Abstract
Halorhodospira (Hlr.) halophila strain BN9622 is an extremely halophilic and alkaliphilic purple phototrophic bacterium and has been widely used as a model for exploring the osmoadaptive and photosynthetic strategies employed by phototrophic extreme halophiles that enable them to thrive in hypersaline environments. Here we present the cryo-EM structures of (1) a unique native Hlr. halophila triple-complex formed from light-harvesting (LH1), the reaction center (RC), and high-potential iron–sulfur protein (HiPIP) at 2.44 Å resolution, and (2) a HiPIP-free LH1–RC complex at 2.64 Å resolution. Differing from the LH1 in the Hlr. halophila LH1–LH2 co-complex where LH1 encircles LH2, the RC-associated LH1 complex consists of 16 (rather than 18) αβ-subunits circularly surrounding the RC. These distinct forms of LH1 indicate that the number of subunits in a Hlr. halophila LH1 complex is flexible and its size is a function of the photocomplex it encircles. Like LH1 in the LH1–LH2 co-complex, the RC-associated LH1 complex also contained two forms of αβ-polypeptides and both dimeric and monomeric molecules of bacteriochlorophyll a. The majority of the isolated Hlr. halophila LH1–RC complexes contained the electron donor HiPIP bound to the surface of the RC cytochrome subunit near the heme-1 group. The bound HiPIP consisted of an N-terminal functional domain and a long C-terminal extension firmly attached to the cytochrome subunit. Despite overall highly negative surface-charge distributions for both the cytochrome subunit and HiPIP, the interface between the two proteins was relatively uncharged and neutral, forming a pathway for electron tunneling. The structure of the Hlr. halophila LH1–RC–HiPIP complex provides insights into the mechanism of light energy acquisition coupled with a long-distance electron donating process toward the charge separation site in a multi-extremophilic phototroph.
Similar content being viewed by others
Introduction
In a previous paper1, we described the structure of a novel type of light-harvesting (LH) complex from the extremely halophilic and alkaliphilic purple phototrophic bacterium Halorhodospira (Hlr.) halophila strain BN96222,3. The LH complex forms a unique double-ring structure with the large LH1 encircling a small LH2 to form a native LH1–LH2 co-complex. In the detergent-solubilized photocomplexes from Hlr. halophila intracytoplasmic membranes (ICM), the LH1–LH2 co-complex coexists with a second and structurally distinct LH1 complex, the LH1–reaction center (RC) core complex. The two complexes could not be completely separated by standard purification techniques1,4, although a small amount of highly purified LH1–LH2 was obtained and used for spectroscopic measurements. As a result, a mixture of the LH1–LH2 and LH1–RC complexes with an approximate ratio of 1:1 (Supplementary Fig. 1a) was subjected to cryo-EM single-particle analysis1. Both complexes were clearly distinct and so their structures could be determined by computational imaging analysis in cryo-EM that can “purify” the complexes in silico when biochemical purification methods have failed5. Here, we present the cryo-EM structures of the Hlr. halophila LH1–RCs, focusing on the structure of the RC complex that was tightly associated with a high-potential iron–sulfur protein (HiPIP) as an electron donor to the RC cytochrome (Cyt) subunit.
Hlr. halophila contains four genes encoding HiPIPs3 of which two encode isozymes, referred to as iso-1 and iso-2 HiPIPs ( ~ 65% sequence identity); the isozymes are abundant in cell extracts and have been purified and characterized6,7,8. The Hlr. halophila RC was reported to exist in a firm complex with iso-2 HiPIP in the membrane fractions and in partially purified protein based on biochemical and spectroscopic analyses9. The bound iso-2 HiPIP exhibited a redox potential of about 100 mV higher than that of the purified protein (~ + 50 mV)6, making it suitable for mediating electrons between the Cyt bc1 complex (~ + 120 mV for the Rieske center) and the RC ( ~ + 270 mV for the special pair)9. The strong affinity toward the membrane-embedded RC seems to be a common feature of HiPIP in purple phototrophic bacteria because HiPIPs have been reported to copurify with membrane fractions from many species9 including Rubrivivax (Rvi.) gelatinosus10,11 and Rhodoferax (Rfx.) fermentans12. It has been proposed that docking of HiPIP to the RC in Rvi. gelatinosus is primarily controlled by hydrophobic contacts between the protein surfaces, based on site-specific mutagenetic studies13. Despite these many studies on HiPIPs, no structure of a native HiPIP–RC co-complex has yet been determined.
The first structure of a reconstituted HiPIP–RC complex was obtained by co-crystallization of individually purified HiPIP and LH1–RC complex from the thermophilic purple sulfur bacterium Thermochromatium (Tch.) tepidum14. The Tch. tepidum HiPIP binds to the surface of the tetraheme Cyt subunit of the RC in close proximity to the low-potential heme-1 group. The binding interface between the two proteins is primarily formed by uncharged residues and is characterized by hydrophobic features14. Here, we compare and contrast the structure of the Hlr. halophila native LH1–RC–HiPIP with that of the reconstituted Tch. tepidum LH1–RC:HiPIP with a focus on their relative geometries and interaction interface between HiPIP and the Cyt subunit in each species.
Results
Cryo-EM imaging analysis revealed that two-thirds (66.7%) of the total LH1–RC from Hlr. halophila strain BN9622 contained bound HiPIP on the surface of the RC Cyt subunit (Supplementary Fig. 1); these complexes copurified with HiPIP-free LH1–RC complexes during DEAE chromatography. The LH1s in both complexes exhibited two absorption maxima at 788 nm and 883 nm (Supplementary Fig. 2a). Light-induced P+/P absorption difference spectra (Supplementary Fig. 2b) of these complexes showed signals primarily from the RC pigments: 1253 nm for P+, 879 nm for P, and 791/815 nm for the accessary bacteriochlorophylls (BChls) a. Cryo-EM structures of the HiPIP-bound and HiPIP-free LH1–RC complexes were determined at resolutions of 2.44 Å and 2.64 Å, respectively (Fig. 1, Table 1, Supplementary Figs. 1, 3, 4). Because the structure of the HiPIP-free LH1–RC was essentially the same as that of the HiPIP-bound LH1–RC, with a root-mean-square deviation (RMSD) of 0.160 Å for the mainchain Cα carbons, we focus here on the higher-resolution structure of the LH1–RC–HiPIP triple complex.
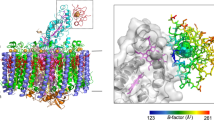
a Side view of the overall structure of the triple-complex. b Expanded view of the region marked in a where HiPIP is shown as sticks and the Cyt subunit is shown in transparent surface representation. Hemes are shown in magenta sticks and the 4Fe–4S cluster is shown as spheres. The residues in HiPIP are color-coded by the values of their B-factor as indicated in the scale bar. c Top view of the triple-complex from the periplasmic side. d Tilted view of cofactors in the complex. Color scheme: HiPIP, orange; RC-Cyt subunit, cyan; RC-L subunit, magenta; RC-M subunit, blue; RC-H subunit, wheat; LH1-α, green; LH1-β, slate blue; BChl a, red sticks; BPhe, orange sticks; carotenoids, yellow sticks; heme, magenta sticks; MQ, cyan sticks; UQ, blue sticks; phospholipids, wheat sticks; Fe, green spheres.
Overall structure of the Hlr. halophila LH1–RC–HiPIP triple complex
The Hlr. halophila RC contains a tetraheme Cyt subunit bound with HiPIP at its surface close to the heme-1 group (Fig. 1a). The bound Hlr. halophila HiPIP contains a long C-terminal domain that is tightly associated with the Cyt subunit (Fig. 1b). The LH1 in the LH1–RC–HiPIP triple complex forms a closed ring structure surrounding the RC with a similar arrangement of the αβ-subunits to those of the LH1s from Tch. tepidum15,16, Thiorhodovibrio frisius (strain 970)17, Rhodospirillum (Rsp.) rubrum18,19, Allochromatium (Alc.) tepidum20 and Rhodopila (Rpi.) globiformis21. However, differing from the LH1 in the Hlr. halophila LH1–LH2 co-complex1, the LH1 in the LH1–RC–HiPIP triple complex is composed of 16 (rather than 18) αβ-subunits (Fig. 1c); this indicates that Hlr. halophila LH1 is capable of forming rings of different numbers of αβ-subunits depending on the photocomplexes they encircle. Phospholipids (presumably phosphatidylglycerol, PG) were identified in the spaces between LH1 β-polypeptides (Fig. 1c).
Similar to the LH1 in the Hlr. halophila LH1–LH2 co-complex1, the LH1 in the LH1–RC–HiPIP triple complex contains two layers of BChl a molecules (Fig. 1d). One layer is formed by the monomeric (isolated) BChls with longer BChl–BChl distances that do not overlap, whereas the second is formed by dimeric BChls that have extensive overlaps and which show strong coupling between their bacteriochlorins. In addition, nine spirilloxanthins were identified in the complex. Similar to the type strain of Hlr. halophila, strain SL1T22, two types of quinones are present in Hlr. halophila BN9622 ICM (Supplementary Fig. 5) with menaquinone-8 (MQ-8) being the dominant component; three ubiquinone-8 (UQ-8) and one MQ-8 were identified in the LH1–RC–HiPIP structure (Supplementary Fig. 6). Four heme groups in the Cyt subunit and the 4Fe–4S cluster in HiPIP are aligned forming an electron transport chain toward the RC special pair (Fig. 1d).
The LH1 and RC in the Hlr. halophila LH1–RC–HiPIP complex
As was found in the LH1 of the Hlr. halophila LH1–LH2 co-complex, two forms of LH1 α- and β-polypeptides were identified in the Hlr. halophila LH1–RC–HiPIP complex (Fig. 2a). Overall, the two complexes showed similar conformation and pigment arrangement, differing only in the number of αβ-subunits. In the LH1–RC–HiPIP, α1/β1- and α2/β2-pairs form an alternating arrangement within the LH1 with a total of eight sets of each pair (Fig. 2a). Thirty-two dimeric BChls a in the Hlr. halophila LH1 form a partially overlapping ring on the periplasmic side of the membrane with average Mg–Mg distances of 9.3 Å within a dimer and 8.3 Å between dimers (Fig. 2b, Supplementary Table 1). The eight monomeric BChls a are located on the cytoplasmic side of the membrane with Mg–Mg distances of 33.5–34.6 Å. The closest Mg–Mg distance between a monomeric BChl a and a nearby dimeric BChl a is 15.7 Å. The BChl–BChl distances along with relative orientations of the LH1 pigments are virtually the same as those for the corresponding pigments in the Hlr. halophila LH1–LH2 co-complex1, indicating that both BChl–BChl distances and pigment arrangements are not significantly affected by the number of αβ-subunits (that is, the LH1 ring size). Eight all-trans-spirilloxanthins span between the α2- and β2-polypeptides. In addition to the BChls a assigned to either LH1 or RC, an isolated BChl a was identified in the inner space between LH1 and RC on the cytoplasmic side of the membrane (Fig. 2a, Supplementary Fig. 7a). This “extra” BChl a is coordinated from the back by an oxygen atom in one of the head groups of a cardiolipin (Supplementary Fig. 7b) with one of its phosphate group in close proximity to the BChl a macrocycle. The “extra” BChl a also interacts with twelve residues in LH1 α1, α2 and the RC H-subunit through van der Waals contacts (Supplementary Fig. 7c) forming a snugly fitted pocket. In particular, the interacting helix in the H-subunit is located in an insertion region specific to the Hlr. halophila complex (Supplementary Fig. 8) and appears to pull both LH1 α1 and α2 polypeptides toward the RC, slightly deviating them from the LH1 arrangement observed in other LH1-RCs (Supplementary Fig. 7a).
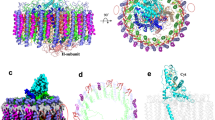
a Tilted view of the LH1 complex looking from the periplasmic side. Multiple polypeptides are shown by cylindrical cartoons and pigments are shown by sticks; the arrow shows an extra BChl a. Color scheme: α1, green; β1, blue; α2, orange; β2, gray; BChl a, red; carotenoids, yellow. b Tilted view of the pigment arrangement in LH1 with representative distances (in Å) between the BChls a. For clarity, phytol chains were omitted. c Side view of the overall structure of the RC complex. The extended C-terminal domain of the Cyt subunit is shown by red color in the dotted ellipse. (d) Side view of the cofactors in the RC. Color schemes are the same as in Fig. 1.
The Hlr. halophila RC reveals a similar protein structure to that of the Tch. tepidum RC (Fig. 2c, Supplementary Fig. 5), with a RMSD of 0.747 Å for the mainchain Cα carbons (PDB: 5Y5S)16. The Hlr. halophila Cyt subunit is truncated at its N-terminal Cys residue and modified by a covalently bound diacylglycerol lipid. A longer C-terminal domain (23 residues) was resolved in the density map for the Hlr. halophila Cyt subunit compared with that of mature Tch. tepidum (Supplementary Fig. 8) and extends around the Cyt subunit surface close to the heme-2 and heme-4 groups. The Hlr. halophila Cyt subunit contains a large number of acidic (Asp and Glu) residues (20% of total amino acids) relative to basic (Lys and Arg) residues (6.8%) as reflected by its highly negative surface charge distribution (Supplementary Fig. 9a). This finding is consistent with that of the strongly acidic Hlr. halophila proteome23, a common characteristic of halophilic bacteria24,25. Co-factor arrangement in the Hlr. halophila RC also resembles that of Tch. tepidum (Fig. 2d, Supplementary Fig. 5b). Based on our cryo-EM density map, one MQ-8 and one UQ-8 were identified unambiguously at the RC QA and QB sites (Supplementary Fig. 4b), respectively. The latter finding is at odds with a conclusion reached by lipophilic analysis and flash-induced absorption changes on the purified LH1–RC complex from a different strain of Hlr. halophila strain SL1T in which MQ-8 was suggested to occupy the QB site22.
The Hlr. halophila iso-3 HiPIP structure
The structure of the Hlr. halophila RC-bound HiPIP was determined from the cryo-EM potential density map with an amino acid sequence of protein ID: WP_201171020, corresponding to a third form (protein ID: WP_011814410) of the HiPIP isozymes encoded in the genome reported for Hlr. halophila strain SL1T 26; this protein is hereafter referred to as iso-3 HiPIP. It is noted that the gene encoding iso-3 HiPIP in the Hlr. halophila BN9622 genome is located within the photosynthetic gene cluster next to bchE and close to bchI and bchJ, suggesting that it is the key photosynthetic electron donor in this species. The Hlr. halophila iso-3 HiPIP is characterized by a long extension of the C-terminus and reveals a two-domain structure composed of an N-terminal functional portion containing the 4Fe–4S cluster and the C-terminus that interacts with the Cyt subunit (Fig. 3a). A fragment between Trp47 and Ala69 was invisible in the cryo-EM density map presumably due to structural disorders. Despite relatively low sequence similarities, the Hlr. halophila RC-bound iso-3 HiPIP exhibited a similar protein conformation and cluster arrangement of its 4Fe–4S-containing functional domain as those of the Tch. tepidum HiPIP27 (PDB: 5D8V, RMSD = 0.728 Å) (Fig. 3) and the iso-1 HiPIP from Hlr. halophila strain BN96268 (PDB: 2HIP, RMSD = 0.783 Å) (Supplementary Fig. 10a). Similar to the unique property reported for the Hlr. halophila iso-1 and iso-2 HiPIPs7, the iso-3 HiPIP also contains a high proportion of acidic residues (20% of total amino acids vs. 7.6% for basic residues) resulting in an overall negatively charged surface (Supplementary Fig. 9b).
a Overall structure of the iso-3 HiPIP. The 4Fe–4S cluster is shown as sticks (S, yellow; Fe, orange). b Superposition of the Cα carbons of the Hlr. halophila iso-3 HiPIP (orange) with those of the Tch. tepidum HiPIP (gray, PDB: 5D8V). c Overlap of the 4Fe–4S clusters between Hlr. halophila iso-3 HiPIP (colored) and Tch. tepidum HiPIP (gray). d Sequence alignment between the Hlr. halophila iso-3 HiPIP and Tch. tepidum HiPIP with identical amino acids (*), conservative (:) and semi-conservative (.). Residues with gray fonts were invisible in the structures. The bridging Leu residues are shown in red fonts.
Interactions between the iso-3 HiPIP and the Cyt subunit
The Hlr. halophila iso-3 HiPIP is tightly associated with the Cyt subunit over its N-terminal functional portion (Pro25–Glu121) and long C-terminal extension (Ala126–Gly142) (Fig. 4a–c, Supplementary Figs. 4b and 11). Most of the residues on the interface involving electron transfer are distributed around the 4Fe-4S cluster and heme-1 group. Despite overall negatively charged surfaces for both HiPIP and the Cyt subunit, the binding surfaces between the two prosthetic groups are essentially neutral (Fig. 4d, e) and formed primarily by uncharged residues: Leu86, Leu87, Ala101, Phe103 and Trp118 in HiPIP (Fig. 4b) and Tyr77, Thr92 and Phe117 in the Cyt subunit (Fig. 4c); this indicates that hydrophobic interactions exist between the two proteins, a feature also reported for the Tch. tepidum HiPIP–Cyt co-complex14. The HiPIP C-terminal extension interacts with surface residues Glu106–Asn161 and Glu298–Arg300 in the Cyt subunit, and several hydrogen bonds were identified between the two proteins in this region (HiPIP132Arg/Cyt109Thr, HiPIP140Arg/Cyt113Asp, HiPIP133Asp/Cyt300Arg) that likely contribute to stabilizing the HiPIP–Cyt complex. This feature may also indicate that iso-3 HiPIP is more tightly bound to the Cyt subunit than is iso-2 HiPIP because only 30–50% of the iso-2 HiPIP was reported to firmly associate with the Cyt subunit9 and two-thirds of total LH1–RC complexes were identified to bind iso-3 HiPIP in our 3D classification analysis.
a Side view of the interface between the Cyt subunit (cyan) and iso-3 HiPIP (orange). Residues with distances <3.8 Å between the two proteins are shown by their sidechains in sticks. Representative close contacts ( < 3.8 Å) are shown by dashed lines. b Front view of the interface for iso-3 HiPIP. c Front view of the interface for Cyt-subunit. d Surface charge distribution on the interface for iso-3 HiPIP with color codes according to the electrostatic potential (red: negative charge, blue: positive charge) in the scale bar. Black dashed ellipse indicates the position of the 4Fe–4S cluster inside. e Surface charge distribution on the interface for the Cyt subunit. Black dashed ellipse indicates the position of the heme-1 group (magenta sticks). f Expanded view of superposition of the Cα carbons for the Cyt subunits between the Hlr. halophila iso-3 HiPIP–Cyt complex (colored) and Tch. tepidum HiPIP–Cyt complex (gray, PDB: 7C52) showing a less tilted angle for the iso-3 HiPIP attached to the Cyt subunit with respect to that of the HiPIP in a Tch. tepidum HiPIP:LH1–RC co-crystal. The angles were measured for the lines connecting the sulfur S1 atom in 4Fe-4S and the Fe atom in heme-1 with respect to the axis connecting the Fe atoms in heme-1 and heme-3.
The Hlr. halophila iso-3 HiPIP binds to the Cyt subunit at a somewhat different angle than that observed in the co-crystallized Tch. tepidum HiPIP–Cyt complex (Fig. 4f, Supplementary Fig. 11d). The Hlr. halophila HiPIP is tilted with an angle of 35.6° for the line connecting the sulfur S1 atom in 4Fe-4S and the Fe atom in heme-1 with respect to the axis connecting the Fe atoms in the heme-1 and heme-3; this angle is reduced by 11° compared with the HiPIP in the Tch. tepidum HiPIP–Cyt co-complex14.
Predicted electron transport pathway
The electron transport chain from Hlr. halophila iso-3 HiPIP to the special pair of the RC via the Cyt subunit spans more than 70 Å and contains 7 redox centers (Fig. 5a). First in this process is a trans-protein electron tunneling step between the 4Fe-4S cluster in HiPIP and the heme-1 in the Cyt subunit over a distance of 11.9 Å between the S1 atom in the 4Fe-4S cluster and the central Fe atom in heme-1 (Fig. 5b). Electron transfer from the 4Fe-4S cluster to heme-1 is likely an energetically “uphill” process because heme-1 in the Hlr. halophila Cyt subunit has a redox potential of +60 mV9 and if we assume a similar potential (~ + 140 mV) for the Hlr. halophila iso-3 HiPIP to that of the iso-2 HiPIP9 based on their compositional similarities and tight association with the Cyt subunit. According to a coarse-grained hopping model calculation (eMap)28, this process is mediated by the Leu87 in HiPIP as a bridging residue (Fig. 5b, Supplementary Fig. 12) located in the middle, ~4 Å away from both S1 in HiPIP and the 21-methyl carbon in heme-1; a Leu-mediated pathway was also predicted for the Tch. tepidum HiPIP–Cyt co-complex14. It is noted that the residue at the corresponding position of this Leu is a Phe in iso-1 and iso-2 HiPIPs (Supplementary Fig. 10c). The following transfer steps within the Cyt subunit involve a through-space sequential inter-heme tunneling process with center-to-center distances of 13.9–16.3 Å (Fig. 5a). The last step of electron transfer to the special pair is relayed by the aromatic ring of Tyr162 in the L-subunit, which is located directly above the BChl a dimer (Fig. 5c). This Tyr residue is conserved in the RC L-subunit of all purple bacteria regardless of whether their RC contains or lacks a Cyt subunit and has been predicted to function as a bridging residue for the electron transfer from either soluble Cyt c229,30 or the membrane-bound Cyt subunit14.
a Predicted trans-protein electron tunneling pathway from the Hlr. halophila iso-3 HiPIP to the RC special pair. Distances were measured as center-to-center distances for the heme groups in the Cyt subunit, distance from the heme-3 Fe atom to the hydroxyl group of Tyr162 in the RC L-subunit and distances from the Tyr162 hydroxyl group to the Mg atoms in the RC BChl a dimer. b Expanded view of the portion of the 4Fe-4S cluster in iso-3 HiPIP and heme-1 in Cyt-subunit; sidechain of the bridging residue Leu87 in the iso-3 HiPIP is shown by sticks. c Expanded view of the portion of heme-3 in the Cyt-subunit and the special pair BChl a dimer. The sidechain of the Tyr162 in the RC L-subunit is shown by sticks.
Discussion
The LH1–RC structure from Hlr. halophila BN9622 described herein is the first for an extremely halophilic and alkaliphilic purple phototroph containing BChl a and is the first native co-complex known that forms with its electron donor, HiPIP. As described in a previous paper1 and this work, the Hlr. halophila strain BN9622 LH1 complex reveals several characteristic features: (i) approximately half of the LH1 complexes form an 18 αβ-subunit ring surrounding an LH2; (ii) the remaining LH1 complexes form a 16 αβ-subunit ring encircling the RC; and (iii) both the LH2- and RC-surrounding LH1 complexes are composed of two pairs of αβ-polypeptides and two layers of BChl a. Despite differences in ring size and the photocomplex they surround, arrangements of the LH1 polypeptides and pigments are almost identical between LH1–LH2 and LH1–RC (within four pairs of LH1, RMSD = 0.299 Å), indicating a variability of the LH1 assemblies only in the number of its subunits, a number that apparently depends on the photocomplex it encircles (Supplementary Fig. 13a). By contrast, superimposition of the LH1 rings between Hlr. halophila and Tch. tepidum (PDB: 5Y5S) revealed large differences with RMSD = 1.361 Å (Supplementary Fig. 13b). However, interactions between the short helix of the RC H-subunit, the LH1 subunits, and the extra molecules of BChl a seem to enhance local stability of the LH1 ring of the Hlr. halophila LH1–RC, allowing it to form rings of different diameters (Supplementary Fig. 7). The structural flexibility of LH1 has been manifestly demonstrated by a Rhodobacter sphaeroides (recently reclassified into the genus Cereibacter) mutant lacking its RC and LH2. The mutant showed that small, medium and large LH1 rings corresponding to 15, 16 or 18 αβ-subunits, respectively, can co-exist in the cell and form a variety of shapes including circles, polygonal rings, open rings, ellipses, and arcs31. No such variations have been observed for LH231. These results together with those obtained herein indicate that assembly of the LH1 is inherently flexible and that the photocomplex that lies within the LH1 likely provides structural constraints guiding formation of a coherent complex with the surrounding LH1, whether or not assisted by the presence of an extra BChl a. The reason why an LH1–LH2 co-complex has not been identified in other purple phototrophs is unknown but may relate to the unusual environment of extremely high salinity and pH in which Hlr. halophila BN9622 thrives (the organism was isolated from a soda lake of nearly 36% total salinity and pH 10.7)2. Several advantages of producing an LH1–LH2 have been discussed in a previous paper1.
Although the Hlr. halophila RC reveals a highly similar structure to those reported for other purple phototrophs (Supplementary Fig. 5), the surface of its periplasm-exposed Cyt subunit is highly negatively charged (Supplementary Fig. 9a) in contrast to the relatively positive surface-charge distributions observed in the acidophilic purple phototroph Rpi. globiformis21 and in the neutrophilic purple phototrophs Tch. tepidum16 and Blastochloris (Blc.) viridis32. The highly negatively charged nature of the Hlr. halophila Cyt subunit is a reflection of its unusually high proportion of acidic residues compared with the RC Cyt subunit from other purple phototrophs33, including alkaliphilic, acidophilic, halophilic, and thermophilic species (Supplementary Fig. 9c). The Hlr. halophila pattern is also characteristic of the Cyt subunit of halophilic, alkaliphilic and thermophilic BChl b-containing species Hlr. halochloris34,35,36 (Supplementary Fig. 9c), suggesting that such a pattern may be restricted to multi-extremophilic purple bacteria. However, it should be noted that while the negatively charged characteristic of the Hlr. halophila Cyt subunit is consistent with the strong acidity of the entire Hlr. halophila proteome23, the Hlr. halochloris proteome does not show such a tendency 23. This may relate to the different major osmoprotectants used by these species for survival in such hypersaline environments: in high salt media, Hlr. halophila cells accumulate KCl23 as a compatible solute, whereas Hlr. halochloris cells accumulate glycine betaine37. At low salinities, Hlr. halophila cells synthesize glycine betaine, and a switching mechanism between KCl and glycine betaine is controlled by salinity in this phototroph38. Generally, acidic residues are more favored than basic residues in terms of water solubility, protein hydration39 and accessible surface area25, leading to an increased protein solubility40 at high ionic strengths. The acidity of the Hlr. halophila proteome is therefore required for protein surface hydration38, and the excessive negative charges on the surface of the Hlr. halophila Cyt subunit may also modulate redox potentials of the heme groups and prevent nonspecific binding with their electron transferring partners (see latter discussion).
Similar to its Cyt subunit, the Hlr. halophila iso-3 HiPIP is also characterized by a negative surface-charge distribution (Supplementary Fig. 9b) from its high proportion of acidic residues (20%). Based on biochemical analyses7, all experimentally examined HiPIPs can be divided into two groups: (1) the HiPIPs from Rpi. globiformis, Rhodovibrio (Rhv.) salinarum (an extreme halophile) and Rvi. gelatinosus which exhibit an average (~ + 330 mV) or higher reduction potential without apparent dependence of protein net charges, and (2) the Hlr. halophila HiPIPs that are highly negatively charged ( ~ 20% acidic residues) and have significantly lower potentials (iso-1: +110 mV; iso-2: +50 mV, measured in isolated forms)6. The potential of the former group is likely a consequence of compensation of the electrostatic forces by modulation of the polarity of the protein matrix, whereas the potential of the latter group is likely due to destabilization of additional negative charges in the reduced state7; that is, excessive negative charges result in lower potentials. Although the exact potential for the Hlr. halophila iso-3 HiPIP identified in our work is unknown, we assume that it has a value within the range between those of iso-1 and iso-2 HiPIPs based on their characteristic amino acid compositions and structural similarities (Supplementary Fig. 10). The redox potentials of all HiPIPs are modulated by the local environment (hydrophobicity, solvation) near the 4Fe–4S cluster7 and the conditions used in measurements9. For example, the Hlr. halophila iso-2 HiPIP shows a significantly higher potential ( + 140 mV) upon binding to the RC compared with that ( + 50 mV) of the isolated protein measured at low salt concentrations9. The isolated HiPIP potential also shifts to a more positive value at high salt concentrations9. These findings indicate that HiPIP reduction potentials can be substantially modified by the properties of the interface formed with electron transferring partners, shifting toward being more positive upon binding of the partner. Because reduction potentials of Hlr. halophila Cyt bc1 and RC are +110 mV (Rieske center) and +270 mV (P+/P)9,22, respectively, the corrected potential of the RC-bound Hlr. halophila HiPIP at physiological salt conditions suggests that it is suitable for shuttling electrons between the two complexes.
Despite the overall negative charge distributions for both the Hlr. halophila Cyt subunit and iso-3 HiPIP as shown here (Supplementary Fig. 9), the interface between the two proteins in their complex is relatively uncharged and hydrophobic (Fig. 4d, e). This is a uniform characteristic of HiPIP–Cyt subunit interactions in purple phototrophs13,14,41,42,43,44 and a required environment for electron tunnelling between the two proteins. The locally hydrophobic nature may also play an important role in facilitating specific binding between Hlr. halophila HiPIP and its Cyt subunit in a well-defined geometry and may also explain the intermediate potential of +60 mV for the outermost heme-1 of the Hlr. halophila Cyt subunit9, a value lower than that of Rvi. gelatinosus (+130 mV)45 but higher than those of Blc. viridis (–60 mV)46 and Alc. vinosum (+10 mV)47.
A half-time (t1/2) of ≤ 11 μsec was determined for electron transfer between the tightly bound Hlr. halophila iso-2 HiPIP and the Cyt subunit9. This value is similar to that observed for Rfx. fermentans (~ 2 μsec)12 but much shorter than half-times measured for Rvi. gelatinosus (300 μsec)10, Rhodocyclus (Rcy.) tenuis (480 μsec)48, Alc. vinosum (~35 msec at low salt concentrations)43 and Tch. tepidum (52 msec)49. The large differences over 4 orders of magnitude between these species may be rationalized by their different binding states (rate-limiting steps)14. The value of t1/2 ≤ 11 μsec observed for the tightly bound Hlr. halophila iso-2 HiPIP–Cyt subunit is in good agreement with that ( ~ 11 μsec) calculated from a docking model based on the Marcus theory9 and similar to that ( ~ 5 μsec) estimated for the Tch. tepidum HiPIP–Cyt subunit from a similar model calculation14. These calculated values represent intrinsic half-time limits for the fastest possible electron transfer between HiPIP and the Cyt subunit in a constantly docking complex. Thus, any t1/2 longer than ~10 μsec may indicate that equilibrium states exist between a firmly complexed form and a freely isolated form. Although we do not know the exact t1/2 value for the iso-3 HiPIP–Cyt subunit complex described here, a fast electron transfer would be predicted on the basis of its firmly associated form (Figs. 1 and 4, Supplementary Fig. 11). In this regard, the Rfx. fermentans HiPIP–Cyt subunit can also be considered a tightly-bound complex like that of Hlr. halophila. By contrast, the HiPIP–Cyt subunits of Rvi. gelatinosus and Rcy. tenuis may form relatively long-lived complexes (slow equilibrium) while the Alc. vinosum and Tch. tepidum HiPIP–Cyt subunits form short-lived complexes in rapid equilibrium. Considering that electron transfer from the Cyt subunit to the special pair is governed by a rate-limiting step typically in the microsecond range50 within the Cyt subunit (heme-2 to heme-4 for Blc. viridis), the electron transfers from HiPIP to the Cyt subunit in Hlr. halophila and Rfx. fermentans with t1/2 ≤ 11 μsec may not be rate-limiting in the donation of electrons to the RC special pair in these species in contrast to those (in the millisecond range) for Alc. vinosum and Tch. tepidum.
Because the Hlr. halophila iso-2 and iso-3 HiPIPs are tightly associated with the Cyt subunit (at least the majorities of them), other soluble electron carriers should be required for donating electrons to the bound HiPIPs. Several c-type soluble Cyt proteins (for example, Cyt c551, Cyt c′) together with HiPIPs, a flavoprotein and a bacterial ferredoxin have been identified in abundance in Hlr. halophila cell extracts9,51. Among these, Cyt c551, Cyt c′ and iso-1 HiPIP have potentials of +58 mV, +30 mV and +110 mV, respectively, suggesting they are all candidates for electron donation to bound HiPIPs, although the functional roles of each of these proteins remain to be elucidated.
Finally, the fact that a novel LH1–LH2 complex1 and a native LH1–RC–HiPIP complex have been discovered in perhaps the most extremophilic of all known purple bacteria is probably no coincidence. The combination of environmental hurdles that Hlr. halophila faces in its punishing natural habitat are unlike those encountered by any other known phototroph. Considering this, it is not surprising that Hlr. halophila has evolved unique structures and metabolic strategies to employ photosynthesis as its means of energy conservation. Hence, continued study of this remarkable phototroph will undoubtedly broaden our understanding of photosynthetic diversity and the limits to which biological photochemistry can occur.
Methods
Preparation and Characterization of the Hlr. halophila LH1–RC-Containing Complexes
Halorhodospira (then Ectothiorhodospira) halophila strain BN9622 was kindly supplied to MTM by Dr. Johannes Imhoff2, GEOMAR Helmholtz Centre for Ocean Research, Kiel, Germany, in November 1988. A confirmation of strain BN9622 as a strain of Hlr. halophila has been published3. The cells were grown phototrophically1. Chromatophores were treated with 1.0% (w/v) n-dodecyl β-D-maltopyranoside (DDM) at 25 °C for 60 min followed by centrifugation at 4 °C and 150,000 × g for 90 min. The extracts were loaded onto a DEAE column (Toyopearl 650S, TOSOH) equilibrated at 8 °C with 20 mM Tris-HCl buffer (pH 8.0) containing 0.1% w/v DDM. The fractions containing LH1–RC–HiPIP and LH1–RC were eluted by a linear gradient of NaCl from 0 mM to 400 mM. The LH1–RC-containing fractions were concentrated for absorption measurements (Supplementary Fig. 2) and assessed by negative-stain EM using a JEM-1010 instrument (JEOL). Light-induced P+/P absorption difference spectra were obtained using a V-780 spectrophotometer (JASCO) by subtracting spectra in the dark from those measured during 940 nm LED illumination at room temperature. Quinones were extracted from chromatophores and analyzed using reverse-phase HPLC52.
Cryo-EM Data Collection
Proteins for cryo-EM were concentrated to ~6 mg/ml, after which 3 microliters of the protein solution were applied on glow-discharged holey carbon grids (200 mesh Quantifoil R2/2 molybdenum) that had been treated with H2 and O2 mixtures in a Solarus II plasma cleaner (Gatan, Pleasanton, USA) for 30 s and then blotted and plunged into liquid ethane at –182 °C using an EM GP2 plunger (Leica, Microsystems, Vienna, Austria). The applied parameters were a blotting time of 5.5 s at 80% humidity and 4 °C. Data were collected on a Titan Krios (Thermo Fisher Scientific, Hillsboro, USA) electron microscope at 300 kV equipped with a Falcon 3 camera (Thermo Fisher Scientific). Movies were recorded using EPU software (Thermo Fisher Scientific) at a nominal magnification of 96 k in counting mode and a pixel size of 0.820 Å at the specimen level with a dose rate of 0.69 e- per physical pixel per second, corresponding to 1.39 e- per Å2 per second at the specimen level. The exposure time was 28.7 s, resulting in an accumulated dose of 40 e- per Å2. Each movie includes 40 fractioned frames.
Image Processing
All of the stacked frames were subjected to motion correction with MotionCor253. Defocus was estimated using CTFFIND454. A total of 331,335 particles were selected from 3194 micrographs using crYOLO55. All selected particles were further analyzed with RELION3.156, and 314,478 particles were selected by 2-D classification and divided into four classes by 3-D classification resulting in only one good class of LH1–RC co-complex containing 135,064 particles. The initial 3-D model was generated in RELION. The 3-D auto refinement without any imposed symmetry (C1) produced a map at 2.48 Å resolution after contrast transfer function (CTF) refinement, Bayesian polishing, masking, and post-processing. Particles were also subjected to subtraction of the detergent micelle density followed by combined focused 3-D classification and 3-D auto refinement to yield the final maps of LH1–RC–HiPIP and HiPIP-free LH1–RC with resolutions of 2.44 Å and 2.64 Å, respectively, according to the gold-standard Fourier shell correlation using a criterion of 0.14357. The local resolution maps were calculated with RELION. Focused 3-D classification58 was conducted for the HiPIP-bound region in the LH1–RC–HiPIP complex using the mask59,60 in RELION until convergence (Supplementary Fig. 1a).
Model Building and Refinement of the LH1–RC–HiPIP and LH1–RC Complexes
The initial atomic model for the LH1–RC–HiPIP structures was generated with MODELLER v10.161 using the atomic models of the Tch. tepidum LH1–RC (PDB: 5Y5S) and HiPIP (PDB: 5D8V). The model was then fitted to the cryo-EM maps obtained for the Hlr. halophila LH1–RC–HiPIP and LH1–RC using Chimera62. Amino acid substitutions and real space refinement for the peptides and cofactors were performed using COOT63. The manually modified models were refined in real-space on PHENIX64, and the COOT/PHENIX refinements were iterated until the refinements converged. Finally, the statistics calculated using MolProbity65 were checked. Figures were drawn using the Pymol Molecular Graphic System (Ver2.5, Schrödinger)66, UCSF Chimera62 and UCSF ChimeraX67.
Electron Tunneling Analysis
The electron transfer pathway from the 4Fe-4S cluster in iso-3 HiPIP to the heme-1 group in Cyt subunit was predicted using eMap (ver. 2.0)28, an online application for identifying and visualizing electron and hole transfer pathways in proteins, which is based on a coarse-grained version of the Pathways model68. In the single protein analysis mode, Leu was included in addition to the default Trp and Tyr as a through-space hopping residue. Default parameters were used throughout the calculation.
Statistics and Reproducibility
Experimental errors for all absorption spectra were within 1 nm. Experimental errors for the HPLC chromatogram were within 0.1 min. The cryo-EM data were collected from multiple grids. Micrographs contaminated with hexagonal ice were excluded from the reconstruction dataset through visual inspection. Data collection, processing, and refinement statistics are summarized in Table 1. All RMSD values for Cα positions between superimposed structures were calculated using PyMOL.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Maps and models of LH1–RC–HiPIP and LH1–RC have been deposited in the EMDB and PDB with the accession codes: EMD-39836, EMD-39837, PDB-8Z82, and PDB-8Z83, respectively. The numerical source values underlying Supplementary Figs. 2, 3 and 5 can be found in supplementary data set 1, 2 and 3, respectively. All other data are available from the authors upon reasonable request.
References
Tani, K. et al. A distinct double-ring LH1–LH2 photocomplex from an extremophilic phototroph. Nat. Commun. https://doi.org/10.1038/s41467-024-55811-9 (2025).
Imhoff, J. F., Hashwa, F. & Trüper, H. G. Isolation of extremely halophilic phototrophic bacteria from the alkaline Wadi Natrun, Egypt. Arch. Hydrobiol. 84, 381–388 (1978).
Imhoff, J. F., Kyndt, J. A. & Meyer, T. E. Genomic comparison, phylogeny and taxonomic reevaluation of the Ectothiorhodospiraceae and description of Halorhodospiraceae fam. nov. and Halochlorospira gen. nov. Microorganism 10, 295 (2022).
Leguijt, T. & Hellingwerf, K. J. Characterization of reaction center/antenna complexes from bacteriochlorophyll a containing Ectothiorhodospiraceae. Biochim. Biophys. Acta 1057, 353–360 (1991).
Lyumkis, D. Challenges and opportunities in cryo-EM single-particle analysis. J. Biol. Chem. 294, 5181–5197 (2019).
Przysiecki, C. T., Meyer, T. E. & Cusanovich, M. A. Circular dichroism and redox properties of high redox potential ferredoxins. Biochemistry 24, 2542–2549 (1985).
Heering, H. A., Bulsink, Y. B. M., Hagen, W. R. & Meyer, T. E. Influence of charge and polarity on the redox potentials of high-potential Iron–sulfur proteins: evidence for the existence of two groups. Biochemistry 34, 14675–14686 (1995).
Breiter, D. R., Meyer, T. E., Rayment, I. & Holden, H. M. The molecular structure of the high potential iron-sulfur protein isolated from Ectothiorhodospira halophila determined at 2.5-Å resolution. J. Biol. Chem. 266, 18660–188667 (1991).
Lieutaud, C., Alric, J., Bauzan, M., Nitschke, W. & Schoepp-Cothenet, B. Study of the high-potential iron sulfur protein in Halorhodospira halophila confirms that it is distinct from cytochrome c as electron carrier. Proc. Natl. Acad. Sci. USA 102, 3260–3265 (2005).
Schoepp, B. et al. In vivo participation of a high potential iron–sulfur protein as electron donor to the photosynthetic reaction center of Rubrivivax gelatinosus. Biochemistry 34, 11736–11742 (1995).
Lieutaud, C., Nitschke, W., Verméglio, A., Parot, P. & Schoepp-Cothenet, B. HiPIP in Rubrivivax gelatinosus is firmly associated to the membrane in a conformation efficient for electron transfer towards the photosynthetic reaction centre. Biochim. Biophys. Acta 1557, 83–90 (2003).
Hochkoeppler, A. et al. Kinetics of photo-induced electron transfer from high-potential iron–sulfur protein to the photosynthetic reaction center of the purple phototroph Rhodoferax fermentans. Proc. Natl. Acad. Sci. USA 93, 6998–7002 (1996).
Osyczka, A. et al. Comparison of the binding sites for high-potential iron-sulfur protein and cytochrome c on the tetraheme cytochrome subunit bound to the bacterial photosynthetic reaction center. Biochemistry 38, 15779–15790 (1999).
Kawakami, T. et al. Crystal structure of a photosynthetic LH1-RC in complex with its electron donor HiPIP. Nat. Commun. 12, 1104 (2021).
Niwa, S. et al. Structure of the LH1-RC complex from Thermochromatium tepidum at 3.0 Å. Nature 508, 228–232 (2014).
Yu, L.-J., Suga, M., Wang-Otomo, Z.-Y. & Shen, J.-R. Structure of photosynthetic LH1-RC supercomplex at 1.9 Å resolution. Nature 556, 209–213 (2018).
Tani, K. et al. Cryo-EM structure of a Ca2+-bound photosynthetic LH1-RC complex containing multiple αβ-polypeptides. Nat. Commun. 11, 4955 (2020).
Tani, K. et al. Cryo-EM structure of the photosynthetic LH1-RC complex from Rhodospirillum rubrum. bioRxiv, https://doi.org/10.1101/2021.05.30.446358 (2021).
Qian, P. et al. Cryo-EM structure of the Rhodospirillum rubrum RC-LH1 complex at 2.5 Å. Biochem. J. 478, 3253–3263 (2021).
Tani, K. et al. A Ca2+-binding motif underlies the unusual properties of certain photosynthetic bacterial core light–harvesting complexes. J. Biol. Chem. 298, 101967 (2022).
Tani, K. et al. An LH1–RC photocomplex from an extremophilic phototroph provides insight into origins of two photosynthesis proteins. Commun. Biol. 5, 1197 (2022).
Schoepp-Cothenet, B. et al. Menaquinone as pool quinone in a purple bacterium. Proc. Natl Acad. Sci. USA 106, 8549–8554 (2009).
Deole, R., Challacombe, J., Raiford, D. W. & Hoff, W. D. An extremely halophilic Proteobacterium combines a highly acidic proteome with a low cytoplasmic potassium content. J. Biol. Chem. 288, 581–588 (2013).
Fukuchi, S., Yoshimune, K., Wakayama, M., Moriguchi, M. & Nishikawa, K. Unique amino acid composition of proteins in halophilic bacteria. J. Mol. Biol. 327, 347–357 (2003).
Tadeo, X. et al. Structural basis for the aminoacid composition of proteins from halophilic archea. PLoS Biol. 7, e1000257 (2009).
Challacombe, J. F. et al. Complete genome sequence of Halorhodospira halophila SL1. Stand. Genomic Sci. 8, 206–214 (2013).
Hirano, Y., Takeda, K. & Miki, K. Charge-density analysis of an iron-sulfur protein at an ultra-high resolution of 0.48 Å. Nature 534, 281–284 (2016).
Tazhigulov, R. N., Gayvert, J. R., Wei, M. & Bravaya, K. B. eMap: A web application for identifying and visualizing electron or hole hopping pathways in proteins. J. Phys. Chem. B 123, 6946–6951 (2019).
Axelrod, H. L. et al. X-ray structure determination of the cytochrome c2:reaction center electron transfer complex from Rhodobacter sphaeroides. J. Mol. Biol. 319, 501–515 (2002).
Tani, K. et al. Cryo-EM structure of the photosynthetic LH1-RC complex from Rhodospirillum rubrum. Biochemistry 60, 2483–2491 (2021).
Bahatyrova, S. et al. Flexibility and size heterogeneity of the LH1 light-harvesting complex revealed by atomic force microscopy. J. Biol. Chem. 279, 21327–21333 (2004).
Qian, P., Siebert, C. A., Wang, P., Canniffe, D. P. & Hunter, C. N. Cryo-EM structure of the Blastochloris viridis LH1-RC complex at 2.9 Å. Nature 556, 203–208 (2018).
Nagashima, K. V. P., Sakuragi, Y., Shimada, K. & Matsuura, K. Comparative analysis of the primary structure of the reaction center-bound cytochrome subunit ini purple bacteria. Photosynth. Res. 55, 349–355 (1998).
Kimura, Y. et al. Electrostatic charge controls the lowest LH1 Qy transition energy in the triply extremophilic purple phototrophic bacterium, Halorhodospira halochloris. Biochim. Biophys. Acta –Bioenergetics 1862, 148473 (2021).
Kimura, Y. et al. Salt- and pH-dependent thermal stability of photocomplexes from extremophilic bacteriochlorophyll b-containing Halorhodospira species. Microorganisms 10, 959 (2022).
Qi, C.-H. et al. Structural insights into the unusual core photocomplex from a triply extremophilic purple bacterium, Halorhodospira halochloris. J. Integr. Plant Biol. 66, 2262–2272 (2024).
Galinski, E. A. & Trüper, H. G. Betaine, a compatible solute in the extremely halophilic phototrophic bacterium Ectothiorhodospira halochloris. FEMS Microbiol. Lett. 13, 357–360 (1982).
Deole, R. & Hoff, W. D. A potassium chloride to glycine betaine osmoprotectant switch in the extreme halophile Halorhodospira halophila. Sci. Rep. 10, 3383 (2020).
Trevino, S. R., Scholtz, J. M. & Pace, C. N. Amino acid contribution to protein solubility: Asp, Glu, and Ser contribute more favorably than the other hydrophilic amino acids in RNase Sa. J. Mol. Biol. 366, 449–460 (2007).
Kramer, R. M., Shende, V. R., Motl, N., Pace, C. N. & Scholtz, J. M. Toward a molecular understanding of protein solubility: increased negative surface charge correlates with increased solubility. Biophys. J. 102, 1907–1915 (2012).
Bertini, I., Gaudemer, A., Luchinat, C. & Piccioli, M. Electron self-exchange in high-potential iron–sulfur proteins. Characterization of protein I from Ectothiorhodospira vacuolata. Biochemistry 32, 12887–12893 (1993).
Osyczka, A. et al. Different mechanisms of the binding of soluble electron donors to the photosynthetic reaction center of Rubrivivax gelatinosus and Blastochloris viridis. J. Biol. Chem. 276, 24108–24112 (2001).
Venturoli, G. et al. Electron transfer from HiPIP to the photooxidized tetraheme cytochrome subunit of Allochromatium vinosum reaction center: new insights from site-directed mutagenesis and computational studies. Biochemistry 43, 437–445 (2004).
Ciurli, S. & Musiani, F. High potential iron-sulfur proteins and their role as soluble electron carriers in bacterial photosynthesis: tale of a discovery. Photosynth. Res. 85, 115–131 (2005).
Nitschke, W., Agalidis, I. & Rutherford, A. W. The reaction-centre associated cytochrome subunit of the purple bacterium Rhodocyclus gelatinosus. Biochim. Biophys. Acta 1100, 49–57 (1992).
Dracheva, S. M. et al. Electrogenic steps in the redox reactions catalyzed by photosynthetic reaction-center complex from Rhodopseudomonas viridis. Eur. J. Biochem. 171, 253–264 (1988).
Nitschke, W., Jubault-Bregler, M. & Rutherford, A. W. The reaction center associated tetraheme cytochrome subunit from Chromatium vinosum revisited: A reexamination of its EPS properties. Biochemistry 32, 8871–8879 (1993).
Menin, L., Schoepp, B., Parot, P. & Verméglio, A. Photoinduced cyclic transfer in Rhodocyclus tenuis cells: participation of HiPIP or Cyt c8 depending on the ambient redox potential. Biochemistry 36, 12183–12188 (1997).
Kobayashi, M., Saito, T., Takahashi, K., Wang, Z.-Y. & Nozawa, T. Electronic properties and thermal stability of soluble redox proteins from a thermophilic purple sulfur photosynthetic bacterium, Thermochromatium tepidum. Bull. Chem. Soc. Jpn. 78, 2164–2170 (2005).
Nitschke, W. & Dracheva, S.M. Reaction center associated cytochromes. in Anoxygenic Photosynthetic Bacteria (eds. Blankenship, R.E., Madigan, M.T. & Bauer, C.E.) 775-805 (Kluwer Academic Publishers, Dordrecht, The Netherlands, 1995).
Meyer, T. E. Isolation and characterization of soluble cytochromes, ferredoxins and other chromophoric proteins from the halophilic phototrophic bacterium Ectothiorhodospira halophila. Biochim. Biophys. Acta 806, 175–183 (1985).
Kimura, Y. et al. Characterization of the quinones in purple sulfur bacterium Thermochromatium tepidum. FEBS Lett. 589, 1761–1765 (2015).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Wagner, T. & Raunser, S. The evolution of SPHIRE-crYOLO particle picking and its application in automated cryo-EM processing workflows. Commun. Biol. 3, 61 (2020).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Bai, X. C., Rajendra, E., Yang, G., Shi, Y. & Scheer, S. H. Sampling the conformational space of the catalytic subunit of human gamma-secretase. eLife 4, e11182 (2015).
Roh, S. H. et al. Subunit conformational variation within individual GroEL oligomers resolved by Cryo-EM. Proc. Natl. Acad. Sci. USA 114, 8259–8264 (2017).
Yamaguchi, H. et al. Structural insights into thermostabilization of leucine dehydrogenase from its atomic structure by cryo-electron microscopy. J. Struct. Biol. 205, 11–21 (2019).
Šali, A. & Blumdell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Cryst. D66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Cryst. D66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr 66, 12–21 (2010).
DeLano, W.L. The PyMOL molecular graphics system, DeLano Scientific, LCC, San Carlos, CA USA. (2004).
Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, editors, and developers. Protein Sci. 30, 70–82 (2021).
Balabin, I. A., Hu, X. & Beratan, D. N. Exploring biological electron transfer pathway dynamics with the Pathways plugin for VMD. J. Comput. Chem. 33, 906–910 (2012).
Acknowledgements
We thank Mahiro Yamashita for providing technical assistance. This research was partially supported by Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Numbers JP20am0101118 (support number 1758) and JP20am0101116 (support number 1878), 17am0101116j0001, 18am0101116j0002 and 19am0101116j0003. R.K., E.R.P, M.H., and B.M.H. acknowledge the generous support of the Okinawa Institute of Science and Technology (OIST), Scientific Computing & Data Analysis Section at OIST and the Japanese Cabinet Office. This work was supported in part by JSPS KAKENHI Grant Numbers 20H05086, 20H02856, 23K05822, 24K01620, 22K06144 and 24H02084, Japan. K.T. was supported in part by the Center for Quantum and Information Life Sciences, University of Tsukuba. L.-J.Y. acknowledges support of the National Key R&D Program of China (No. 2019YFA0904600). M.T.M. was supported in part by NASA Cooperative Agreement 80NSSC21M0355. R.K. acknowledges support from Prof. Tsumoru Shintake.
Author information
Authors and Affiliations
Contributions
Z.-Y.W.-O., Y.K., and K.T. designed the work, M.T.M. provided materials, K.T., R.K., K.V.P.N., M.K., N.H., K.N., S.N., K.I., S.T., E.R.P. and M.H. performed the experiments, K.T., R.K., K.V.P.N., L.-J.Y., Y.K., M.T.M., A.M., B.M.H. and Z.-Y.W.-O. analyzed data, Z.-Y.W.-O., K.T., Y.K. and M.T.M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Laura Rodríguez Pérez and Christina Karlsson-Rosenthal. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tani, K., Kanno, R., Nagashima, K.V.P. et al. A Native LH1–RC–HiPIP Supercomplex from an Extremophilic Phototroph. Commun Biol 8, 42 (2025). https://doi.org/10.1038/s42003-024-07421-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-07421-w
This article is cited by
-
A distinct double-ring LH1–LH2 photocomplex from an extremophilic phototroph
Nature Communications (2025)