Abstract
Organisms’ responses to environmental changes involve complex, coordinated responses of multiple tissues and potential parental influences. Here using a multi-tissue approach we determine how variation in parental behavioural tolerance and exposure to elevated CO2 influences the developmental and intergenerational molecular responses of their offspring in the coral reef fish Acanthochromis polyacanthus to future ocean acidification (OA) conditions. Gills and liver showed the highest transcriptional response to OA in juvenile fish regardless of parental OA conditioning, while the brain and liver showed the greatest intergenerational acclimation signals. Developmentally induced signals of OA, such as altered neural function in the brain, were restored to control levels after intergenerational exposure. Intergenerational CO2 exposure also enabled the offspring to adjust their metabolic processes, potentially allowing them to better meet the energetic demands of a high CO2 environment. Furthermore, offspring of OA-exposed parents differentially expressed a new complement of genes, which may facilitate intergenerational acclimatory responses. A genetic component of intergenerational plasticity also played a crucial role, with the parental behavioural phenotype largely determining the offspring’s transcriptional signals. Overall, our results reveal tissue-specific transcriptional changes underlying intergenerational plastic responses to elevated CO2 exposure, enhancing understanding of organismal acclimation to OA throughout the whole body.
Similar content being viewed by others
Introduction
Given the ongoing rapid human-induced global change, organisms need to acclimate and adapt to the changing environments in order to survive. Ocean acidification (OA), driven by the absorption of anthropogenic CO2, has increased the amount of dissolved CO2 in the oceans. Projections estimate a rise in CO2 levels from a present-day value of 400 µatm to ~900 µatm by the end of the century1. Ocean acidification (OA) is reported to negatively impact the physiology and behaviour of various marine organisms including fish2,3. However, increasing evidence suggests that multi-generational exposure to elevated CO2 conditions could influence the adaptive capacity of future generations to OA conditions4. In fact, several studies have reported transgenerational acclimation in a number of fish species as well as some invertebrates to OA3. Examples include Atlantic silverside, certain species of anemonefish, oysters, mussels, sea urchins, and copepods3,5,6,7,8. Specifically, transgenerational exposure to elevated CO2 conditions has been shown to facilitate acclimation of metabolism, growth, survival, neuronal plasticity and behaviour in independent studies6,9,10,11,12,13,14,15, however, we are still learning about the underlying molecular mechanisms of such acclimation processes.
Furthermore, there exists considerable variability both within and across species in the biological responses to OA, due to differences in their evolutionary and environmental history. This variability in sensitivity to elevated CO2 within populations could be crucial in long-term adaptation by favouring the selection of more tolerant individuals. Indeed, variation in behavioural tolerance to elevated CO2 exposure has been reported to be heritable and hence could facilitate rapid selection of tolerant genotypes in the population16,17. Such selection for CO2 tolerance has been shown to occur in nature, leading to populations consisting of individuals with greater behavioural tolerance to elevated CO218. Furthermore, inter-individual variation in sensitivity to ocean acidification could have an epigenetic basis19,20. Several studies have reported the expression levels of genes involved in epigenetic processes to be altered upon exposure to elevated CO2 conditions13,21. Transfer of epigenetic factors that influence gene expression profiles from parents to offspring (epigenetic inheritance) could be one of the potential mechanisms of inter- and trans-generational acclimation and eventual adaptation to OA.
Adaptive responses of organisms to environmental changes involve integrated activity of various tissues, with each tissue undergoing changes in its transcriptional landscape resulting in the overall response of the organism. However, to date, research has mainly focused on individual tissue functional changes in response to OA with less emphasis on how these changes integrate to create a whole-body response. Several studies have examined the effects of OA on brain and neurosensory systems since the discovery of impaired behavioural responses in various fish species in elevated CO2 conditions. The altered behavioural responses have been linked to changes in the functioning of the GABAergic signalling pathway22 and the circadian rhythm in the brain of fish exposed to elevated CO214,23,24. Previous studies have also focused on the effects of OA on the gill transcriptome as gills are the primary organ involved in acid-base regulation, immune defence, and stress response, and hence play a vital role in maintaining cellular homeostasis under conditions of CO2 stress25,26,27. These processes are energetically expensive and indeed changes in the aerobic metabolic scope28,29,30,31 and expression levels of key metabolic genes32 have been reported in fish exposed to elevated CO2. Therefore, exposure to elevated CO2 affects various aspects of fish physiology such as metabolism, cellular redox status, ion transport and acid-base homeostasis, neurological functioning and behaviour thereby exerting whole-body functional reprogramming33. Therefore, a systematic transcriptomic analysis is needed to determine how the biological processes associated with each tissue interact to drive adaptive responses of the whole organism to elevated CO2 environments.
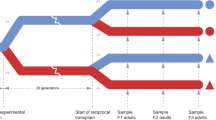
In this study, we conducted an intergenerational CO2 exposure experiment and performed a systematic analysis of gene expression changes in response to elevated CO2 across three tissues, the brain, the gills, and the liver, using the spiny chromis damselfish Acanthochromis polyacanthus. Given the functional specificity of different tissues, we hypothesise that certain molecular pathways would exhibit tissue-specific regulation, while others would show coordinated regulation involving multiple tissues. While A. polyacanthus can be sensitive to increases in water temperature and CO2 levels, it has the potential to acclimate to the changing environmental conditions across multiple generations13,14,34,35, making it an ideal model to explore the molecular basis of both developmental and intergenerational plasticity. In fact, this species has been used as a model to study the impacts of climate change and investigate the molecular basis of intergenerational plasticity to environmental changes. This is due to its advantageous life-history traits, such as the formation of monogamous breeding pairs, direct development of larvae, and suitability for breeding and rearing in captivity36. However, past studies have only focused on single tissues13,14,19. Here, by using a multi-tissue transcriptomic approach we demonstrate how dynamic cross-talk between tissues underlies the general and common elevated CO2 response, as well as the organism’s developmental and intergenerational specific response to future ocean acidification conditions (Fig. 1). In particular, we explored genes with three specific expression patterns – (i) genes that show significantly altered expression in both the developmental and intergenerational CO2 conditions compared to control, (ii) genes that are differentially regulated in the developmental CO2 condition but whose expression is similar to control levels in the intergenerational CO2 condition, and (iii) genes whose expression is significantly altered exclusively in the intergenerational CO2 condition (Fig. 1). This enabled us to tease apart distinct patterns of gene regulation critical for understanding the mechanisms of intergenerational acclimation. Additionally, we also reveal how the acclimation response of offspring, mediated by altered gene expression profiles across multiple tissues, is influenced by variation in parental sensitivity to elevated CO2 and parental environment. Offspring of behaviourally sensitive parents no longer showed signatures of altered neural activity, observed during within-generation exposure to OA, when the parents had also been previously exposed to OA. Interestingly, offspring of parents with behaviourally tolerant phenotype showed an overall greater magnitude of transcriptional response to elevated CO2 exposure and increased intergenerational plastic responses compared to offspring with behaviourally sensitive parents. This intergenerational plasticity was evident from the upregulation of cellular stress response genes in the gills and liver with both within- and intergeneration exposure, and additional upregulation in the brain only upon intergenerational exposure. Moreover, several genes associated with energy metabolism were differentially regulated in the brain and liver of offspring with behaviourally tolerant parents only in the intergenerational treatment. Overall, through systemic characterisation of the effects of OA, we show how the molecular responses within each tissue integrate to drive intergenerational acclimation to OA at the organismal level.
Schematic graph representing the gene expression profiles. Expression profile of (i) CO2-response genes: genes with significantly higher or lower expression in developmental CO2 treatment (D) and intergenerational CO2 treatment (I) compared to control (C), (ii) genes showing a rescue pattern: genes with significantly higher or lower expression in developmental CO2 treatment (D) compared to control (C), but whose expression is not significantly different in intergenerational CO2 treatment (I) compared to control (C), and (iii) intergeneration-specific genes: genes with significantly higher or lower expression in intergenerational CO2 treatment (I) compared to control (C), but whose expression is not significantly different in developmental CO2 treatment (D) compared to control (C).
Results
Molecular processes affected by all elevated CO2 treatments
To understand the general effects of elevated CO2 exposure regardless of the time of exposure to elevated CO2, we identified genes that were commonly differentially expressed (DE) in both the developmental and intergenerational treatments compared to control (Fig. 1(i)), which are considered the general “CO2 response genes”. Irrespective of the parental environment, the liver showed the greatest transcriptional response to elevated CO2 exposure in offspring of both sensitive and tolerant parental behavioural phenotypes, with 63 and 986 differentially expressed (DE) genes, respectively (Fig. 2 and Supplementary Table S3 and S4). In the offspring of tolerant parental phenotype, the gills followed with 883 DE genes, while the brain had 44 DE genes (Fig. 2 and Supplementary Table S3). Conversely, in the offspring of sensitive parental phenotype, the brain displayed a higher number of DE genes (37) compared to the gills (9; Supplementary Table S3). Overall, offspring of parents with tolerant behavioural phenotype had a higher number of DE genes, indicating increased gene expression regulation across all three tissues in response to elevated CO2 exposure compared to offspring with behaviourally sensitive parents. However, the CO2 response genes showed high tissue specificity with no genes being commonly DE across the three tissues in the sensitive parental phenotype and only 3 and 81 genes being shared between the brain and gills, and liver and gills respectively in the tolerant parental phenotype (Supplementary Fig. S3a).
Functional enrichment analysis of the CO2 response genes in offspring with sensitive parental phenotype revealed translation and amino-acid synthesis to be important in the brain, while no significantly enriched functions were found in the gills and liver (Fig. 3 and Supplementary Table S5). For the offspring of tolerant parental phenotype, biosynthetic processes were commonly enriched in the brain and gills (Fig. 3). Few other functional pathways were commonly enriched between the gills and liver, including metabolism, translation, protein transport, immune and stress response (Fig. 3 and Supplementary Table S5). Interestingly, while genes associated with metabolic pathways such as glycolysis, TCA cycle, and mitochondrial electron transport chain were commonly differentially regulated in both the gills and liver, pentose phosphate pathway genes showed upregulation exclusively in the gills. Additionally, immune response and translation showed tissue-specific regulation with downregulation in the liver but upregulation in the gills (Supplementary Table S5). Furthermore, RNA processing and primary active transmembrane transport were enriched only in the liver (Supplementary Table S5). This suggests intricate and tissue-specific regulation of various functions in response to elevated CO2 exposure.
Parental exposure to elevated CO2 “rescues” developmental effects
Genes whose expression is altered upon developmental exposure to elevated CO2 (i.e. are DE in developmental treatment compared to control and intergeneration) but returned to control levels when parents were previously exposed to elevated CO2 (i.e. are not DE when comparing intergeneration treatment with control) are considered to show a “rescue” pattern suggesting cross-generation plasticity (Fig. 1a(ii)). In offspring with sensitive parental phenotype, a total of 883, 55, and 108 genes in the brain, gills and liver, respectively, showed a “rescue” pattern. Similarly, in offspring with tolerant parental phenotype 458, 284, and 434 genes in the brain, gills, and liver, respectively, showed a “rescue” pattern (Supplementary Tables S3 and S6). Parental conditioning had the largest effect on brain gene expression followed by the liver (Fig. 2). Similar to the CO2-response genes, there were very few genes commonly DE across tissues (Supplementary Fig. S3b).
Genes exhibiting a rescue pattern in the brains of offspring of behaviourally sensitive parents showed the highest degree of specificity in functional regulation with pathways involved in cytoskeleton organisation, apoptosis, synaptic signalling, sodium and calcium channel activity, transcription regulation, cellular transport, and small GTPase signalling being exclusively enriched only in this tissue (Fig. 4 and Supplementary Table S7). Gills were found to play a key role in regulating ion transport in offspring of both behaviourally sensitive and tolerant parents. Specifically, the expression of genes encoding sodium and potassium channel transporters was altered in the gills upon developmental exposure to elevated CO2 levels but returned to control levels in intergenerationally exposed fish (Fig. 4 and Supplementary Tables S6 and S7). Additionally, purine ribonucleoside salvage pathway and DNA replication were identified as key functions among the “rescue” genes in the offspring of parents with sensitive phenotype in the gills and liver respectively (Fig. 4 and Supplementary Table S7). This suggests that the molecular responses of the offspring are influenced by their parental environment, with specific functional pathways being selectively regulated in each tissue due to parental conditioning to elevated CO2 levels.
Intergenerational specific response to elevated CO2
We found a large transcriptional response to elevated CO2 that was only seen in the intergenerationally exposed fish and not in fish with only developmental (within-generation) exposure to elevated CO2. This indicates plasticity of the offspring transcriptome due to parental conditioning to elevated CO2 and was especially marked in offspring of tolerant parents (Fig. 2). These are genes that were only differentially expressed in the intergenerational treatment (compared to control and development) but were at control levels in the developmental treatment (Fig. 1(iii)). Specifically, 383, 207, and 1226 genes were DE in the brain, gills, and liver respectively in offspring with tolerant parents, while offspring with sensitive parents had 36, 7, and 8 DE genes in the brain, gills, and liver respectively that were specific to the intergenerational treatment (Supplementary Tables S3 and S8). Offspring of both tolerant and sensitive parents showed high tissue specificity in the intergenerational specific transcriptional signature to elevated CO2 with only one and five genes being commonly DE across all three tissues in the sensitive and tolerant parental phenotypes respectively (Supplementary Fig. S3c).
The intergeneration specific response was substantially higher in offspring of behaviourally tolerant parents with various functions showing tissue-specific regulation. Stress response and metabolic pathways were commonly differentially regulated in the brain and liver (Fig. 5), but the liver showed a much larger number of DE genes (~400) associated with metabolism compared to the brain (11 DE genes; Supplementary Table S9). Additionally, signal transduction pathways were enriched only in the brain while several functions such as biosynthetic pathways, endopeptidase activity, lipid binding/ transport, transcriptional regulation, proteolysis, and spindle organisation were exclusive to the liver (Fig. 5, Supplementary Table S9). Interestingly, molecular signatures indicating pH regulation by bicarbonate retention were identified only in the intergenerationally treated fish with tolerant parental phenotype, indicated by the downregulation of SLC4A1, CFTR, SLC12A2, and SLC9A3 in the gills and upregulation of SLC4A4 in the brain (Supplementary Table S8). Therefore, offspring of parents with tolerant behavioural phenotype showed increased molecular signatures of intergenerational plasticity and this was especially evident in the liver. This change in transcriptional signature could regulate the above mentioned “rescue” pattern and equip offspring to better cope with OA conditions.
Parental variability in CO2 sensitivity impacts the offspring transcriptome
Parental behavioural phenotype was found to have a substantial influence on the offspring’s transcriptional response. Across all three tissues, offspring with behaviourally tolerant parents when faced with elevated CO2 had larger changes in gene expression levels (log2FC > 5; Fig. 6) and a greater number of DE genes involved in the overall CO2 response (common in developmental and intergenerational CO2 exposure compared to control; Fig. 2). There were also very few genes involved in the overall CO2 response shared between the two parental phenotypes, specifically only six, three, and eleven common DE genes in the brain, gills, and liver respectively (Supplementary Fig. S3a). When considering genes involved in intergenerational plastic responses, there were more differentially expressed genes in the offspring of tolerant parents compared to those of sensitive parents, except for genes showing a rescue pattern in the brain (Fig. 2). Additionally, none of the DE genes involved in intergenerational plasticity were shared in the liver tissue between the two parental phenotypes while the brain and gills had a small proportion of common DE genes (specifically, 121 and 11 common DE genes showing a rescue pattern in the brain and gills respectively and only two and one common DE genes in the intergeneration specific response in the brain and gills respectively (Supplementary Fig. S3b, c).
Log2 fold change in expression of all differentially expressed genes. Log2 fold change profiles for genes involved in (a) overall CO2 response, (b) rescue pattern, and (c) intergenerational specific response across all three tissues. SP indicates samples with a sensitive parental phenotype and TP indicates samples with a tolerant parental phenotype.
The difference in transcriptional response between offspring of the two parental phenotypes was especially pronounced when considering genes showing an intergeneration-specific signature. Offspring of tolerant parental phenotype had more DE genes with a much higher magnitude of gene expression changes (log2FC > 5; Fig. 6), compared to those with behaviourally sensitive parents. While there were some common transcriptional responses between offspring with sensitive and tolerant parents, offspring of tolerant parents showed a much stronger transcriptional response to elevated CO2 in general and also had a stronger signature of intergenerational plasticity. As these differences between the parental behavioural phenotype already suggest, we also found an interaction between parental phenotype and offspring gene expression response to the different CO2 conditions. Specifically, 158 genes in the brain, 157 in the gills, and 1635 in the liver exhibited a significant interaction effect (Supplementary Table S10).
Discussion
Changes in the offspring transcriptional landscape in response to elevated CO2 exposure exhibited tissue-specific signatures which were largely influenced by previous parental exposure to elevated environmental CO2, but also by the parental behavioural phenotype. In all elevated CO2 treatments, we found that gills are critical in activating stress and immune response pathways and the brain and liver had the greatest signal of intergenerational plastic response. In fact, intergenerationally treated fish no longer showed molecular signatures of altered neural signalling in the brain that were seen in the developmental (within-generation) treatment. Additionally, differential regulation of a new complement of metabolic genes exclusively in the brain and liver of offspring from parents with prior exposure to elevated CO2 suggests that parental conditioning may influence the offspring’s capacity to regulate metabolic processes in response to elevated CO2 exposure. A. polyacanthus is known to have a highly plastic genome enabling it to respond and acclimate to environmental changes37,38 and our results show that this persists across generations. One limitation of our study is the relatively small number of family lines, which may confound treatment effects with inherent family line differences. However, our findings suggest that this species has the potential to rapidly acclimate to the changing ocean environments.
Genes that are always differentially expressed in elevated CO2 conditions, regardless of the timing of exposure (within-generation or intergeneration), are key genes in the general response to ocean acidification (OA). The gills and liver exhibited a higher transcriptional response in all elevated CO2 treatments suggesting that these tissues play an important role in the overall response of the fish to elevated CO2. In both the developmental and intergenerational CO2 treatments, these tissues exhibited differential regulation of key functional pathways such as cellular metabolism, stress response, and immune function. However, while stress response genes were upregulated in both the gills and the liver, genes involved in immune response were upregulated in the gills but downregulated in the liver. This suggests tissue-specific regulation of key pathways, with the gills and liver working in synergy to mitigate oxidative stress and maintain cellular redox balance. Similar differences in tissue-specific regulation of specific metabolic pathways were observed between the gills and liver in elevated CO2 treatments. While TCA cycle genes were elevated in both tissues, the pentose phosphate pathway was upregulated only in the gills. Furthermore, the cytochrome complex and glycolytic pathway were downregulated in the hepatic transcriptome. This suggests a shift in metabolic priorities in response to elevated CO2 exposure potentially redirecting carbon fluxes to support cellular demands for various metabolites and cofactors needed for other biological processes such as biosynthetic pathways and the cellular stress response machinery39,40,41. Overall, our results indicate a common functional response in the gills and liver that are always activated in elevated CO2 conditions irrespective of previous parental conditions.
Parental exposure to altered environmental conditions can pre-acclimate the offspring transcriptome to these new conditions via intergenerational plasticity. In juvenile A. polyacanthus, parental conditioning to elevated CO2 was found to “rescue” the effects of developmental exposure to the same conditions. This “rescue pattern” was predominantly evident in the brains of offspring with sensitive parental phenotype. Specifically, the expression of genes involved in synaptic plasticity and calcium channel activity, initially altered in the developmental treatment, were similar to control levels in the intergenerationally treated fish. Exposure to OA conditions has been shown to affect neural plasticity and neurogenesis in some fish species resulting in changes in neural circuitry and signalling42,43, which could result in behavioural alterations22. However, the “rescue pattern” of synaptic plasticity genes along with cell adhesion and cytoskeleton-related genes, which mediate synaptic plasticity44,45, in intergenerationally treated fish hints at the restoration of altered neural signalling in the offspring facilitated by previous parental exposure to elevated CO2. This suggests an enhanced capacity for intergenerational plasticity in A. polyacanthus. In fact, a comparison of six species living in wild CO2 seeps with long-term elevated CO2 exposures revealed A. polyacanthus to have substantially larger brain transcriptional plasticity compared to the other species37. Therefore, intergenerational CO2 exposure potentially restores the dynamic equilibrium of cytoskeletal proteins, thereby re-establishing synaptic signalling processes to control levels.
This “rescue pattern” in the brain was also associated with transcriptional regulation such as transcription factors and RNA-mediated gene silencers. Changes in the external environment can trigger reprogramming of transcriptional networks resulting in dynamic regulation of gene expression46 as also suggested in wild fish populations naturally exposed to elevated CO247. Therefore, while these regulatory genes play a key role in developmental plastic responses to elevated environmental CO2 levels, these are no longer needed in intergenerationally treated fish revealing acclimation. Similarly, in the gills, this ‘rescue’ pattern was seen for genes encoding ion transporters including potassium channels which are known for their significance in pH regulation and association with CO2 chemoreception48. While the differential expression in the developmental treatment is likely triggered by hypercapnia prompting downstream compensatory responses to maintain pH homeostasis48,49, the restoration of expression levels to control levels in intergenerationally treated fish suggests that intergenerational exposure may enhance and facilitate improved pH regulatory mechanisms. As pH defence is a key mechanism in the gills50, this “rescue pattern” may reveal an acclimation process in the offspring facilitated by parental conditioning to elevated CO2.
To facilitate this intergenerational acclimation, other adjustments specific to the intergenerational treatment may be needed. Changes in lipid metabolism have been shown to facilitate acclimation to elevated CO2 as well as temperature3,35,51 and here we see metabolic adjustments such as upregulation of fatty-acid metabolism in the brain and liver and increased lipid synthesis in the liver only with previous parental exposure. Metabolic changes have also been seen in juvenile A. melanopus exposed to elevated CO2 when their parents were also exposed to the same CO2 conditions6. Therefore, an increase in lipid metabolism in both the brain and liver in intergenerationally treated fish combined with the observed redirection of metabolic carbon fluxes when considering the overall CO2 response, might indicate a similar switch from carbohydrate to lipid metabolism as the primary metabolic pathway for ATP production in elevated CO2 conditions. This could present a key intergenerational acclimation process, where parental exposure equips their offspring to better manage the increased metabolic demands associated with changing environments.
Our data suggests a certain degree of predictability of offspring plasticity from the parental behavioural phenotype, which suggests that plasticity could partially be a genetic trait. Offspring of tolerant parental phenotype showed a stronger transcriptional response to elevated CO2 exposure and increased intergenerational plastic responses, predominantly in the brain and liver, compared to offspring of behaviourally sensitive parents. Additionally, an interaction between parental behavioural phenotype and offspring transcriptional responses to the different CO2 treatments was observed, with the liver having the highest number of genes with an interaction effect. Parental behavioural phenotype has been shown to influence the offspring’s brain transcriptional response to elevated CO2 in A. polyacanthus10, with behavioural tolerance being heritable17. Selection experiments have indicated a genetic basis for individual variation in OA induced responses in a variety of animals52,53,54,55, including the behavioural phenotype to chemical alarm cues in elevated CO2 in A. polyacanthus16. Therefore, there exists a complex interplay between parental phenotype and environmental CO2 conditions in shaping the offspring’s molecular responses. This intraspecific variation in organismal response is key in driving future adaptive evolution. Our results suggest that offspring of parents with a tolerant behavioural phenotype have an increased capacity for intergenerational plasticity and potential acclimation to future ocean acidification conditions, with genetic variation possibly playing a role.
This study used a systematic approach considering parental behaviour, environment, and multiple tissues to assess molecular responses of a coral reef fish to future ocean conditions. The gill and liver transcriptomes exhibited substantial changes, with a certain level of tissue specificity in the regulation of key cellular processes, such as stress and immune responses, in both developmental and intergenerational elevated CO2 conditions. This highlights their fundamental roles in acclimatory responses to OA, regardless of previous parental conditions. Additionally, parental conditioning to elevated CO2 facilitated intergenerational acclimation responses in the offspring, as evidenced by the “rescue” of neural activity in the brain and pH regulation in the gills. This implies that intergenerational CO2 exposure facilitates efficient pH regulation upon within-generation elevated CO2 exposure. Previous parental exposure to OA conditions also equipped their offspring to better manage metabolic needs associated with a new environment with elevated CO2. While plastic acclimatory processes with previous parental exposure may facilitate coping mechanisms with OA, these vary depending on the parental phenotype also suggesting a genetic component to the future survival of the species. Overall, our study reveals how intergenerational plasticity is facilitated from a whole-organism perspective and illustrates how transcriptional changes across multiple tissues integrate to drive the plastic responses of fish to the changing ocean chemistry.
Methods
Sample collection, behavioural testing, and experimental design
All sample collection and the experimental procedures were carried out in accordance with institutional and national law guidelines. We have complied with all relevant ethical regulations for animal use. Ethics approval for the study was granted by James Cook University with the approval number A1828.
Adult Acanthochromis polyacanthus were collected from the wild on the Great Barrier Reef, Australia (18°38'24.3“S, 146°29'31.8“E) and transported to the James Cook University’s Experimental Marine Aquarium Facility as described previously14,17. Briefly, upon arrival, the adult fish were left to acclimate to the tank environment for five days after which they were exposed to elevated CO2 (754 ± 92 µatm) for seven days. To obtain the desired CO2 conditions, three header tanks (60 L) fed water into 32 L aquaria where fish were held across three separate systems. 100% CO2 was diffused in the header tanks (for control ambient air was diffused). pH controllers (Aqua Medic, Germany) maintained the desired pH in the header tanks that supplied the tanks in each system with pHNBS (National Bureau of Standards. Temperature measurements were taken daily in each tank using a pH electrode (SevenGo Pro, Mettler Toledo, Switzerland) and a temperature probe (Cormark C26, Norfolk, UK). Further details are described in Welch & Munday17. Following the CO2 exposure we tested their behavioural sensitivity to conspecific chemical alarm cues (CAC) using a two-chamber flume as described previously14,17. The behavioural trials lasted nine minutes in total (2 min acclimatisation period, 2 min testing, 1 min water switch, 2 min acclimatisation, 2 min testing). The fish were classified as being behaviourally sensitive or tolerant to elevated CO2 based on the amount of time spent in water containing the CAC10,13,14,17. Sensitive individuals spent ≥ 70% (89.69 ± 15.69%) of time in CAC whereas tolerant individuals spent ≤ 30% of time (7.35 ± 11.1%) in CAC, with approximately 38% of collected fish falling into each category. Individuals of similar size displaying the same behavioural phenotype (sensitive or tolerant) were then grouped into breeding pairs. Three sensitive and three tolerant pairs were held in control conditions (414 ± 46 µatm; similar to the pCO2 levels in the wild56) and two sensitive and two tolerant pairs were held in elevated CO2 conditions (754 ± 92 µatm) for three months prior to the breeding season. Each parental pair formed a distinct family line, and five different family lines for tolerant as well as sensitive pairs were used to ensure that the effects seen were not due to a single parental pair (Supplementary Table S1). To further minimise potential genetic bias, one sensitive and one tolerant parental pair, initially exposed to control conditions for three months and bred at control levels, were subsequently exposed to elevated CO2 for three months after which they bred. Therefore, control and developmentally treated fish are siblings from three different parental pairs for both sensitive and tolerant behavioural phenotypes. Additionally, one parental pair has offspring in control, developmental and intergenerational CO2 treatment. In this experiment, similar to previous studies, we did not observe any significant effect of elevated CO2 on reproductive output or juvenile mortality10. Offspring clutches from each parental pair were placed into three different experimental treatments resulting in three combinations of parent-offspring conditions for each parental phenotype: (1) Control treatment – Parents and offspring held at control condition (414 ± 46 µatm; N = 9 each for sensitive and tolerant (3 from each parental pair)); (2) Developmental treatment – Parents held at control condition and offspring exposed to elevated CO2 (754 ± 92 µatm; N = 9 each for sensitive and tolerant (3 from each parental pair)) immediately after hatching; and (3) Intergenerational treatment – Parents and offspring exposed to elevated CO2 (754 ± 92 µatm; N = 9 each for sensitive and tolerant (three from each parental pair)). The offspring were held in their respective conditions until they were five months old after which nine fish from each parental behavioural phenotype (sensitive or tolerant), from each treatment condition (N = 27 from each parental phenotype; N = 54 total fish sampled) were euthanized and the brain, gills and liver were dissected, snap frozen in liquid nitrogen and stored at −80 °C until further processing (Supplementary Fig. S1). On average all the sampled fish weighed 2.04 ± 0.43 g. A one-way ANOVA revealed no significant difference in the weight of the offspring across the three CO2 conditions.
RNA extraction, sequencing, and gene expression analyses
Total RNA was extracted from the fish brains, livers, and gills using the AllPrep DNA/RNA Mini kit from Qiagen following the manufacturer’s instructions, with a slight modification for the liver tissues where a lower ethanol concentration (50%) was used in the washing step. RNA quality was determined using nanodrop and Agilent Bioanalyzer and samples having an RNA integrity value (RIN) ≥ 8 were sequenced using Illumina HiSeq 2500 to get paired-end reads of 100 bp at Macrogen Inc., South Korea. A total of 1614.25 ± 3.05, 2367.03 ± 5.19, and 2227.23 ± 6.37 million raw paired-end reads were obtained from the 162 sequenced libraries from brain, gills, and liver, respectively which included nine control, nine developmental, and nine intergenerational samples for each parental phenotype for each tissue (Supplementary Table S1). The quality of the raw reads was examined using FastQC57 v0.11.8 and adaptors and low quality sequences were trimmed using Trimmomatic58 v0.39 (ILLUMINACLIP: adaptors.fa:2:30:15:8:true; SLIDINGWINDOW:4:20; MINLEN:32). Only those sequences ≥ 32 bp in length with both the forward and reverse reads retained after trimming were used for further analysis. Potential contaminant sequences were identified using kraken59 v2.0.8-beta, with a confidence score of 0.3, using the bacteria, fungi and virus RefSeq genomic libraries as reference and removed from further analyses. A total of 1510.51 ± 2.62, 2254.16 ± 4.95, and 2116.25 ± 6.04 million high-quality sequences were retained after the filtering process (Supplementary Table S1). These sequences were mapped to the Acanthochromis polyacanthus reference genome (unpublished) using HISAT260 v2.1.0. On average, 84 ± 1.83%, 91.22 ± 0.66%, and 93.33 ± 0.81% reads mapped to the reference genome from the brain, gills, and liver respectively (Supplementary Table S1). Raw read counts per gene were obtained using featureCounts61 v2.0.0 (parameters: -B -J -M –fraction), assigning fractional counts to multi-mapped reads. Exploring the gene expression patterns across the whole dataset (162 samples) using principal component analysis (PCA) revealed a clear clustering of samples by tissues indicating that tissues vary greatly in their gene expression patterns (Supplementary Fig. S2). Subsequent analysis of differences in gene expression levels was therefore carried out separately for each tissue using the DESeq262 v1.32.0 package in R63 v4.2.1.
Principal component analysis (PCA) using the regularised log transformed (rlog) counts was done in R v4.2.1 to detect and remove outlier samples. For samples with sensitive parental phenotype, two developmental CO2 treatment samples were outliers - one in the gills (SF1-1-D) and one in the liver (SF2-1-D) and three different samples from the intergenerational CO2 treatment were outliers in the brain, gills and liver (SF1-1-T in brain, SF3-2-T in gills, SF2-3-T in liver). Additionally, for samples with tolerant parental phenotype, three outlier samples were identified in the liver – one from control (TF3-2-C), one from the developmental CO2 treatment (TF2-3-D), and one from the intergenerational CO2 treatment (TF1-1-T). The outlier samples were distributed across all five family lines, with no single family line being predominant. A likelihood ratio test (LRT) using a model comparison approach was then used to determine the effect of the family line in driving the gene expression patterns and to determine the best design formula for the final DE analysis. First, significant differences in gene expression were measured by comparing a model including treatment and family line against a reduced model without the family line factor separately for each tissue. For a total of 924, 910, and 923 genes in the brain, gills, and liver respectively, the model including family line better explained the observed differences in gene expression compared to the reduced model excluding this factor (FDR corrected p-value < 0.05; Supplementary Table S2). Pair-wise comparisons between the control, developmental and intergenerational treatment were then carried out in DESeq2 (accounting for the family effect, using Wald test (design = ~family + condition) separately for each parental phenotype for each tissue to determine the effect of parental environment and parental tolerance to CO2 on the molecular responses of the offspring to elevated CO2. Despite accounting for family effects, the limited number of families in our study may still confound treatment effects with family line differences. For each pair-wise comparison, the genes were considered to be significantly differentially expressed (DE) if the False Discovery Rate (FDR) adjusted p-value was <0.05, the absolute log 2-fold change in expression was >0.3 and baseMean was >10, as done in previous studies10,13,14. Functional enrichment analysis of the significant DE genes was carried out in OmicsBox64 (https://www.biobam.com/omicsbox) v1.4.11 using Fisher’s Exact Test (FDR corrected p-value < 0.05) with the option of reducing to most specific GO terms to reduce redundancy. The genes associated with the enriched GO terms were further categorised into broader functional groups based on their functional description from the UniProt knowledgebase (UniProtKB; https://www.uniprot.org/). All figures are made using ggplot in R v4.2.1. We also conducted a Likelihood Ratio Test to assess the interaction between parental phenotype and CO2 exposure conditions of the offspring. Genes with a FDR adjusted p-value <0.05 and baseMean >10 were considered to exhibit a significant interaction effect.
Statistics and reproducibility
The details about experimental design, bioinformatic software tools, and statistics performed in this study are described in detail in the methods section. Sample sizes for each experimental treatment, along with the final counts remaining after the removal of outliers are described in detail in the methods section. Replicates are defined as individual fish.
All statistical analyses were performed in R v4.2.1. Firstly, a one-way ANOVA was used to confirm that there were no significant differences in weight of the fish across all experimental treatments. Outlier samples were identified through principal component analysis (PCA) of regularised log transformed (rlog) counts in R. Differential gene expression analysis was performed using DESeq2 v1.32.0 in R, beginning with a likelihood ratio test (LRT) to assess whether family line influenced the gene expression patterns. Subsequently, pairwise comparisons between control, developmental and intergenerational treatments were conducted for each parental phenotype and tissue separately, using the Wald test in DESeq2 with the design formula ~ family + condition. Lastly, functional enrichment analysis of all significantly differentially expressed genes was conducted in OmicsBox using Fisher’s Extact Test.
Data availability
All supplementary tables are in the Supplementary Data 1 file. The brain RNA-Seq raw sequences are deposited in NCBI under BioProject ID PRJNA311159. The gills and liver RNA-Seq raw sequences are deposited in NCBI under BioProject ID PRJNA989422. The data can be accessed via SRA Entrez (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA989422) or SRA Run selector (https://trace.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA989422).
References
Sundin, J. The effects of ocean acidification on fishes–history and future outlook. J. Fish. Biol. 103, 765–772 (2023).
Heuer, R. M. & Grosell, M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 307, R1061–R1084 (2014).
Strader, M. E., Wong, J. M. & Hofmann, G. E. Ocean acidification promotes broad transcriptomic responses in marine metazoans: A literature survey. Front. Zool. 17, 1–23 (2020).
Nagelkerken, I. et al. The effects of climate change on the ecology of fishes. PLoS Clim. 2, e0000258 (2023).
Goncalves, P. et al. Rapid transcriptional acclimation following transgenerational exposure of oysters to ocean acidification. Mol. Ecol. 25, 4836–4849 (2016).
Miller, G. M., Watson, S. A., Donelson, J. M., McCormick, M. I. & Munday, P. L. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Change 2, 858–861 (2012).
Murray, C. S., Malvezzi, A., Gobler, C. J. & Baumann, H. Offspring sensitivity to ocean acidification changes seasonally in a coastal marine fish. Mar. Ecol. Prog. Ser. 504, 1–11 (2014).
Thor, P. & Dupont, S. Transgenerational effects alleviate severe fecundity loss during ocean acidification in a ubiquitous planktonic copepod. Glob. Change Biol. 21, 2261–2271 (2015).
Allan, B. J. M., Miller, G. M., Mccormick, M. I., Domenici, P., & Munday, P. L. Parental effects improve escape performance of juvenile reef fish in a high-CO2 world. Proc. R. Soc. B: Biol. Sci. https://doi.org/10.1098/RSPB.2013.2179 (2014).
Monroe, A. A., Schunter, C., Welch, M. J., Munday, P. L., & Ravasi, T. Molecular basis of parental contributions to the behavioural tolerance of elevated pCO2 in a coral reef fish. Proc. R. Soc. B: Biol. Sci. https://doi.org/10.1098/rspb.2021.1931 (2021).
Munday, P. L. Transgenerational acclimation of fishes to climate change and ocean acidification. F1000Prime Rep. https://doi.org/10.12703/P6-99 (2014).
Schade, F. M., Clemmesen, C., Wegner, M. & Wegener, A. Within-and transgenerational effects of ocean acidification on life history of marine three-spined stickleback (Gasterosteus aculeatus). Mar. Biol. 161, 1667–1676 (2014).
Schunter, C. et al. An interplay between plasticity and parental phenotype determines impacts of ocean acidification on a reef fish. Nat. Ecol. Evol. 2, 334–342 (2018).
Schunter, C. et al. Molecular signatures of transgenerational response to ocean acidification in a species of reef fish. Nat. Clim. Change 6, 1014–1018 (2016).
Stiasny, M. H. et al. Effects of parental acclimation and energy limitation in response to high CO2 exposure in Atlantic cod. Sci. Rep. 8, 1–8 (2018).
Lehmann, R., et al. Genetic architecture of behavioural resilience to ocean acidification. bioRxiv https://doi.org/10.1101/2022.10.18.512656 (2022).
Welch, M. J. & Munday, P. L. Heritability of behavioural tolerance to high CO2 in a coral reef fish is masked by nonadaptive phenotypic plasticity. Evol. Appl. 10, 682–693 (2017).
Munday, P. L. et al. Selective mortality associated with variation in CO2 tolerance in a marine fish. Ocean Acidif. 1, 1–5 (2013).
Ryu, T., Veilleux, H. D., Donelson, J. M., Munday, P. L. & Ravasi, T. The epigenetic landscape of transgenerational acclimation to ocean warming. Nat. Clim. Change 8, 504–509 (2018).
Turner, B. M. Epigenetic responses to environmental change and their evolutionary implications. Philos. Trans. R. Soc. B: Biol. Sci. 364, 3403–3418 (2009).
Huang, R. et al. A potential role for epigenetic processes in the acclimation response to elevated pCO2 in the model diatom Phaeodactylum tricornutum. Front. Microbiol. 9, 3342 (2019).
Schunter, C., Ravasi, T., Munday, P. L., & Nilsson, G. E. Neural effects of elevated CO2 in fish may be amplified by a vicious cycle. Conserv. Physiol. https://doi.org/10.1093/CONPHYS/COZ100 (2019).
Lee, D. W., Song, J. A., Park, H. S. & Choi, C. Y. Circadian rhythm disturbances due to exposure to acidified conditions and different photoperiods in juvenile Olive Flounder (Paralichthys olivaceus). Ocean Sci. J. 56, 198–206 (2021).
Williams, C. R. et al. Elevated CO2 impairs olfactory-mediated neural and behavioral responses and gene expression in ocean-phase coho salmon (Oncorhynchus kisutch). Glob. Change Biol. 25, 963–977 (2019).
De Souza, K. B., Jutfelt, F., Kling, P., Förlin, L. & Sturve, J. Effects of increased CO2 on fish gill and plasma proteome. PLoS ONE 9, e102901 (2014).
Deigweiher, K., Hirse, T., Bock, C., Lucassen, M. & Pörtner, H. O. Hypercapnia induced shifts in gill energy budgets of Antarctic notothenioids. J. Comp. Physiol. B 180, 347–359 (2010).
Deigweiher, K., Koschnick, N., Pörtner, H. O. & Lucassen, M. Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 295, R1660–R1670 (2008).
Crespel, A. et al. Long-term effects of ocean acidification upon energetics and oxygen transport in the European sea bass (Dicentrarchus labrax, Linnaeus). Mar. Biol. 166, 116 (2019).
Gräns, A. et al. Aerobic scope fails to explain the detrimental effects on growth resulting from warming and elevated CO2 in Atlantic halibut. J. Exp. Biol. 217, 711–717 (2014).
Pimentel, M., Pegado, M., Repolho, T. & Rosa, R. Impact of ocean acidification in the metabolism and swimming behavior of the dolphinfish (Coryphaena hippurus) early larvae. Mar. Biol. 161, 725–729 (2014).
Rummer, J. L. et al. Elevated CO2 enhances aerobic scope of a coral reef fish. Conserv. Physiol. 1, cot023 (2013).
Frommel, A. Y. et al. Differential gene expression patterns related to lipid metabolism in response to ocean acidification in larvae and juveniles of Atlantic cod. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 247, 110740 (2020).
Grosell M., Munday P. L., Farrell A. P., Brauner C. J. Fish Physiology Volume 37: Carbon Dioxide (Elsevier, 2019).
Donelson, J. M., Munday, P. L., McCormick, M. I. & Pitcher, C. R. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30–32 (2012).
Veilleux, H. D. et al. Molecular processes of transgenerational acclimation to a warming ocean. Nat. Clim. Change 5, 1074–1078 (2015).
Robertson, D. R. Field observations on the reproductive behaviour of a pomacentrid fish, Acanthochromis polyacanthus. Z. Für Tierpsychol. 32, 319–324 (1973).
Kang, J. et al. Rapid evolution fuels transcriptional plasticity to ocean acidification. Glob. Change Biol. 28, 3007–3022 (2022).
Bernal, M. A., et al. Species-specific molecular responses of wild coral reef fishes during a marine heatwave. Sci. Adv. https://doi.org/10.1126/sciadv.aay3423 (2020).
Gansemer, E. R. et al. NADPH and glutathione redox link TCA cycle activity to endoplasmic reticulum homeostasis. IScience 23, 101116 (2020).
Rokitta, S. D., John, U. & Rost, B. Ocean acidification affects redox-balance and ion-homeostasis in the life-cycle stages of Emiliania huxleyi. PLoS ONE 7, e52212 (2012).
Walsh, P. J. & Milligan, C. L. Roles of buffering capacity and pentose phosphate pathway activity in the gas gland of the gulf toadfish Opsanus beta. J. Exp. Biol. 176, 311–316 (1993).
Costa, R. A., Olvera, A., Power, D. M., & Velez, Z. Ocean acidification affects the expression of neuroplasticity and neuromodulation markers in seabream. Biol. Open https://doi.org/10.1242/bio.059073 (2022).
Lai, F. et al. Responses of neurogenesis and neuroplasticity related genes to elevated CO2 levels in the brain of three teleost species. Biol. Lett. https://doi.org/10.1098/rsbl.2017.0240 (2017).
Gordon-Weeks, P. R. & Fournier, A. E. Neuronal cytoskeleton in synaptic plasticity and regeneration. J. Neurochem. 129, 206–212 (2014).
Zapara, T. A., Simonova, O. G., Zharkikh, A. A. & Ratushnyak, A. S. The effects of the dynamic state of the cytoskeleton on neuronal plasticity. Transl. Ross. Fiziol. Zh. Im. I. M. Sechenova, I/Ol. 30, 128–138 (2000).
Swift, J. & Coruzzi, G. M. A matter of time — How transient transcription factor interactions create dynamic gene regulatory networks. Biochim. Biophys. Acta- Gene Regul. Mech. 1860, 75–83 (2017).
Petit-Marty, N., Nagelkerken, I., Connell, S. D. & Schunter, C. Natural CO2 seeps reveal adaptive potential to ocean acidification in fish. Evol. Appl. 14, 1794–1806 (2021).
Qin, Z., Lewis, J. E. & Perry, S. F. Zebrafish (Danio rerio) gill neuroepithelial cells are sensitive chemoreceptors for environmental CO2. J. Physiol. 588, 861–872 (2010).
Suresh, S., Mirasole, A., Ravasi, T., Vizzini, S. & Schunter, C. Brain transcriptome of gobies inhabiting natural CO2 seeps reveal acclimation strategies to long-term acidification. Evol. Appl. 16, 1345–1358 (2023).
Claiborne, J. B., Edwards, S. L. & Morrison‐Shetlar, A. I. Acid–base regulation in fishes: cellular and molecular mechanisms. J. Exp. Zool. 293, 302–319 (2002).
Liu, S. et al. RNA-Seq reveals expression signatures of genes involved in oxygen transport, protein synthesis, folding, and degradation in response to heat stress in catfish. Physiol. Genom. 45, 462–476 (2013).
Langer, G., Nehrke, G., Probert, I., Ly, J. & Ziveri, P. Strain-specific responses of Emiliania huxleyi to changing seawater carbonate chemistry. Biogeosciences 6, 2637–2646 (2009).
Parker, L. M., Ross, P. M. & O’Connor, W. A. Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Mar. Biol. 158, 689–697 (2011).
Pistevos, J. C., Calosi, P., Widdicombe, S. & Bishop, J. D. Will variation among genetic individuals influence species responses to global climate change? Oikos 120, 675–689 (2011).
Sunday, J. M., Crim, R. N., Harley, C. D. & Hart, M. W. Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS ONE 6, e22881 (2011).
Albright, R., Langdon, C. & Anthony, K. R. N. Dynamics of seawater carbonate chemistry, production, and calcification of a coral reef flat, central Great Barrier Reef. Biogeosciences 10, 6747–6758 (2013).
Andrews, S. FastQC: A Quality Control Tool For High Throughput Sequence Data (2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Wood, D. E., & Salzberg, S. L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biol. https://doi.org/10.1186/gb-2014-15-3-r46 (2014).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 1–21 (2014).
R Core Team. R: A Language And Environment For Statistical Computing (R Foundation for Statistical Computing, 2021).
OmicsBox – Bioinformatics Made Easy, BioBam Bioinformatics. https://www.biobam.com/omicsbox (2019).
Acknowledgements
CS and SS were supported through the HKU start-up to C.S. The study was partially funded by a General Research Fund (17300721) and the NSFC Excellent Young Scientist Award (AR225205) to C.S. P.L.M. was supported by the ARC Centre of Excellence for Coral Reef Studies and T.R. was supported by the Okinawa Institute of Science and Technology (OIST). We would like to thank all members of Celia Schunter’s Lab for their invaluable support and feedback on this work.
Author information
Authors and Affiliations
Contributions
The experiment was designed and run by M.J.W. and P.L.M. Molecular lab work was performed by C.S. and sequenced by T.R.; S.S. carried out the transcriptome expression analysis with input from C.S.; S.S. led the writing of the manuscript with input from C.S. and all authors read, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors declare they have no competing interests.
Ethical approval
Sample collection was carried out following all institutional and national law guidelines. The experiment was completed under James Cook University ethics approval A1828.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Luke Grinham and Johannes Stortz. [A peer review file is available.]
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Suresh, S., Welch, M.J., Munday, P.L. et al. Cross-talk between tissues is critical for intergenerational acclimation to environmental change in Acanthochromis polyacanthus. Commun Biol 7, 1531 (2024). https://doi.org/10.1038/s42003-024-07241-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-07241-y