus a standard deviation of zero (Supplementary Table S4). We multiplied the SES by -1 to obtain an index for specialization and averaged across species of each plot and elevational zone.
Species richness and beta diversity
For taxa sampled through metabarcoding, DNA barcodes (bacteria: 16S, fungi: ITS, insects: CO1) were clustered into Operational Taxonomic Units (OTUs) using a similarity threshold of 98% for bacteria and fungi and 97% for insects. We used OTUs as a proxy for bacterial and fungal species, whereas insect OTUs were assigned to a globally unique identifier (Barcode Index Numbers, BINs) from the Barcode of Life data system (BOLD)83,84, which we used as a proxy for insectBIN species. The utility of BINs in assessing biodiversity has been largely demonstrated85,86,87. We calculated species richness as the sum of all species found at a study plot. To quantify beta diversity, we calculated the Jaccard dissimilarity between each set of two plots from different forest developmental stages for each elevational zone, respectively.
Statistics and reproducibility
We first analysed how the SES of niche breadth (H I), species richness (H II), and beta diversity (H III) changed with elevation (Fig. 2). In a second step, we analysed how differences in species richness between forest developmental stages differed between the three elevational zones (H IV). To do so, we fitted individual multilevel models for each taxonomic group within a Bayesian framework using the brms package88. We z-transformed (mean = 0, SD = 1) all continuous predictor variables to increase sampling efficiency.
To analyse habitat specialization along elevation, we used the SES of specialization as the response variable and the elevational zone as a three-level categorical predictor variable. Due to differences in the dispersion of the SES across the elevational zones, we fitted distributional models with a Gaussian error distribution and used the elevational zone to model the dispersion \({phi}\). We used multivariate Gaussian processes with x and y UTM coordinates as joint predictors to model spatial autocorrelation. We applied 15 basis functions for bacteria and 20 for all other taxa to minimize computation time and avoid overfitting but adequately account for spatial autocorrelation89.
To address patterns of species richness along gradients of elevation and forest development, we fitted negative-binomial models with species richness as a response and an interaction term between forest developmental stage (categorical) and elevation (continuous) as predictors. For bacteria, fungi, and plants, we added the day of year as a continuous covariate to adjust for phenological effects due to different sampling dates. We added a variable that groups neighbouring study plots as random intercepts to account for spatial autocorrelation (groups are shown in Supplementary Fig. S1).
To address patterns of beta diversity among plots from different forest developmental stages of each elevational zone, we fitted models with the pairwise Jaccard dissimilarities as response and elevational zone as a categorical predictor. We added the elevational and spatial distance between each set of the two plots to all models, as well as the difference between the sampling day to the models for bacteria, fungi, and plants as continuous covariates, to adjust for spatial and phenological differences. In the case of a quadratic relationship between beta diversity and a covariate, we added the covariate as a quadratic term using the poly function. We fitted the models using a beta probability distribution.
We provide further information about model specifications (priors, iterations), evaluations (convergence, residual checks, goodness-of-fit (Supplementary Table S2), posterior predictive checks, VIF), and summaries (Supplementary Tables S5, S8a–S8c, S10, S12, and S13) in the statistical analyses and results of the supplementary material.
To assess the effect of elevation on habitat specialization (H I), we calculated the pairwise differences of the predictions between each elevational zone. To assess the overall effect of elevation on species richness (H II), we added the baseline elevation coefficient (gap stage) to all developmental stage and elevation interaction coefficients and averaged over all. To assess whether beta diversity decreases with increasing elevation (H III), we computed the differences of predictions between the elevational zones. To assess whether the differences among forest developmental stages are strongest at low elevations and decrease towards the tree line (H IV), we first computed the absolute differences between predictions of the optimum and each other developmental stage, separately for each elevational zone. We then computed a “difference-of-differences” across the elevational zones: We subtracted the absolute differences of each optimum-[developmental stage]-comparison of the montane and subalpine zone from the absolute difference of the corresponding optimum-[developmental stage]-comparison of the submontane zone. For a better understanding, we provide a graphical concept of our calculations in the supplemental material (Supplementary Fig. S6).
We summarize all posterior distributions using the Maximum A Posteriori (MAP) and the 95% Highest Density Interval (HDI), representing the value with the highest probability density (mode) and the interval containing 95% of the highest probability density (95% Credible Interval, CI). To assess the existence and importance of an effect, we calculated the probability of direction (pd) and the ROPE percentage for each predictor90,91 using the bayestestR package92. The pd is strongly correlated with the frequentist p-value and represents a robust and model-independent index ranging from 50% to 100% that indicates the certainty of an effect’s direction (positive or negative), i.e., the existence of an effect. However, the pd does not assess the magnitude or importance of an effect, which is better evaluated using the ROPE percentage. The Region of Practical Equivalence (ROPE) defines an area around the null value, enclosing values equivalent to the null and thus of negligible magnitude and importance. The ROPE percentage indicates the proportion of the 95% HDI within this area, which here is defined as a range from –0.1 * SDy to 0.1 * SDy90. All changes in species richness correspond to a unit increase in the standard deviation of elevation, which is 357 m across our plots. We provide all pd and ROPE percentage measures together with the model summaries in the supplementary results (Supplementary Tables S4-S12).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data used in our analysis have been deposited in a publicly accessible archive on Dryad: https://doi.org/10.5061/dryad.bk3j9kdkp93
Code availability
All analysis were conducted in R, and the code has been deposited in the same Dryad archive as the data: https://doi.org/10.5061/dryad.bk3j9kdkp93
References
Lomolino, M. V. Elevation gradients of species‐density: Historical and prospective views. Glob. Ecol. Biogeogr. 10, 3–13 (2001).
McCain, C. M. & Grytnes, J.-A. Elevational Gradients in Species Richness; https://doi.org/10.1002/9780470015902.a0022548 (2010).
Rahbek, C. The role of spatial scale and the perception of large‐scale species‐richness patterns. Ecol. Lett. 8, 224–239 (2005).
Hodkinson, I. D. Terrestrial insects along elevation gradients: species and community responses to altitude. Biol. Rev. Camb. Philos. Soc. 80, 489–513 (2005).
Körner, C. Plant adaptation to cold climates. F1000Research 5; https://doi.org/10.12688/f1000research.9107.1 (2016).
Weiher, E. & Keddy, P. A. Assembly rules, null models, and trait dispersion: new questions from old patterns. Oikos 74, 159 (1995).
Rasmann, S., Alvarez, N. & Pellissier, L. The altitudinal niche-breadth hypothesis in insect-plant interactions. In Annual Plant Reviews (John Wiley & Sons, Ltd. 2014), pp. 339–359.
Belmaker, J., Sekercioglu, C. H. & Jetz, W. Global patterns of specialization and coexistence in bird assemblages. J. Biogeogr. 39, 193–203 (2012).
MacArthur, R. H. Patterns of species diversity. Biol. Rev. 40, 510–533 (1965).
MacArthur, R. H. & Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Naturalist 101, 377–385 (1967).
Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 (2000).
Mermillon, C. et al. Variations in niche breadth and position of alpine birds along elevation gradients in the European alps. Ardeola 69, 41–58 (2022).
Schellenberger Costa, D. et al. Plant niche breadths along environmental gradients and their relationship to plant functional traits. Divers. Distrib. 24, 1869–1882 (2018).
Granot, I. & Belmaker, J. Niche breadth and species richness: Correlation strength, scale and mechanisms. Glob. Ecol. Biogeogr. 29, 159–170 (2020).
Swanson, M. E. et al. The forgotten stage of forest succession: Early‐successional ecosystems on forest sites. Front. Ecol. Environ. 9, 117–125 (2011).
Wohlgemuth, T., Jentsch, A. & Seidl, R. Disturbance Ecology. 1st ed. (Springer International Publishing; Imprint Springer, Cham, 2022).
Franklin, J. F. et al. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For. Ecol. Manag. 155, 399–423 (2002).
Lehnert, L. W., Bässler, C., Brandl, R., Burton, P. J. & Müller, J. Conservation value of forests attacked by bark beetles: Highest number of indicator species is found in early successional stages. J. Nat. Conserv. 21, 97–104 (2013).
Pulsford, S. A., Lindenmayer, D. B. & Driscoll, D. A. A succession of theories: purging redundancy from disturbance theory. Biol. Rev. 91, 148–167 (2016).
Hilmers, T. et al. Biodiversity along temperate forest succession. J. Appl. Ecol. 55, 2756–2766 (2018).
Pepin, N. et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Change 5, 424–430 (2015).
Turner, M. G. et al. The magnitude, direction, and tempo of forest change in Greater Yellowstone in a warmer world with more fire. Ecol. Monogr. 92; https://doi.org/10.1002/ecm.1485 (2022).
Thom, D. & Seidl, R. Accelerating mountain forest dynamics in the Alps. Ecosystems 25, 603–617 (2022).
Kraft, N. J. B., Godoy, O. & Levine, J. M. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl Acad. Sci. USA 112, 797–802 (2015).
Vandewiele, M. et al. Mapping spatial microclimate patterns in mountain forests from LiDAR. Agric. Forest Meteorol. 341, 109662; (2023).
Glasmann, F., Senf, C., Seidl, R. & Annighöfer, P. Mapping subcanopy light regimes in temperate mountain forests from Airborne Laser Scanning, Sentinel-1 and Sentinel-2. Sci. Remote Sens. 8, 100107 (2023).
Dobrowski, S. Z. A climatic basis for microrefugia: The influence of terrain on climate. Glob. Change Biol. 17, 1022–1035 (2011).
Haesen, S. et al. Microclimate reveals the true thermal niche of forest plant species. Ecol. Lett. 26, 2043–2055 (2023).
Frei, K. et al. Topographic depressions can provide climate and resource microrefugia for biodiversity. iScience 26, 108202 (2023).
Bátori, Z. et al. Karst dolines provide diverse microhabitats for different functional groups in multiple phyla. Sci. Rep. 9, 7176 (2019).
Lenoir, J., Hattab, T. & Pierre, G. Climatic microrefugia under anthropogenic climate change: Implications for species redistribution. Ecography 40, 253–266 (2017).
Finocchiaro, M. et al. Bridging the gap between microclimate and microrefugia: A bottom-up approach reveals strong climatic and biological offsets. Glob. Change Biol. 29, 1024–1036 (2023).
Lladó, S., López-Mondéjar, R. & Baldrian, P. Drivers of microbial community structure in forest soils. Appl Microbiol. Biotechnol. 102, 4331–4338 (2018).
Baldrian, P. Forest microbiome: diversity, complexity and dynamics. FEMS Microbiol. Rev. 41, 109–130 (2017).
Siles, J. A. & Margesin, R. Abundance and diversity of bacterial, archaeal, and fungal communities along an altitudinal gradient in Alpine forest soils: what are the driving factors? Micro. Ecol. 72, 207–220 (2016).
Shen, C. et al. Contrasting patterns and drivers of soil bacterial and fungal diversity across a mountain gradient. Environ. Microbiol. 22, 3287–3301 (2020).
Chauvier, Y. et al. Influence of climate, soil, and land cover on plant species distribution in the European Alps. Ecol. Monogr. 91, e01433 (2021).
Bryant, J. A. et al. Microbes on mountainsides: contrasting elevational patterns of bacterial and plant diversity. Proc. Natl Acad. Sci. USA 105, 11505–11511 (2008).
Peters, M. K. et al. Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat. Commun. 7, 13736 (2016).
Classen, A. et al. Temperature versus resource constraints: which factors determine bee diversity on Mount Kilimanjaro, Tanzania? Glob. Ecol. Biogeogr. 24, 642–652 (2015).
Currie, D. J. Energy and large-scale patterns of animal- and plant-species richness. Am. Naturalist 137, 27–49 (1991).
Khaliq, I., Hof, C., Prinzinger, R., Böhning-Gaese, K. & Pfenninger, M. Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc. R. Soc. B. 281, 20141097 (2014).
Brown, J. H. Why are there so many species in the tropics? J. Biogeogr. 41, 8–22 (2014).
Birkett, A. J., Blackburn, G. A. & Menéndez, R. Linking species thermal tolerance to elevational range shifts in upland dung beetles. Ecography 41, 1510–1519 (2018).
Sunday, J. M. et al. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610–5615 (2014).
Dormann, C. F. et al. Plant species richness increases with light availability, but not variability, in temperate forests understorey. BMC Ecol. 20, 43 (2020).
Hurlbert, A. H. Species–energy relationships and habitat complexity in bird communities. Ecol. Lett. 7, 714–720 (2004).
Penone, C. et al. Specialisation and diversity of multiple trophic groups are promoted by different forest features. Ecol. Lett. 22, 170–180 (2019).
Renner, S. C. et al. Divergent response to forest structure of two mobile vertebrate groups. For. Ecol. Manag. 415-416, 129–138 (2018).
Müller, J. et al. Aggregative response in bats: Prey abundance versus habitat. Oecologia 169, 673–684 (2012).
Clavel, J., Julliard, R. & Devictor, V. Worldwide decline of specialist species: toward a global functional homogenization? Front. Ecol. Environ. 9, 222–228 (2010).
Kulakowski, D. et al. A walk on the wild side: Disturbance dynamics and the conservation and management of European mountain forest ecosystems. For. Ecol. Manag. 388, 120–131 (2017).
Thom, D. & Seidl, R. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol. Rev. Camb. Philos. Soc. 91, 760–781 (2016).
Viljur, M.-L. et al. The effect of natural disturbances on forest biodiversity: An ecological synthesis. Biol. Rev. Camb. Philos. Soc. 97, 1930–1947 (2022).
Moning, C. & Müller, J. Critical forest age thresholds for the diversity of lichens, molluscs and birds in beech (Fagus sylvatica L.) dominated forests. Ecol. Indic. 9, 922–932 (2009).
Winter, M.-B. et al. On the structural and species diversity effects of bark beetle disturbance in forests during initial and advanced early-seral stages at different scales. Eur. J. For. Res 136, 357–373 (2017).
Winter, M.-B. et al. Multi-taxon alpha diversity following bark beetle disturbance: Evaluating multi-decade persistence of a diverse early-seral phase. For. Ecol. Manag. 338, 32–45 (2015).
Allouche, O., Kalyuzhny, M., Moreno-Rueda, G., Pizarro, M. & Kadmon, R. Area-heterogeneity tradeoff and the diversity of ecological communities. Proc. Natl Acad. Sci. USA 109, 17495–17500 (2012).
Tews, J. et al. Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J. Biogeogr. 31, 79–92 (2004).
Law, B. S. & Dickman, C. R. The use of habitat mosaics by terrestrial vertebrate fauna: implications for conservation and management. Biodivers. Conserv 7, 323–333 (1998).
Orrock, J. L. & Watling, J. I. Local community size mediates ecological drift and competition in metacommunities. Proc. R. Soc. B. 277, 2185–2191 (2010).
Siqueira, T. et al. Community size can affect the signals of ecological drift and niche selection on biodiversity. Ecology 101, e03014 (2020).
Zenner, E. K., Peck, J. E., Hobi, M. L. & Commarmot, B. Validation of a classification protocol: Meeting the prospect requirement and ensuring distinctiveness when assigning forest development phases. Appl Veg. Sci. 19, 541–552 (2016).
Larrieu, L. et al. Deadwood and tree microhabitat dynamics in unharvested temperate mountain mixed forests: A life-cycle approach to biodiversity monitoring. For. Ecol. Manag. 334, 163–173 (2014).
Begehold, H., Rzanny, M. & Flade, M. Forest development phases as an integrating tool to describe habitat preferences of breeding birds in lowland beech forests. J. Ornithol. 156, 19–29 (2015).
Hilmers, T., Biber, P., Knoke, T. & Pretzsch, H. Assessing transformation scenarios from pure Norway spruce to mixed uneven-aged forests in mountain areas. Eur. J. For. Res 139, 567–584 (2020).
Beck, E., Bendix, J., Kottke, I., Makeschin, F. & Mosandl, R. Gradients in a Tropical Mountain Ecosystem of Ecuador (Springer Science & Business Media, 2008).
Gradstein, S. R., Homeier, J. & Gansert, D. The tropical mountain forest (Göttingen University Press, Göttingen, 2008).
Haddad, N. M. et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. American Association for the Advancement of Science (2015).
Walentowski, H. et al. Handbuch der natürlichen Waldgesellschaften Bayerns. Ein auf geobotanischer Grundlage entwickelter Leitfaden für die Praxis in Forstwirtschaft und Naturschutz. 4th ed. (Verlag Geobotanica, Freising, 2020).
McDowell, N. G. et al. Pervasive shifts in forest dynamics in a changing world. Science 368; https://doi.org/10.1126/science.aaz9463 (2020).
Seidl, R. et al. Forest disturbances under climate change. Nat. Clim. Change 7, 395–402 (2017).
Senf, C., Sebald, J. & Seidl, R. Increasing canopy mortality affects the future demographic structure of Europe’s forests. One Earth 4, 749–755 (2021).
Thom, D. et al. The impacts of climate change and disturbance on spatio-temporal trajectories of biodiversity in a temperate forest landscape. J. Appl. Ecol. 54, 28–38 (2017).
Mandl, L., Stritih, A., Seidl, R., Ginzler, C. & Senf, C. Spaceborne LiDAR for characterizing forest structure across scales in the European Alps. Remote Sens. Ecol. Conserv 9, 599–614 (2023).
Thom, D. et al. Will forest dynamics continue to accelerate throughout the 21st century in the Northern Alps? Glob. Change Biol. 28, 3260–3274 (2022).
Zierl, H. History of forest and forestry in the Berchtesgaden National Park - from primeval forest via 800 years of forest use to natural forest. Forstliche Forschungsberichte München 206, 155–177 (2009).
De Frenne, P. et al. Global buffering of temperatures under forest canopies. Nat. Ecol. Evol. 3, 744–749 (2019).
Dollinger, C., Rammer, W. & Seidl, R. Climate change accelerates ecosystem restoration in the mountain forests of Central Europe. J. Appl Ecol. 60, 2665–2675 (2023).
Müller, J. & Brandl, R. Assessing biodiversity by remote sensing in mountainous terrain: the potential of LiDAR to predict forest beetle assemblages. J. Appl Ecol. 46, 897–905 (2009).
R Core Team. R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2021).
Oksanen, J. et al. Vegan: Community Ecology Package R package version 2.6-4. (2022).
Ratnasingham, S. & Hebert, P. D. N. A DNA-based registry for all animal species: the barcode index number (BIN) system. PLOS ONE 8, e66213 (2013).
Ratnasingham, S. & Hebert, P. D. N. bold: The Barcode of life data system. Mol. Ecol. Notes 7, 355–364, http://www.barcodinglife.org (2007).
Pentinsaari, M., Hebert, P. D. N. & Mutanen, M. Barcoding beetles: a regional survey of 1872 species reveals high identification success and unusually deep interspecific divergences. PloS One 9, e108651 (2014).
Schmidt, S., Schmid-Egger, C., Morinière, J., Haszprunar, G. & Hebert, P. D. N. DNA barcoding largely supports 250 years of classical taxonomy: identifications for Central European bees (Hymenoptera, Apoidea partim). Mol. Ecol. Resour. 15, 985–1000 (2015).
Hausmann, A. et al. Genetic patterns in European geometrid moths revealed by the Barcode Index Number (BIN) system. PloS one 8, e84518 (2013).
Bürkner, P.-C. brms: An R Package for Bayesian multilevel models using Stan. J. Stat. Soft. 80, 1–28 (2017).
Dormann, C. F. et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628 (2007).
Kruschke, J. K. Rejecting or accepting parameter values in Bayesian estimation. Adv. Methods Pract. Psychol. Sci. 1, 270–280 (2018).
Makowski, D., Ben-Shachar, M. S., Chen, S. H. A. & Lüdecke, D. Indices of effect existence and significance in the Bayesian framework. Front. Psychol. 10, 2767 (2019).
Makowski, D., Ben-Shachar, M. & Lüdecke, D. bayestestR: Describing effects and their uncertainty, existence and significance within the Bayesian Framework. JOSS 4, 1541 (2019).
Richter, T. et al. Data from: Effects of climate and forest development on habitat specialization and biodiversity in Central European mountain forests. Dryad; https://doi.org/10.5061/dryad.bk3j9kdkp (2024).
Acknowledgements
We thank Robin Reiter, Anna-Maria Bachleitner, August Schellmoser, Verena Styrnik, Ake Geiß, and a large number of colleagues from the National Park staff as well as student helpers for their contribution to field work. We further thank Alexander Szallies and Torben Kölkebeck (beetles), Wolfgang H. O. Dorow (ants), Jörg Spelda (woodlice, millipedes, centipedes), David Stille and Stefan Linzmaier (small mammals), and Alfred Haslberger (moths) for identification of species from pitfall and light traps. We thank Christoph Moning, Ralph Martin, Johannes Urban, Sascha Homburg, Lukas Griem, and Thomas Kuhn for bird species identification and Milenka Reiter-Mehr for bat species validations from sound recordings. We thank Lena Fleckenstein for DNA extraction and Torsten Hothorn for statistical consulting. This work was part of the “Climate Change Research Initiative of the Bavarian National Parks” funded by the Bavarian State Ministry of the Environment and Consumer Protection. Kristin Braziunas and Rupert Seidl acknowledge support from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (Grant Agreement 101001905, FORWARD).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
S.S. and R.S. designed the overall framework with inputs of C.B. and J.M. T.R. and S.S. developed the concept of the study with inputs from R.S. and J.M. L.G. and T.R. selected the study plots with help from S.S. and D.T. L.G. and T.R. collected the biodiversity data with help from S.S. and R.S. C.B. coordinated the laboratory work for DNA extraction. T.R. coordinated the taxonomic and L.G. and S.S. the molecular identification process. C.S. processed the LiDAR data. K.H.B. prepared the trait data for the plant species. L.G. and T.R. curated the data. T.R. analysed the data with support from S.S. and C.S. T.R. and S.S. led the writing with inputs from S.K.. All authors critically revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks David Costa and Monica Berdugo for their contribution to the peer review of this work. Primary Handling Editors: Shouli Li and Joao Valente. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Richter, T., Geres, L., König, S. et al. Effects of climate and forest development on habitat specialization and biodiversity in Central European mountain forests. Commun Biol 7, 1518 (2024). https://doi.org/10.1038/s42003-024-07239-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-07239-6