Abstract
Purple phototrophic bacteria produce two kinds of light-harvesting complexes that function to capture and transmit solar energy: the core antenna (LH1) and the peripheral antenna (LH2). The apoproteins of these antennas, encoded respectively by the genes pufBA and pucBA within and outside the photosynthetic gene cluster, respectively, exhibit conserved amino acid sequences and structural topologies suggesting they were derived from a shared ancestor. Here we present the structures of two photosynthetic complexes from Roseospirillum (Rss.) parvum 930I: an LH1–RC complex and a variant of the LH1 complex also encoded by pufBA that we designate as LH1′. The LH1–RC complex forms a closed elliptical structure consisting of 16 pairs of αβ-polypeptides that surrounds the RC. By contrast, the LH1′ complex is a closed ring structure composed of 14 pairs of αβ-polypeptides, and it shows significant similarities to LH2 complexes both spectrally and structurally. Although LH2-like, the LH1′ complex is larger than any known LH2 complexes, and genomic analyses of Rss. parvum revealed the absence of pucBA, genes that encode classical LH2 complexes. Characterization of the unique Rss. parvum photocomplexes not only underscores the diversity of such structures but also sheds new light on the evolution of light-harvesting complexes from phototrophic bacteria.
Similar content being viewed by others
Introduction
Photosynthesis converts solar energy into chemical energy and ultimately provides energy for all life on Earth. Among phototrophic organisms, purple bacterial photosynthetic metabolism is anoxygenic, and their light reactions occur in pigment–protein complexes within intracytoplasmic membranes (ICM). These complexes include a light-harvesting 1 complex (LH1), light-harvesting 2 complex (LH2), a reaction center (RC), cytochrome bc1 (Cyt bc1), and ATP synthases (ATPases). The RC is surrounded by the LH1 complex to form the LH1–RC core complex present in all purple phototrophic bacteria. By contrast, the LH2 complex is located outside the LH1–RC and is called the peripheral antenna and present in only some species1,2. LH1 and LH2 complexes are encoded by pufBA genes within the photosynthetic gene cluster and pucBA genes located outside the photosynthetic gene cluster, respectively, but exhibit conserved amino acid sequences and structures3,4,5.
In recent years, many structures of LH1–RC and LH2 complexes have been solved using single-particle cryo-electron microscopy (cryo-EM), and essential structural principles have emerged that have significantly advanced our understanding of these complexes from a functional standpoint. Complexes from different species exhibit significant diversity in both composition and topology. All core complexes consist of LH1 surrounding the RC to form monomeric or dimeric open or closed structures, whereas all LH2 complexes are closed circular structures that vary considerably in their specific details. Most LH1–RC complexes exist as monomers with the RC surrounded by a closed LH1 ring composed of 16–17 pairs of αβ-polypeptides6,7,8,9,10,11,12,13 or an open ring composed of 10–15 pairs of αβ-polypeptides plus auxiliary small transmembrane polypeptides11,14,15,16,17,18,19,20. Dimeric LH1–RC core structures are present in some Rhodobacter species21,22 and the alkaliphilic purple bacterium Rhodobaca (Rca.) bogoriensis23 and are characterized by continuous S-shaped LH1 arrays surrounding two RCs. Additionally, the LH1–RC core complex of Gemmatimonas (G.) phototrophica consists of two concentric rings surrounding the reaction center including an inner ring of 16 subunits and an outer ring of 24 subunits24.
In contrast to LH1 complexes, all characterized LH2 complexes form closed rings composed of 7–9 pairs of αβ-polypeptides25,26,27,28,29. Nevertheless, LH2 complexes share several structural features in common with LH1 complexes; both are large oligomers formed from multiple pairs of αβ-heterodimers and non-covalently bound molecules of bacteriochlorophyll (BChl) and carotenoid. Because of this structural similarity, it has been proposed that a common ancestor gave rise to both LH1 and LH2 complexes, with LH2 genes arising from gene duplications in an LH1-containing ancestor3,30,31. Although an appealing hypothesis, there is currently little experimental evidence to firmly support this scenario, leaving this important aspect of photosynthetic evolution unresolved.
Roseospirillum (Rss.) parvum is a mesophilic marine purple bacterium within the Alphaproteobacteria32, and its unusual absorption spectrum indicates that its light-harvesting complexes contain two types of BChl a with Qy transitions at 909 nm (corresponding to LH1-Qy) and 805 nm (corresponding to LH2-Qy but lacking the characteristic Qy at ~850 nm of classical LH2 complexes)33. Compared with the thoroughly investigated LH1 complexes from Thermochromatium (Tch.) tepidum34 and Thiorhodovibrio (Trv.) frisius strain 9708 in which calcium ions bound within the complex are critical to the red shift of LH1-Qy, the red-shifted photocomplexes of Rss. parvum result from a mechanism distinct from that of calcium binding based on preliminary analyses of amino acid sequence alignments35. Moreover, the Rss. parvum LH1–RC complex also differs from previously reported properties of the LH1–RC complex from the BChl a-containing filamentous and thermophilic phototroph Roseiflexus (Rfl.) castenholzii, a complex that absorbs at 804 and 880 nm14,20.
To explore the structural basis for the unusual absorption of Rss. parvum, we detail here the cryo-EM structures of the two photosynthetic complexes produced by this unusual phototroph. In addition to its LH1–RC core complex, Rss. parvum produces an LH1-only complex that we have designated LH1′. Although encoded by pufBA, LH1′ is smaller than LH1 in the Rss. parvum core complex, does not encircle a RC, is ring-shaped rather than elliptical, and functionally resembles LH2 of purple bacteria. Collectively, our results provide structural details of hitherto unseen photocomplexes, reveal mechanisms that underlie the unique absorption properties of both Rss. parvum photocomplexes and offer fresh insight into the evolution of purple bacterial photosynthetic complexes.
Results
Overall structure of the Rss. parvum LH1–RC and LH1′ complexes
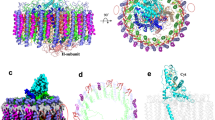
The LH1–RC core complex and the LH1′ complex purified from Rss. parvum using one-step solubilization followed by DEAE chromatography and characterized by a series of biochemical methods, exhibited absorption maxima (Qy) at 908 and 905 nm, respectively (Supplementary Fig. 1); the cryo-EM structures of these complexes were then determined at resolutions of 2.65 Å and 2.35 Å, respectively (Table 1 and Supplementary Figs. 2–4). The LH1 complex of Rss. parvum consists of 16 pairs of helical αβ-polypeptides (αβ-dimer), 47 BChls a, and 16 trans-spirilloxanthins, forming a closed elliptical structure surrounding the RC (Fig. 1a, b). The long and short dimensions of the outer LH1-β polypeptide ring (distances between the outer edge of opposite helices) were 116.3 Å and 109.7 Å, respectively (Fig. 1b). The RC is accommodated in the LH1 ellipsoid and fits the shape of the inner LH1-α ring with a height of approximately 126.2 Å (Fig. 1a). The structure of the Rss. parvum RC subunits are nearly identical to those of corresponding subunits from the Tch. tepidum RC with a root-mean-square deviation (RMSD) value of 0.786 Å (Supplementary Fig. 5a). However, a major difference between them is that the Rss. parvum RC contains a complete N-terminus of its Cyt subunit (Supplementary Fig. 4c, Supplementary Fig. 5a, and see later discussion).
a Side view of the LH1–RC complex parallel to the membrane plane with the periplasmic C-subunit above and the cytoplasmic H-subunit below. b Top view of the LH1–RC from the periplasmic side of the membrane. c Tilted view of the cofactors arrangement in LH1–RC complex. Dotted circle indicates the position that lacks a B803 molecule. d Side view of the LH1′ complex parallel to the membrane plane. e Top view of the LH1′ complex from the periplasmic side of the membrane. f Tilted view of the cofactors arrangement in LH1′ complex. The dimensions of the two complexes are represented and the color scheme is shown below the panels. Phospholipids are omitted for clarity. Abbreviations: BPhe a, bacteriopheophytin a; Spx, spirilloxanthin; MQ-8, menaquinone-8.
Cofactors in the Rss. parvum RC include 4 BChls a, 2 bacteriopheophytins (BPhe) a, 4 heme molecules, one 15-cis-spirilloxanthin and one menaquinone (MQ)-8 at the QA site; no ubiquinone (UQ) was observed at the QB site (Supplementary Fig. 5b, Supplementary Fig. 6). In contrast to most purple bacterial LH1–RC complexes, the BChls a in the Rss. parvum LH1 ring are distributed in a double-layered ring, similar to that in the LH1–RC complex of Rfl. castenholzii. One group of Rss. parvum BChls consists of closely stacked dimeric BChls a with a Qy maximum at 908 nm (B908), whereas the other group is composed of monomeric BChl a, with maximum absorption at 803 nm (B803) (Fig. 1c, Supplementary Fig. 1c). Each pair of LH1 αβ-polypeptides binds two B908 molecules on the periplasmic side, one B803 molecule on the cytoplasmic side, and one trans-spirilloxanthin that spans the transmembrane region. Notably, however, there is no B803 located between the first and sixteenth pairs of αβ-polypeptides, and this likely forms the channel necessary for quinone exchange (Fig. 1c).
The overall structure of the Rss. parvum LH1′ complex is a closed double ring consisting of 14 pairs of αβ-polypeptides encoded by pufBA, 42 BChls a, and 14 trans-spirilloxanthins (Fig. 1d, e). The inner ring formed by 14 α-polypeptides has a diameter of 58.8 Å, and the outer ring formed by β-polypeptides has a diameter of 103.8 Å (Fig. 1e). Like in its LH1, BChls a in the Rss. parvum LH1′ complex are also arranged in a double-layered ring, with the bacteriochlorophyll ring clustered near the periplasmic side composed of 14 pairs of dimeric BChls a with maximum absorption at 905 nm (B905) (Fig. 1f, Supplementary Fig. 1d). The latter Qy is slightly blue-shifted compared to B908 of LH1 but redshifted ~50 nm compared to the maximum absorption of LH2 complexes, most of which absorb near 850 nm. The bacteriochlorophyll ring clustered near the cytoplasmic side of the membrane consists of 14 monomeric BChls a, with maximum absorption at 803 nm (B803) (Fig. 1f, Supplementary Fig. 1d), similar to all previously reported LH1 and LH2 complexes. Additionally, 14 trans-spirilloxanthin molecules are positioned between each pair of LH1′ αβ-polypeptides with no apparent interactions with these polypeptides or with BChls a.
Unique features of the Rss. parvum LH1 complex
Within each pair of Rss. parvum LH1 αβ-polypeptides, B908 molecules are coordinated with α-His33 and β-His40, and the C31-acetyl groups of BChl a molecules form hydrogen bonds with α-Trp44 and β-Trp50 (Fig. 2a, b). Sequence alignment reveals that the conserved alanine, glycine or valine at position 36 of the β-polypeptide is replaced by cysteine in Rss. parvum36, although cysteine is rarely found in purple bacterial light-harvesting antenna proteins (Fig. 3). Base on the structure, we found that the thiol group of β-Cys36 can form two hydrogen bonds with the C131-keto and C132-ester groups of the α-B908 molecule (Fig. 2b). These additional hydrogen bonds further strengthen the interaction between pigments and their neighboring polypeptides, stabilizing the conformation of BChls a. In addition to β-Cys36, there is another Cys residue at position α-Cys36, which is similar to that found in the LH1 α1 polypeptide of Halorhodospira (Hlr.) halochloris37 (Fig. 3). Although the thiol group of α-Cys36 in Rss. parvum is distant from the bacteriochlorophyll ring and does not form hydrogen bonds, it is in the vicinity of the conserved His coordinating α-BChl a, which likely also contributes to the redshift of the LH1 Qy band.
a Arrangement of cofactors in an LH1 unit. The coordinated amino acid residues with BChl a molecules are shown by sticks and labeled for clarity. The color codes of polypeptides and pigments are the same as in Fig. 1. b Hydrogen bonding site around B908 in LH1 αβ-polypeptide. Both the residues involved in hydrogen bond interaction and hydrogen bond length are labeled. c Hydrogen bonding site around B803 in LH1 αβ-polypeptide. An aromatic residue involved in hydrogen bonding with B803 is also labeled. d Comparison of BChl a molecules orientation of Rss. parvum (green) with those of Rba. sphaeroides (light pink, PDB:7PBW), Rbl. acidophilus (pale yellow, PDB:1NKZ), Phs. molischianum (blue, PDB:1LGH), Mch. purpuratum (red, PDB:6ZXA), Rfl. castenholzii (cyan, PDB:8IUG) and G. phototrophica (pale cyan, PDB:7O0U). Their αβ-polypeptides are shown by grey white cartoon and carotenoid molecules are omitted for clarity. e Side view of a surface representation showing a channel (red dashed circle) in the LH1 ring which lacks a B803 molecule. Other channels are sealed by the B803 molecules (cyan). f The quinone-exchange channel between Rss. parvum LH1 α1- and α16-polypeptides. The surface representation LH1 α1- and α16-polypeptides are colored in pale cyan and are shown by the yellow ribbon simultaneously. The residues forming this hydrophobic channel are shown by magenta sticks.
The sequences are aligned relative to the histidine (or asparagine) residues coordinating BChl molecules. The conserved residues that coordinate and/or hydrogen-bond (mainly C31-acetyl) to dimeric BChl molecules are shown in red and green, respectively. The non-conserved residues that coordinate and/or hydrogen-bond (mainly C31-acetyl) to monomeric BChl molecules (Qy absorption at 800 or 820 nm) are shown in blue and orange, respectively. The acidic residues of α- and β-polypeptides are colored in purple. The cysteine residues in Rss. parvum and Hlr. halochloris are colored in magenta. The WxxDxI or WxxDxV motifs present in Ca2+-binding LH1 polypeptides are marked by the dashed box.
A total of 15 monomeric B803 molecules exist in the Rss. parvum LH1 complex, and the porphyrin plane of B803 is nearly perpendicular to that of B908 (Fig. 1c). Nevertheless, the orientation and position of the B803 bacteriochlorophyll ring differs (Fig. 2d). The average distance between Mg in adjacent LH1 B803 molecules is 19.77 Å (Supplementary Table 1), shorter than that in all known LH2 complexes and indicating relatively strong coupling between monomeric molecules of BChl a in Rss. parvum. Although the conformation of the Rss. parvum dimeric BChl a is similar to that in the Rfl. castenholzii LH1–RC and G. phototrophica LHh (outer ring), the orientation of the Rss. parvum monomeric BChl a molecules differs as shown in Fig. 2d and is more similar to that of LH2 complexes25,26,27,28,29. In the Rfl. castenholzii LH1–RC14, the B804 molecule is coordinated with β-His26, whereas in G. phototrophica LHh24, there is no evident coordination for the central Mg2+ of the B800 BChl a. By contrast, in Rss. parvum, the central Mg2+ of the B803 molecule is coordinated with an α-N-terminal methionine that is not acylated (Fig. 2a, c). Furthermore, a strong hydrogen bond (2.6 Å) is formed between the C31-acetyl group of B803 and the nearby β-Tyr25 that further stabilizes B803 and likely affects its Qy absorption (Fig. 2c).
Another distinctive characteristic of the Rss. parvum LH1–RC and LH1′ complexes is the presence of several negative charges on the periplasmic side of the membrane (Supplementary Fig. 7a, b). A similar distribution is also observed in Hlr. halochloris LH1–RC complex38, which has a higher content of acidic residues compared to other phototrophic bacteria (Fig. 3). Supplementary Fig. 7c, d illustrate the pH-dependent spectral changes observed in the purified LH1–RC and LH1′ complexes. As the pH decreases (below 7.5), the intensity of the LH1 Qy band at 908 nm undergoes a significant decrease and gradual blue-shift. When the pH drops by two full units to 5.5, the LH1 Qy band at 893 nm does not further blue-shift. At the same time, the LH1 Qy band at 803 nm remains virtually unaffected by this pH decrease (Supplementary Fig. 7c). Similarly, the LH1′ Qy at 905 nm blue-shifted to 890 nm at pH 5.5 or 5.0 (Supplementary Fig. 7d). All of these changes are nearly reversible when the pH returns to neutrality (Supplementary Fig. 8a, b). Moreover, it was found that relatively slight spectral changes (6 and 9 nm) occurred when the LH1–RC and LH1′ complexes were suspended in an acidic buffer (pH 5.0) containing 0.03% DDM, and these changes were also reversible (Supplementary Fig. 8c, d).
As previously mentioned, due to the absence of the 16th B803 molecule, a pore forms between the first and sixteenth pairs of αβ-polypeptides in Rss. parvum LH1 (Figs. 1c, 2e). This likely functions as a channel for quinone exchange and as such is surrounded by multiple hydrophobic amino acids including Leu18, Val19, Ala22, and Val26 in α1 and Phe12, Thr17, Ala20, Leu21, and Phe24 in α16 (Fig. 2f). Furthermore, the tail of the α-B908 molecule and the polyene chain of spirilloxanthin, which are bound to the first pair of αβ-polypeptides, lie close to this channel, contributing to its hydrophobicity.
The Rss. parvum RC complex
The structure of the Rss. parvum RC is similar to known purple bacterial RCs; however, the N-terminal domain of the Cyt subunit differs. Hydrophobicity analysis of the Cyt subunit revealed a transmembrane helix from Ala23 to Met45 (Supplementary Fig. 9a), consistent with the cryo-EM map, where a helix formed by 17 amino acids (Val27–Val43) inserts into the membrane, functioning as an anchor (Supplementary Fig. 4c, Supplementary Fig. 5a). This arrangement has also been observed in the RC Cyt subunits of Rfl. castenholzii14 and the acidophilic purple bacterium Rhodopila (Rpi.) globiformis13. Structurally, this unique configuration not only tightly binds the Cyt subunit to the membrane but also stabilizes the entire LH1–RC complex. Positionally, the N-terminal transmembrane helix is located near the first and second LH1 α-polypeptides, enhancing the connectivity of the entire complex without affecting the binding of B908 and B803 molecules (Fig. 4a, b). Comparison of the Rss. parvum RC with the Rpi. globiformis RC reveals slight differences in the length and position of the transmembrane helix of the Cyt subunit, but both are embedded in the membrane at similar angles and their N-terminal loop regions are highly similar (Fig. 4c, d). Previous studies have shown that the C-terminus of the LH1–RC protein PufX in Rhodobacter (Rba.) sphaeroides overlaps with the N-terminal loop region of the Cyt subunit in Rpi. globiformis in terms of conformation and position, suggesting a potential evolutionary link between PufX and the N-terminal region of the RC-bound Cyt subunit13. Similarly, aligning the Rss. parvum RC with the Rba. sphaeroides LH1–RC also reveals overlap between the position and conformation of the C-terminus of PufX (Leu55-Gly69) and the N-terminal loop region of the Rss. parvum Cyt subunit (Met44-Leu58) (Fig. 4e, f). Additionally, the Cyt subunit of Rfl. castenholzii also has a complete N-terminus, but this portion is located in a different position than is PufX (Supplementary Fig. 9b). In the Rss. parvum RC, only MQ-8 was identified at the QA site, whereas no quinones were observed at the QB site (Supplementary Fig. 6). This observation was common in early crystal structure studies of RC complexes39,40,41 mainly due to the dissociation of QB from its binding site during the purification process, and this is likely the explanation for the absence of a quinone in this site in the Rss. parvum RC.
a, b The features of the N-terminus of Rss. parvum Cyt subunit. The color codes of αβ-polypeptides are gray, while all other color codes are the same as in Fig. 1. c Superposition of the Cα carbons of RC subunits between Rpi. globiformis (PDB: 7XXF) and the Rss. parvum (this work). The N-terminus of the Cyt subunits of Rss. parvum and Rpi. globiformis are shown by light blue and pale-yellow cartoons, respectively. The other portions of the Rss. parvum RC are colored the same as those in Fig. 1, while those of Rpi. globiformis are colored gray, except for the heme groups, which are shown as yellow sticks. d Expanded view marked in (c) showing that the N-terminal loop region (Met44-Asp65) of the Rss. parvum Cyt subunit overlaps well with that (Phe27-Tyr48) of Rpi. globiformis. For clear presentation, (d) has been slightly rotated relative to the dash box in (c). e Superposition of the Cα carbons of the RC-M subunits between the Rba. sphaeroides monomeric LH1–RC (PDB: 7F0L) and Rss. parvum RC. PufX is shown as a cyan cartoon and the other portions of the complex are shown as gray ribbons, except for Protein-U, which is shown as a hot pink ribbon. f Expanded view marked in (e) showing that the C-terminus (Leu55–Gly69) of PufX overlaps positionally and conformationally with the N-terminal loop region (Met44-Gly58, main chain shown) of the Rss. parvum Cyt subunit.
Unique features of the Rss. parvum LH1′ complex
Although the Rss. parvum LH1′ shows features common in LH2 complexes, genome analysis failed to reveal pucBA, the genes that normally encode LH2 complexes. Because there is only one set of pufBA in the Rss. parvum genome, the LH1′ αβ- polypeptides must also be encoded by pufBA. From a structural point of view, however, Rss. parvum LH1 and LH1′ are not identical; the major differences are that LH1′ does not encircle a RC and is smaller than LH1. As regards the latter, there are 14 αβ-polypeptides in the LH1′ complex compared with 16 in LH1 (Fig. 1d, e). Other than these two major differences, the arrangement and orientation of pigment molecules and the binding mode of BChl a molecules and their hydrogen bonding interactions with surrounding polypeptides in the Rss. parvum LH1′ complex are similar to those in the LH1 complex.
In the Rss. parvum LH1′ complex, the average Mg–Mg distances were 9.30 Å within a dimer and 8.32 Å between dimers (Supplementary Table 1), almost the same as those in the LH1–RC complex. However, unlike the LH1–RC complex that lacks one B803 molecule to form an open cavity, both layers of bacteriochlorophyll rings in the LH1′ complex are intact, similar to that of classical LH2 complexes. The average Mg–Mg distance between B803 BChl monomers was 20.05 Å (Supplementary Table 1), which is slightly shorter than that of classical LH2 complexes. In summary, then, although encoded by the same genes as those that encode LH1, Rss. parvum LH1′ is smaller than its LH1 and neither contacts nor interacts with the RC. Thus, Rss. parvum LH1′ functionally resembles LH2 complexes, and its unique structure may be a prerequisite for capturing wavelengths above 900 nm, radiation virtually unabsorbed by classical LH2 complexes.
Discussion
The presence of two light-harvesting antenna complexes in purple phototrophic bacteria is not uncommon, but when present, the two antenna complexes are typically encoded by distinct gene sets. Here we have solved the high-resolution structures of the LH1–RC complex and an accompanying RC-free complex we refer to as LH1′, both encoded by the genes pufBA. Together, the two complexes constitute the unique photosystem of Rss. parvum.
These two complexes show several highly unusual features, and in particular, a significant red shift in Qy transition from that of LH1 and LH2 complexes of most purple bacteria. There are several factors that could account for this red shift as well as some factors known to trigger a red shift that are likely not involved; both factors are considered here. Although the Qy transitions of the Rss. parvum LH1 and LH1′ complexes lie beyond 900 nm—more than 30 nm and 50 nm red-shifted compared with other LH1 and LH2 complexes, respectively, the average Mg–Mg distances in the Rss. parvum LH1 complex were 9.30 Å within a dimer and 8.25 Å between dimers (Supplementary Table 1). These values are similar to those of Rhodospirillum (Rsp.) rubrum and Rba. sphaeroides9,16 and the orientations of the Qy dipole moments of dimeric BChl a molecules are identical, indicating that exciton coupling is unlikely to be the decisive trigger of the Rss. parvum Qy red shift. In addition, although the LH1 Qy absorption of Rss. parvum is similar to that of Tch. tepidum42, in this thermophilic purple sulfur bacterium a Ca2+-coordination network contributes significantly to the Qy red shift6,43,44; such a network also contributes to the LH1 red shifts observed in Trv. frisius strain 9708 and Allochromatium (Alc.) tepidum12. However, other than the Mg2+ in BChl a and unbound Mg2+ in the RC, no Ca2+ or other metal ions were detected in the LH1–RC complex of Rss. parvum and so the mechanism underlying the Qy red shift in Rss. parvum is clearly distinct from that in these calcium-dependent purple bacteria.
The red shift in both Rss. parvum photocomplexes is likely related to hydrogen-bonding interactions between BChls and neighboring proteins and specific polypeptide sequences that collectively create an environment that affects the Qy transition. Previous studies have shown that an interacting triangle formed with BChl a–Trp47–His48–BChl a in Trv. frisius strain 970 enhances hydrogen-bonding interactions between BChl a and polypeptides and promotes a red shift of the LH1 Qy8,45. In Rss. parvum, besides the conserved hydrogen-bonding interactions between α-Trp44 and β-Trp50 with the BChl a C31-acetyl group, the sulfhydryl group of β-Cys36 can form two additional hydrogen bonds with the C131-keto and C132-ester groups of α-B908 molecules; these enhanced hydrogen-bonding interactions may play an important role in the Rss. parvum Qy red shift.
In some LH1–RC complexes, the extended C-terminal domains of the LH1 αβ-polypeptides are homogeneously arranged to form rigid structures, which may be one of the significant factors contributing to the red shift of the LH1 Qy band8,46. Although the N/C-terminus of Rss. parvum LH1αβ-polypeptides was not fully resolved in the two complexes, the C-terminal chain of the LH1 β-polypeptides was slightly longer and contained several neutral and nonpolar amino acids (Fig. 3). Together, these provide a strong hydrophobic environment on the periplasmic side that stabilizes BChl a and likely contributes to the Qy red shift. Furthermore, there were several negative charges on the periplasmic side of the two complexes of Rss. parvum (Supplementary Fig. 7a, b). Similar distributions were observed in the Hlr. halochloris LH1–RC complex, which included a large number of negatively charged amino acids at the C-terminus of the αβ-polypeptides38,46. As the pH decreased, the Qy band of LH1–RC and LH1′ complexes of Rss. parvum and the LH1–RC band of Hlr. halochloris exhibited reversible spectral changes likely associated with the distribution of electrostatic charges around BChl molecules44,46 (Supplementary Fig. 8). However, the Qy shift is a complicated process, and it was found that both pH and detergents can play a role in it47,48 (Supplementary Fig. 8). Additionally, the unusual cysteine residues of Rss. parvum, especially α-Cys36, located near the conserved His and virtually identical in position to the cysteine residue of LH1 α1 in Hlr. halochloris46, represents a potential candidate for modulating the point charge surrounding BChl a molecules, which is also responsible for the pH-dependent reversible spectral changes in LH1 Qy absorption. Given that the subunit composition and pigment arrangement of the Rss. parvum LH1′ complex is very similar to that of its LH1–RC complex, the above factors also explain the red shift in the LH1′ complex. In addition, however, due to subtle differences in Mg-Mg distances and pigment-polypeptide interactions between the two complexes, the LH1′ Qy is blue-shifted by 3 nm compared with the LH1–RC complex, revealing a possible mechanism for energy transfer from LH1′ to the core complex.
Another unusual feature of the Rss. parvum LH1 complex is the binding of two layers of BChl a of which the B803 molecules close off the channel between adjacent LH1 αβ-polypeptides, as is also the case in the Rfl. castenholzii LH1–RC complex14. However, the absence of a B803 molecule between the 1st and 16th LH1 subunits of Rss. parvum creates a distinct pore, quite likely the channel necessary for quinone exchange (Fig. 2e, Supplementary Fig. 10a). Such a gap is analogous to the pore formed in the Rpi. globiformis and Blastochloris (Blc.) viridis LH1–RCs by the absence of the γ-like polypeptide13 or γ-polypeptide7, respectively (Supplementary Fig. 10b, c). In the Rfl. castenholzii LH1–RC complex, the gap created by the insertion of the Cyt-TM (C-subunit transmembrane helix) and TMx (the subunit X) between the 1st and 15th pairs of αβ-polypeptides is considered to be the quinone channel14,20 (Supplementary Fig. 10d); this is similar to the LH1 ring gap formed by PufX or protein-W in Rba. sphaeroides15,16,21,22,49 and Rps. palustris11, respectively. Additionally, the quinone channel in the Rss. parvum LH1 complex is formed by several hydrophobic amino acids in the 1st and 16th α-polypeptides (Fig. 2f). Sequence alignments show that amino acids near the quinone channels (formed by adjacent LH1 αβ-polypeptides) in LH1 complexes are not identical but do share similar hydrophobic properties (Supplementary Fig. 11). Therefore, this structural feature may be one of the inherent characteristics of core complex quinone channels in purple bacteria.
Previous studies have shown that the RC Cyt subunit in the aerobic purple bacterium Roseobacter (Rsb.) denitrificans has a complete N-terminus, and sequence alignments revealed a phylogenetic relationship between this portion of the Cyt subunit and PufX from both Rba. sphaeroides and Rba. capsulatus50. Moreover, structural studies of the Rpi. globiformis LH1–RC complex have revealed that the N-terminus of the Cyt subunit forms a helix to anchor the subunit into the membrane and shows a high similarity between its N-terminus and the C-terminus of PufX13. The Cyt subunit in the Rss. parvum RC also shows this pattern, with several amino acid residues at its N-terminus overlapping well with the C-terminus of PufX (Fig. 4e, f). Furthermore, a close overlap exists between the N-terminal 13/14 residues of the truncated Cyt subunit and the C-terminus of PufX (Supplementary Fig. 9c–g), and a long-standing hypothesis suggests that the RC of the common ancestor of phototrophic purple bacteria likely contained a full-length four-heme Cyt subunit that subsequently evolved through partial deletion of the pufC gene13.
The overall structure of the Rss. parvum LH1′ complex resembles the heterologously expressed circular LH1-only complex from Tch. tepidum, as both structures comprise 14 pairs of αβ-polypeptides encoded by pufBA51. However, there are also major differences between the two complexes. The Tch. tepidum LH1-only complex was expressed in a photosynthetically incompetent strain of Rsp. rubrum that lacked genes encoding its RC complex. By contrast, in Rss. parvum, a fully functional LH1–RC complex exists with the LH1′ complex being a separate and distinct entity, an arrangement typical of purple bacteria that contain peripheral (LH2) complexes. Despite the fact that the LH1-only complex from Tch. tepidum and Rss. parvum LH1′ are both circular and composed of an identical number of subunits, the complexes do not completely overlap due to differences in the orientation of the αβ-polypeptides and their associated pigments (Supplementary Fig. 12). This also suggests that the function of the Rss. parvum LH1′ complex is distinct from that of its LH1, and in this connection, we propose that LH1′ harvests light energy analogous to classical LH2s. If the Rss. parvum LH1′ does function as a peripheral antenna, it would be the largest LH2-like complex known. The lack of a B850 spectral component in the Rss. parvum LH1′ and the red shift of its Qy transition would also make it a unique peripheral antenna. Conversely, it is possible that LH1′ is a compact and functionally independent version of Rss. parvum LH1. There is a precedent for this, as an LH1-only complex has been obtained during purification of the LH1–RC complexes from Rsp. rubrum9,52 and Rfl. castenholzii53. Other studies using freeze-fracture electron microscopy to investigate the intracytoplasmic membranes of Allochromatium (Alc.) vinosum also revealed different forms of an LH2 complex, one of which was composed of 13 pairs of αβ-heterodimers54. Moreover, atomic force microscopy observations of the intracytoplasmic membranes of Pararhodospirillum (Par.) photometricum have shown the possible presence of an LH2 complex composed of 14 subunits55,56. However, all of these studies lacked confirmation from corresponding high-resolution structures. By contrast, our results with Rss. parvum are based on the atomic structure of the native 14-fold LH1′ complex, and thus the number of subunits per complex is known precisely. Regardless of how it is labeled—an LH1′ or an LH2—the Rss. parvum LH1′ complex likely communicates in some way with its LH1–RC to ultimately funnel light energy into the core complex. The absorption differences reported herein could form the basis of such communication, as energy transfer, albeit slight, from the Rss. parvum LH1′ (Qy 905 nm) to its LH1–RC (Qy 908 nm) would be an energetically “downhill” (and thus favorable) reaction.
All purple bacteria contain an LH1–RC complex while the peripheral LH2 antenna is present in many but not all species. Moreover, genes encoding LH1 are located within the photosynthetic gene cluster (PGC) while LH2 genes are typically located outside of the PGC. Therefore, one could argue that LH1 and LH2 originated from a common ancestor and that the LH1 complex emerged first and gave rise to the LH2 complex through gene duplication followed by horizontal gene transfer to certain species3. Although there is scant firm evidence to support this hypothesis, the fact that the Rss. parvum LH1–RC and LH1′ complexes are both encoded by pufBA provides fresh support for this scenario. Early evolving purple bacteria may have used pufBA to encode LH1 and produced only an LH1–RC complex. And then, possibly in response to the colonization of light-limiting environments and the need to exploit a greater breath of wavelengths, early forms of LH2 such as the Rss. parvum LH1′ complex evolved while still encoded by pufBA. In such a scenario, pucBA would have evolved from pufBA roots to encode smaller LH2 complexes composed of variable numbers of αβ-polypeptides that had different absorption properties25,26,27,28,29. Increasing the structural diversity of LH2 complexes would have broadened the light-harvesting range of purple bacteria throughout the near infrared region. The light-harvesting complexes of Rss. parvum not only fit well into such an evolutionary scenario but in addition enrich our understanding of purple bacterial light-harvesting complexes and expand the diversity of known light-harvesting antenna complexes in general.
Methods
Purification and characterization of the Rss. parvum LH1–RC and LH1′ complexes
Rss. parvum cells (purchased from DSMZ) were cultivated phototrophically at 30 °C for 7 days under incandescent light (50–60 μmol m–2 s–1) as described previously32. The cells were harvested by centrifugation at 5500 rpm (5353 × g) for 10 min and chromatophores were isolated by sonication (Ultrasonic Homogenizer JY92-IIN, SCENTZ, China) of pelleted cells suspended in 20 mM Tris-HCl buffer (pH 7.5) followed by ultracentrifugation (OptimaTM L-100 XP Ultracentrifuge, BECKMAN COULTER, USA) at 208,000 × g for 90 min. Chromatophores were treated with 1.0% (w/v) n-dodecyl β-D-maltopyranoside (β-DDM) at room temperature for 60 min and then centrifugated at 208,000 × g for 60 min. The resulting supernatant was loaded onto a DEAE anion-exchange column (Toyopearl 650S, TOSOH) equilibrated with 20 mM Tris-HCl buffer (pH 7.5) containing 0.03% (w/v) β-DDM. The LH1′ and LH1–RC complexes were eluted by a linear gradient of NaCl from 50 mM to 250 mM (Supplementary Fig. 1a). After washing the mixture of impurities, the LH1′ complex was eluted first, followed by the LH1–RC complex; purities were assessed by sucrose density gradient ultracentrifugation, SDS-PAGE, BN-PAGE and negative-stain electron microscopy (Supplementary Fig. 1, Supplementary Fig. 2a). Fractions with ratios of R908/280 and R905/280 larger than 2.0 and 2.4, respectively, were collected and concentrated with Amicon 100,000 molecular weight cutoff (MWCO) centrifugal filters (Millipore). Absorption spectra were measured using a UV-1900i spectrophotometer (Shimadzu). The purified complexes were diluted with buffers of different pH values (pH 5.0, 5.5, 6.5, 7.5) for pH-dependent spectroscopic measurements. Subsequently, reversible tests were conducted by adding a large amount of buffer containing 20 mM Tris-HCl (pH 7.5) and 0.03% (w/v) β-DDM until neutral pH was reached.
The complete genome of Rss. parvum
The complete genome sequence of Rss. parvum was sequenced using Nanopore Oxford Nanopore Technologies (ONT). After trimming low-quality reads by Trimmomatic v0.33 software57, about 911 Mb of clean sequences were used to assemble the genome by CANU v2.2 software58. Two versions of genomic sequences were generated by different parameters, resulting in sequence sizes of 3,589,066 and 3,578,963, respectively. To ensure accuracy, sequence correction was performed using RACON v1.5.0 software59 (https://github.com/lbcb-sci/racon). Both sequences had a length of above one cycles and were the same in alignment, which was employed using MINIMAP2 v2.24-r112260 for comparisons. Only one cycle was retained as the complete genome after removing overlapped bases, and the finished cycled genome had a length of 3,548,410. Additionally, the genome was polished using NextPolish v1.4.161 with Illumina paired-reads. Finally, a high-quality, complete, circular genome with a length of 3,555,720 bp was assembled. Prodigal V2.6.3 software62 (Assembly, Annotation, and Comparative Analysis of Bifidobacterial Genomes) was employed for annotation using the cycled assembly genome.
Cryo-EM data collection
Proteins for cryo-EM were concentrated to 4 mg/ml. Then 5 microliters of protein solution were applied on glow-discharged holey carbon grids (M01 Au300-R1.2/1.3, Nanodim Tech) previously treated with H2 and Ar mixtures in a Solarus plasma cleaner (Gatan, Pleasanton, USA) for 60 s and then blotted and plunged into liquid ethane at –182 °C using Vitrobot Mark IV (Thermo Fisher Scientific). The applied parameters were a blotting time of 4 s at 100% humidity and 4 °C. Data were collected on a Titan Krios (Thermo Fisher Scientific, Hillsboro, USA) electron microscope at 300 kV equipped with a K3 camera (Gatan, Pleasanton, USA) (Supplementary Fig. 2b). Movies were recorded using EPU software (Thermo Fisher Scientific) at a nominal magnification of 81 k in counting mode and a pixel size of 1.04 Å at the specimen level with a dose rate of 39 e- per physical pixel per second, corresponding to 36 e- per Å2 per second at the specimen level. The exposure time was 1.71 s, resulting in an accumulated dose of 61.6 e- per Å2. Each movie includes 32 fractioned frames.
Image processing of the LH1–RC complex
All of the stacked frames were subjected to motion correction, and defocus was estimated using cryoSPARC63. A total of 788,173 particles were selected from 8150 micrographs using the crYOLO64 (Supplementary Fig. 3). All of the picked particles were further analyzed with cryoSPARC63, and 400,267 particles were selected by 2-D classification and divided into four classes by 3-D classification resulting in only one good class containing 281,288 particles. The initial 3-D model was generated in cryoSPARC. The non-uniform refinement without any imposed symmetry (C1) produced a map at 2.65 Å resolution after contrast transfer function refinement, masking, and post-processing, according to the gold-standard Fourier shell correlation using a criterion of 0.14365,66 (Supplementary Fig. 3b). The local resolution maps were calculated on cryoSPARC.
Image processing of the LH1′ complex
All of the stacked frames were subjected to motion correction, and defocus was estimated using cryoSPARC61. A total of 826,886 particles were selected from 7387 micrographs using crYOLO64 (Supplementary Fig. 3). All of the picked particles were further analyzed with cryoSPARC63, and 521,006 particles were selected by 2-D classification. All particles were subjected to a low-pass filtering of 3 Å before 3D reconstruction. Four initial 3-D models were generated in cryoSPARC and then subjected to Hetero-Refinement, with one good class containing 397,127 particles selected. These particles were then reextracted without filtering, then the non-uniform refinement without any imposed symmetry (C1) produced a map at 2.72 Å resolution65. After applying C14 symmetry, contrast transfer function refinement, masking, and post-processing, a final map of 2.35 Å was produced, according to the gold-standard Fourier shell correlation using a criterion of 0.14366 (Supplementary Fig. 3b). The local resolution maps were calculated on cryoSPARC.
Model building and refinement of the LH1–RC and LH1′ complexes
The atomic model of the Tch. tepidum LH1–RC (PDB: 5Y5S) was fitted to the cryo-EM map obtained for the Rss. parvum LH1–RC using Chimera67. Amino acid substitutions and real space refinement for the polypeptides and cofactors were performed using COOT68. Whole regions of the LH1 αβ-polypeptides as well as both terminal regions of the Cyt subunit and LH1 αβ-subunit were modelled ab-initio based on the density. For LH1′ complex, the atomic model of LH1 αβ-polypeptide with cofactors was fitted to the cryo-EM map sequentially to obtain the final structure. The manually modified model was refined in real-space on PHENIX69, and the COOT/PHENIX refinement was iterated until the refinements converged. Finally, the statistics calculated using MolProbity70 were checked. Figures were drawn with the Pymol Molecular Graphic System (Ver2.5, Schrödinger)71, UCSF Chimera67 and UCSF Chimera X72. Surface charge distributions were calculated using the APBS Electrostatics plugin in the Pymol.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The complete genome sequence of Rss. parvum 930I has been deposited at the National Microbiology Data Center (NMDC) with the accession No. NMDC60154775. Maps and models have been deposited in the EMDB and PDB with the accession codes: EMD-60165 and PDB-8ZK2 for the LH1–RC complex and EMD-60158 and PDB-8ZJW for the LH1′ complex. The numerical source values underlying Supplementary Fig. 1c, d, g, 7c, d and 8 can be found in Supplementary Data 1, and the uncropped and unprocessed images of the gels are in Supplementary Fig. 13. The numerical source values supporting Supplementary Fig. 3b can be found in Supplementary Data 2. All other data are available from the authors upon reasonable request.
References
Hu, X., Ritz, T., Damjanović, A., Autenrieth, F. & Schulten, K. Photosynthetic apparatus of purple bacteria. Q. Rev. Biophys. 35, 1–62 (2002).
Miller, L. C., Martin, D. S., Liu, L. N. & Canniffe, D. P. Composition, organisation and function of purple photosynthetic machinery. In Microbial Photosynthesis (ed. Wang, Q.) 73–114 (Springer Singapore, 2020).
Youvan, D. C. & Ismail, S. Light-harvesting II (B800-B850 complex) structural genes from Rhodopseudomonas capsulata. Proc. Natl. Acad. Sci. USA 82, 58–62 (1985).
Alberti, M., Burke, D. H. & Hearst, J. E. Structure and sequence of the photosynthesis gene cluster. In Anoxygenic Photosynthetic Bacteria (eds. Blankenship, R. E., Madigan, M. T. & Bauer, C. E.) 1083–1106 (Kluwer Academic Dordrecht, 1995).
Swingley, W. D., Blankenship, R. E. & Raymond, J. Evolutionary relationships among purple photosynthetic bacteria and the origin of proteobacterial photosynthetic systems. In The Purple Phototrophic Bacteria (eds. Hunter, C. N., Daldal, F., Thurnauer, M. C. & Beatty, J. T.) 17–29 (Springer Dordrecht, 2009).
Yu, L. J., Suga, M., Wang-Otomo, Z. Y. & Shen, J. R. Structure of photosynthetic LH1–RC supercomplex at 1.9 Å resolution. Nature 556, 209–213 (2018).
Qian, P., Siebert, C. A., Wang, P., Canniffe, D. P. & Hunter, C. N. Cryo-EM structure of the Blastochloris viridis LH1–RC complex at 2.9 Å. Nature 556, 203–208 (2018).
Tani, K. et al. Cryo-EM structure of a Ca2+-bound photosynthetic LH1–RC complex containing multiple αβ-polypeptides. Nat. Commun. 11, 4955 (2020).
Tani, K. et al. Cryo-EM structure of the photosynthetic LH1–RC complex from Rhodospirillum rubrum. Biochemistry 60, 2483–2491 (2021).
Qian, P. et al. Cryo-EM structure of the Rhodospirillum rubrum RC–LH1 complex at 2.5 Å. Biochem. J. 478, 3253–3263 (2021).
Swainsbury, D. J. K. et al. Structures of Rhodopseudomonas palustris RC–LH1 complexes with open or closed quinone channels. Sci. Adv. 7, eabe2631 (2021).
Tani, K. et al. A Ca2+-binding motif underlies the unusual properties of certain photosynthetic bacterial core light-harvesting complexes. J. Biol. Chem. 298, 101967 (2022).
Tani, K. et al. An LH1–RC photocomplex from an extremophilic phototroph provides insight into origins of two photosynthesis proteins. Commun. Biol. 5, 1197 (2022).
Xin, Y. et al. Cryo-EM structure of the RC–LH core complex from an early branching photosynthetic prokaryote. Nat. Commun. 9, 1568 (2018).
Qian, P. et al. Cryo-EM structure of the monomeric Rhodobacter sphaeroides RC–LH1 core complex at 2.5 Å. Biochem. J. 478, 3775–3790 (2021).
Tani, K. et al. A previously unrecognized membrane protein in the Rhodobacter sphaeroides LH1–RC photocomplex. Nat. Commun. 12, 6300 (2021).
Bracun, L. et al. Cryo-EM structure of the photosynthetic RC-LH1-PufX supercomplex at 2.8-Å resolution. Sci. Adv. 7, eabf8864 (2021).
Bracun, L., Yamagata, A., Christianson, B. M., Shirouzu, M. & Liu, L. N. Cryo-EM structure of a monomeric RC-LH1-PufX supercomplex with high-carotenoid content from Rhodobacter capsulatus. Structure 31, 318–328 (2023).
Tani, K. et al. Rhodobacter capsulatus forms a compact crescent-shaped LH1–RC photocomplex. Nat. Commun. 14, 846 (2023).
Qi, C. H. et al. New insights on the photocomplex of Roseiflexus castenholzii revealed from comparisons of native and carotenoid-depleted complexes. J. Biol. Chem. 299, 105057 (2023).
Cao, P. et al. Structural basis for the assembly and quinone transport mechanisms of the dimeric photosynthetic RC–LH1 supercomplex. Nat. Commun. 13, 1977 (2022).
Tani, K. et al. Asymmetric structure of the native Rhodobacter sphaeroides dimeric LH1–RC complex. Nat. Commun. 13, 1904 (2022).
Semchonok, D. A. et al. Cryo-EM structure of the Rhodobaca bogoriensis RC-LH1-PufX dimeric complex at 2.9 Å. https://doi.org/10.1101/2022.02.25.481955 (2022).
Qian, P. et al. 2.4-Å structure of the double-ring Gemmatimonas phototrophica photosystem. Sci. Adv. 8, eabk3139 (2022).
Mcdermott, G. et al. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature 374, 517–521 (1995).
Koepke, J., Hu, X., Muenke, C., Schulten, K. & Michel, H. The crystal structure of the light-harvesting complex II (B800–850) from Rhodospirillum molischianum. Structure 4, 581–597 (1996).
Gardiner, A. T. et al. The 2.4 Å cryo-EM structure of a heptameric light-harvesting 2 complex reveals two carotenoid energy transfer pathways. Sci. Adv. 7, eabe4650 (2021).
Qian, P. et al. Cryo-EM structure of the Rhodobacter sphaeroides light-harvesting 2 complex at 2.1 Å. Biochemistry 60, 3302–3314 (2021).
Qian, P. et al. Cryo-EM structures of light-harvesting 2 complexes from Rhodopseudomonas palustris reveal the molecular origin of absorption tuning. Proc. Natl. Acad. Sci. USA 119, e2210109119 (2022).
Henry, S. L. & Cogdell, R. J. The evolution of the purple photosynthetic bacterial light-harvesting system. In Genome Evolution of Photosynthetic Bacteria (ed. Beatty, J. T.) 205–226 (Elsevier Amsterdam, 2013).
Lang, A. S., Harwood, C. S. & Beatty, J. T. Evolutionary relationships among antenna proteins of purple phototrophic bacteria. In Functional Genomics and Evolution of Photosynthetic Systems (eds. Burnap, R. L. & Vermaas, W. F. J.) 253–264 (Springer Dordrecht, 2012).
Glaeser, J. & Overmann, J. Selective enrichment and characterization of Roseospirillum parvum, gen. nov and sp nov., a new purple nonsulfur bacterium with unusual light absorption properties. Arch. Microbiol. 171, 405–416 (1999).
Permentier, H. P., Neerken, S., Schmidt, K. A., Overmann, J. & Amesz, J. Energy transfer and charge separation in the purple non-sulfur bacterium Roseospirillum parvum. Biochim. Biophys. Acta Bioenerg. 1460, 338–345 (2000).
Niwa, S. et al. Structure of the LH1–RC complex from Thermochromatium tepidum at 3.0 Å. Nature 508, 228–232 (2014).
Rucker, O., Kohler, A., Behammer, B., Sichau, K. & Overmann, J. Puf operon sequences and inferred structures of light-harvesting complexes of three closely related Chromatiaceae exhibiting different absorption characteristics. Arch. Microbiol. 194, 123–134 (2012).
Tuschak, C., Beatty, J. T. & Overmann, J. Photosynthesis genes and LH1 proteins of Roseospirillum parvum 930I, a purple non-sulfur bacterium with unusual spectral properties. Photosynth. Res. 81, 181–199 (2004).
Tsukatani, Y., Hirose, Y., Harada, J., Yonekawa, C. & Tamiaki, H. Unusual features in the photosynthetic machinery of Halorhodospira halochloris DSM 1059 revealed by complete genome sequencing. Photosynth. Res. 140, 311–319 (2019).
Qi, C. H. et al. Structural insights into the unusual core photocomplex from a triply extremophilic purple bacterium, Halorhodospira halochloris. J. Integr. Plant Biol. 66, 2262–2272 (2024).
Deisenhofer, J., Epp, O., Miki, K., Huber, R. & Michel, H. X-ray structure analysis of a membrane protein complex: electron density map at 3 Å resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J. Mol. Biol. 180, 385–398 (1984).
Deisenhofer, J., Epp, O., Miki, K., Huber, R. & Michel, H. Structure of the protein subunits in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3Å resolution. Nature 318, 618–624 (1985).
Nogi, T., Fathir, I., Kobayashi, M., Nozawa, T. & Miki, K. Crystal structures of photosynthetic reaction center and high-potential iron-sulfur protein from Thermochromatium tepidum: thermostability and electron transfer. Proc. Natl. Acad. Sci. USA 97, 13561–13566 (2000).
Suzuki, H. et al. Purification, characterization and crystallization of the core complex from thermophilic purple sulfur bacterium Thermochromatium tepidum. Biochim. Biophys. Acta Bioenerg. 1767, 1057–1063 (2007).
Kimura, Y. et al. Calcium ions are involved in the unusual red shift of the light-harvesting 1 Qy transition of the core complex in thermophilic purple sulfur bacterium Thermochromatium tepidum. J. Biol. Chem. 283, 13867–13873 (2008).
Yu, L. J., Kawakami, T., Kimura, Y. & Wang-Otomo, Z. Y. Structural basis for the unusual Qy red-shift and enhanced thermostability of the LH1 complex from Thermochromatium tepidum. Biochemistry 55, 6495–6504 (2016).
Imanishi, M. et al. A dual role for Ca2+ in expanding the spectral diversity and stability of light-harvesting 1 reaction center photocomplexes of purple phototrophic bacteria. Biochemistry 58, 2844–2852 (2019).
Kimura, Y. et al. Electrostatic charge controls the lowest LH1 Qy transition energy in the triply extremophilic purple phototrophic bacterium, Halorhodospira halochloris. Biochim. Biophys. Acta Bioenerg. 1862, 148473 (2021).
Huo, Y. et al. Spectroscopic properties of LH2 from Thermochromatium tepidum in liposome and detergent micelles. Chem. J. Chin. Univ. 37, 1678–1685 (2016).
Saga, Y., Sasamoto, Y., Inada, K., Wang-Otomo, Z.-Y. & Kimura, Y. Spectral modulation of B850 bacteriochlorophyll a in light-harvesting complex 2 from purple photosynthetic bacterium Thermochromatium tepidum by detergents and calcium ions. Biochim. Biophys. Acta Bioenerg. 1865, 149503 (2024).
Qian, P. et al. Cryo-EM structure of the dimeric Rhodobacter sphaeroides RC–LH1 core complex at 2.9 Å: the structural basis for dimerisation. Biochem. J. 478, 3923–3937 (2021).
Hucke, O., Schiltz, E., Drews, G. & Labahn, A. Sequence analysis reveals new membrane anchor of reaction centre‐bound cytochromes possibly related to PufX. FEBS Lett. 535, 166–170 (2003).
Yan, Y. H. et al. Molecular structure and characterization of the Thermochromatium tepidum light-harvesting 1 photocomplex produced in a foreign host. Biochim. Biophys. Acta Bioenerg. 1865, 149050 (2024).
Picorel, R., Belanger, G. & Gingras, G. Antenna holochrome B880 of Rhodospirillum rubrum S1. Pigment, phospholipid, and polypeptide composition. Biochemistry 22, 2491–2497 (1983).
Collins, A. M. et al. Light-harvesting antenna system from the phototrophic bacterium Roseiflexus castenholzii. Biochemistry 49, 7524–7531 (2010).
Kereïche, S. et al. The peripheral light-harvesting complexes from purple sulfur bacteria have different ‘ring’ sizes. FEBS Lett. 582, 3650–3656 (2008).
Scheuring, S., Rigaud, J. L. & Sturgis, J. N. Variable LH2 stoichiometry and core clustering in native membranes of Rhodospirillum photometricum. EMBO J. 23, 4127–4133 (2004).
Scheuring, S. & Sturgis, J. N. Chromatic adaptation of photosynthetic membranes. Science 309, 484–487 (2005).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Koren, S. et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 27, 722–736 (2017).
Vaser, R., Sović, I., Nagarajan, N. & Šikić, M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 27, 737–746 (2017).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018).
Hu, J., Fan, J., Sun, Z. & Liu, S. NextPolish: a fast and efficient genome polishing tool for long-read assembly. Bioinformatics 36, 2253–2255 (2020).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinf. 11, 119 (2010).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr. 66, 12–21 (2010).
DeLano, W. L. The PyMOL Molecular Graphics System. (DeLano Scientific, LCC, San Carlos, CA USA, 2004).
Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci. 32, e4792 (2023).
Acknowledgements
We are grateful to staff of the Institute of Physics, Chinese Academy of Sciences and Beijing Branch of Songshan Lake Materials Laboratory for instrument support and technical assistance during cryo-EM data collection. This work was supported in part by the National Key R&D Program of China (No. 2022YFC3401800), National Natural Science Foundation of China (32070264), Shandong Provincial Natural Science Foundation, China (ZR2019ZD48), the Strategic Priority Research Program of CAS (XDA26050402) and the Science & Technology Specific Project in Agricultural High-tech Industrial Demonstration Area of the Yellow River Delta (2022SZX12), the Innovation Center for Academicians of Hainan Province, and the Specific Research Fund of the Innovation Center for Academicians of Hainan Province (No. YSPTZX202309). M.T.M. was supported in part by NASA Cooperative Agreement 80NSSC21M0355.
Author information
Authors and Affiliations
Contributions
L.-J.Y. designed the research, X.-P.W. and G.-L.W. performed the experiments, X.-P.W., G.-L.W., Y.F., A.M., M.-J.Z., F.M., B.X., Z.-Y.W.-O., Y.K., M.T.M. and L.-J.Y. analyzed data, L.-J.Y., X.-P.W., M.T.M. and J.O. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Seiji Akimoto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Janesh Kumar and Laura Rodríguez Pérez. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, XP., Wang, GL., Fu, Y. et al. Insights into the divergence of the photosynthetic LH1 complex obtained from structural analysis of the unusual photocomplexes of Roseospirillum parvum. Commun Biol 7, 1658 (2024). https://doi.org/10.1038/s42003-024-07354-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-07354-4