Abstract
The retrosplenial cortex (RSP) is a complex brain region with multiple interconnected subregions that plays crucial roles in various cognitive functions, including memory, spatial navigation, and emotion. Understanding the afferent and efferent connectivity of the RSP is essential for comprehending the underlying mechanisms of its functions. Here, via viral tracing and fluorescence micro-optical sectioning tomography (fMOST), we systematically investigated the anatomical organisation of the upstream and downstream circuits of glutamatergic and GABAergic neurons in the dorsal and ventral RSP. The cortical connections of the RSP show laminar organisation in which the input neurons are distributed more in the deeper layers of the upstream cortex. Although different types of neurons have similar upstream circuits, GABAergic neurons show bidirectional connections with the hippocampus, whereas glutamatergic neurons only show unidirectional connections. Moreover, GABAergic neurons receive more inputs from the primary sensory cortex than from the prefrontal cortex and association cortex. The dorsal and ventral subregions have preferred circuits such that the dorsal RSP exhibits spatially topological connections with the dorsal visual cortex and lateral thalamus. The systematic study on long-range connections across RSP subregions and cell types may provide useful information for future revealing of RSP working mechanisms.
Similar content being viewed by others
Introduction
The retrosplenial cortex (RSP) is a large area in the cortex that functions as a hub in many core networks of brain regions1, such as the hippocampal formation, the anterior thalamic nuclei2, and the frontal cortex3. Through different connections, the RSP is associated with a range of cognitive functions, including episodic memory, navigation, imagination, and future planning4,5,6,7. To reveal how the RSP is precisely organised into cognitive behavioural processes, the anatomical structure of the complex circuits should be dissected.
The subregions of the RSP integrate interoceptive and exteroceptive information from various brain regions8. It is uncontroversial that the dorsal and ventral subregions of the RSP preferentially connect with different curcuits9. Notably, the dorsal RSP (RSPd) is primarily connected to the visual cortex (VIS)10, where it receives visual cues from the VIS and is involved in feature extraction of retinotopic information and the generation of spatiotemporal visual information11. In contrast, the ventral RSP (RSPv) serves as a node for information exchange, connecting the hippocampal formation, the midline limbic, and the thalamus, which contribute to the modulation of functions, such as spatial and contextual information12. However, the specific contribution of the RSP in functional networks is still not clear and may require a high level of anatomical precision. Moreover, both glutamatergic (GLU) and GABAergic neurons span different layers of the RSP13,14. Several types of glutamatergic neurons receive long-range inputs from hippocampal inhibitory and thalamic excitatory sources15. These networks contribute to the long-lasting storage of fear-inducing context memories16. GABAergic neurons are also involved in cortico-cortical interactions17,18,19. It is evident that understanding the long-range projection preferences of type-specific neurons is also critical to the hierarchical information flow of the brain20. Nevertheless, current studies have focused mainly on circuit resolution in glutamatergic neurons and an understanding of GABAergic neuronal connectivity is lacking. Therefore, the systematic dissection of upstream and downstream circuit connectivity of type-specific neurons in RSP subregions is beneficial to help illustrate the specific contributions of the RSP in cognitive functional networks to understand its combined effects, paving the way for subsequent experimental explorations from various perspectives, such as lesion-induced impairments and electrophysiology. Owing to differences in the functional networks involved in the RSP at different positions on the A‒P axis, we chose the intermediate position for our study.
In this study, we employed a combination of viral tracing and the fluorescence micro-optical sectioning tomography (fMOST) system21 to acquire high-resolution and continuous input‒output circuit maps of type-specific neurons in the RSP throughout the entire brain. The results provide valuable insights into target specificity, cell-type specificity, and the topological organisation of the dorsal and ventral subregions of the RSP in relation to their connectivity with the isocortex, hippocampus, and thalamus. Moreover, we also observed that different subregions of the isocortex exhibit distinct layer-specific and hierarchical cortical coherence patterns in their innervation of the RSP. Dissecting the brain-wide connectivity patterns of type-specific neurons in the RSP is extremely important for further systematic interpretation of the complexity in terms of network mechanisms and functions.
Results
Distribution pattern of inputs and outputs of RSP in the whole brain
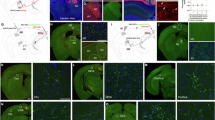
To comprehensively map the connectome of the RSP, we used viral tracing to label the upstream and downstream circuits of the dorsal and ventral RSP. Emx1-ires-Cre and Vgat-ires-Cre mice were used because of their high selectivity for glutamatergic and GABAergic neurons, respectively22,23. To explore the upstream circuits, we utilised modified rabies virus (RV-EnvA-△G-eGFP) with helper viruses, rAAV-EF1α-DIO-mCherry-F2A-TVA-WPRE-hGH, and rAAV-EF1α-DIO-RG24, which were injected into the RSPd or RSPv (Fig. 1a). For accuracy, we counted the number of starter cells, and apart from a small number of starter cells with low counts, most of the counts were within the range of 100–500, in which case the input strength obtained can ignore the effect of the number of starter cells25 (Supplementary Fig. 1a). We found a small number of starter cells located outside the RSP, including in the ACA, MOs, PTLp, SUB, etc. (Supplementary Fig. 1b). To ensure accuracy, we counted the distribution of starter cells in >70% of the RSP. The majority of starter cells in the RSPd and RSPv were distributed at −1.15 to −4.08 mm from bregma (Supplementary Fig. 1d). The eGFP-labelled input cells were widely distributed throughout the entire brain. To evaluate the possibility of virus leakage, we injected rAAV-EF1α-DIO-mCherry-F2A-TVA-WPRE-hGH, rAAV-EF1α-DIO-RG, and RV-EnvA-△G-eGFP into wild-type mice. Weak fluorescent signals were present only at the injection sites (Supplementary Fig. 1e–g), indicating the specificity of the viruses.
a, b Schematic illustrating the viral tracing for input (a) and output (b) circuits to type-specific neurons in RSP. c Schematic of whole-brain mapping and data processing. Step 1: viral tracing and sample preparation; Step 2: fMOST imaging; Step 3: data processing including soma identification (upper) and axonal fibre analysis (bottom); Step 4: 3D visualisation and quantitative statistics. d Anatomical location of injection sites in two subregions of RSP. The coronal section showed the labelled neurons with mCherry and eGFP. Scale bar: 100 μm. e, f Location of all injection sites corresponding panels of the reference atlas. e represents input inject sites; f represents output inject sites. Different colours represent different RSP subregions and neuron types. g Three-dimensional visualisation depicting input cells and axonal projection of both glutamatergic and GABAergic neurons in RSPd and RSPv in representative samples. The graph in the upper left represents the proportion of cells corresponding to the bregma position. The x-axis represents the bregma position, and the y-axis represents the proportion of cells. Colours indicate input cells or fibres from different types of neurons: RSPd GLU, pink; RSPd GABA, blue; RSPv GLU, orange; RSPv GABA, green.
Similarly, to label the axonal projections of glutamatergic and GABAergic neurons in the RSP, we injected sites in Emx1-ires-Cre and Vgat-ires-Cre mice, respectively (Fig. 1b). The labelled sites are depicted in Fig. 1d, f. Notably, the axons were widely distributed across various cortical and subcortical regions (Supplementary Fig. 2). We similarly counted the distribution of output starter cells and found that, with the exception of a small number of starter cells located in the RSP (Supplementary Fig. 1c), both the input and output starter cells were widely distributed throughout the layers of the RSP. Most of the output starter cells were able to cover from −1.15 to −2.88 mm on the A‒P axis and a few were able to cover from −1.15 to −4.08 mm (Supplementary Fig. 1d).
To obtain a high-resolution dataset of whole-brain inputs and outputs of the RSP, the labelled mouse brains were embedded in resin and subjected to continuous high-resolution whole-brain imaging via the fMOST system21. To summarise the information from the imaging results, pipelines were established for the automatic analysis of the connectivity strength between the RSP and other brain regions (Fig. 1c). Specifically, the eGFP-labelled cells and the fluorescent signals of the efferent fibres were analysed separately.
The three-dimensional distribution revealed that the upstream areas of both types of neurons in the same target region were similar (Fig. 1g). Glutamatergic neurons in the RSP prefer to receive input from distal regions, such as the isocortex, hypothalamus, midbrain, and hindbrain. In contrast, GABAergic neurons tend to receive input from adjacent regions around the injection site. There were almost no RV-eGFP-labelled input cells found in the hypothalamus, midbrain, or hindbrain regions among the GABAergic neurons (Fig. 1g).
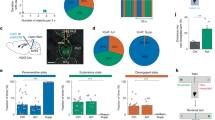
In particular, we identified 50 upstream brain anatomical regions that displayed strong connectivity with both glutamatergic and GABAergic neurons in RSP subregions. To account for the variability in the total number of input cells among the different samples, we normalised the data by calculating the fraction of input cells (which represented the input strength) in each upstream region. To assess the strength of the connections from major functional regions to the RSP, a quantitative brain-wide input matrix across all experiments was constructed. The vast majority of the areas upstream of the RSP originated from the isocortex, accounting for approximately 60% of the total inputs to the whole brain (Fig. 2a). Notably, specific subregions of the isocortex, such as the primary motor area (MOs), primary somatosensory area (SSp), visual cortex (VIS), anterior cingulate area (ACA), and posterior parietal association areas (PTLp), provided the strongest inputs to the RSP. The RSP also received significant inputs from the subcortex, primarily in regions such as the CA1, subiculum (SUB), claustrum (CLA), anteroventral nucleus (AV), anteromedial nucleus (AM), anterodorsal nucleus (AD), and lateral dorsal nucleus (LD) (Fig. 2a and Supplementary Fig. 2a), which is consistent with previous reports26,27.
a Matrix displaying the brain regions projecting to RSP subregions. b The axonal projections from glutamatergic and GABAergic neurons. Each row represents the mean fraction of total inputs or projection signals per structure in each sample. c, d Comparison of inputs (c) and projection (d) between glutamatergic and GABAergic neurons within the same target region and between different target regions. Each point represents the proportion of neuronal inputs or outputs for each subregion or specific type of neuron. The red line indicates a 95% confidence interval.
Moreover, Pearson correlation coefficients (R) were calculated to evaluate the consistency of the strength of the upstream inputs (Fig. 2c). The results demonstrated that the correlation coefficients between the input patterns of different neuron types within the same target (rho = 0.7942, one-way ANOVA) were greater than those of the same neuron type in different targets (rho = 0.7167, one-way ANOVA). Additionally, the correlation coefficients were greater both within and between different neuron types in the different targets. This finding revealed the existence of similar global input patterns across different subregions of the RSP. Although glutamatergic neurons in the RSP receive a greater proportion of inputs from distal brain regions than GABAergic neurons do, there was still a high degree of consistency in the pattern of presynaptic inputs involved in type-specific neurons within the same RSP subregion.
A three-dimensional model revealed that the axonal projections of glutamatergic neurons covered a wider range, extended through various regions, including the striatum, basal forebrain, thalamus, and hypothalamus, and ultimately reached more distal brain regions, such as the midbrain and hindbrain (Fig. 1g). In contrast, the axonal fibres of GABAergic neurons were almost exclusively distributed in forebrain regions, particularly in areas such as the olfactory, CA1, and medial septal (MS) nuclei (Fig. 1g).
We then quantified the output strengths (normalised to the fraction of projecting fibres, defined as the output strength) of both glutamatergic and GABAergic neurons in each RSP subregion projecting to 50 downstream regions (Fig. 2b). Compared with GABAergic neurons, glutamatergic neurons showed a broader fibre distribution, and the projection fibres of the former were more distributed in the regions of the caudoputamen (CP), globus pallidus (GP), thalamus, hypothalamus, superior colliculus (SC), and hindbrain (Fig. 2b), which is consistent with the results observed in the 3D model. With respect to the downstream regions, both the dorsal and ventral RSP axon fibres were concentrated in isocortical areas, including the MOp, MOs, SSp, VIS, ACA, and PTLp, followed by subcortical regions such as the CA1 and CP. Interestingly, in the basal forebrain, the GP, thalamus, hypothalamus, midbrain, and hindbrain regions received the majority of axon fibres from the RSPd, whereas the number of axon fibres originating from the RSPv were fewer or even absent in these regions. Conversely, the axon fibres of the RSPv were primarily distributed in the olfactory, postsubiculum (POST) and SUB, MS and diagonal band nucleus (NDB) (Fig. 2b and Supplementary Fig. 2b). These results suggest that differences in the Cre line influence the breadth of neuronal involvement in connectivity, whereas differences in subregions allow neurons to participate in specific network connections.
Although there were significant spatial differences in the projection target areas of the two types of specific neurons from the same neuronal source (Fig. 1g and Supplementary Fig. 2b), there was some consistency in their overall projection patterns (rho = 0.9161, one-way ANOVA) (Fig. 2d). Furthermore, when we examined the Pearson correlation coefficients of the same neuron type between different sources, we found it to be only 0.5359 (rho = 0.5359, one-way ANOVA) (Fig. 2d). Our results indicated that the projection patterns of the two RSP subregions and their downstream targets significantly differed from each other.
Topological connectivity between RSP and isocortex
To better understand the differences among the mapping relationships in the isocortex and the RSP in different Cre lines in the isocortex and the RSP, we analysed the strength of the upstream and downstream connectivity between different regions of the 38 modules in the isocortex and two RSP subregions (Fig. 3a and Supplementary Fig. 3a). We found that the patterns of cortical inputs to the two RSP subregions were similar in both intensity and topography (Fig. 3a, b). Clustered inputs were observed in the ACA and MOs, followed by the SSp, VIS, and PTLp. However, there were regions from which the fraction of inputs to the RSPd was significantly greater than that to the RSPv (Fig. 3a). These regions included the medial orbital area (ORBm) (p = 0.0048), dorsal auditory area (AUDd) (p = 0.0238), primary auditory area (AUDp) (p = 0.015), and ventral auditory area (AUDv) (p = 0.0224). Furthermore, the layer distributions of the monosynaptic inputs in the isocortex revealed that most functional subregions, such as the MOp, MOs, SSp, VIS, ACA, and PTLp, had strong inputs to both the RSPd and RSPv at L2/3, L5, and L6, whereas other subregions supplied fewer or no inputs from L5 or L6 (Fig. 3d).
a The 39 subregions in the isocortex projects to RSPd (left) and RSPv (right). Two colours represent inputs to glutamatergic and GABAergic neurons, respectively. One-way ANOVA followed by Tukey’s post hoc tests. *p < 0.05, **p < 0.01, ****p < 0.0001. Mean ± SEM. RSPd GLU, n = 4; RSPd GABA, n = 4; RSPv GLU, n = 3; RSPv GABA, n = 4. b, c Topographic distribution of isocortex-RSP inputs (b) and axonal projections (c) to RSP subregions. The shade of the colour indicates the mean fraction of connection strength. d The connectivity between RSP and layer distribution of the whole isocortex. e, f Correlation and hierarchical clustering analysis depicting the similarities and variances in isocortex-RSP connectivity between RSP subregions. The heatmap represents Pearson’s correlation coefficient matrix. g Representative images of input cells (upper) and projecting axons (bottom) from RSP subregions to the visual area (100 μm thick coronal slice). Scale bar: 100 μm. h The connectivity between RSPd and layer distribution of the VIS. i, j Correlation and hierarchical clustering illustrating the variances in brain regions connected with inputs (i) and projections (j) in VIS.
To investigate whether there were multiple input subnetworks to the RSP from each subregion in the isocortex, we conducted unsupervised hierarchical clustering of the Pearson correlation coefficient matrices of the inputs between each of the ten major subregions in the isocortex and the RSP (Fig. 3e). The analysis revealed the possibility of four input subnetworks in the RSP, the first consisting of the prefrontal module of the MOs and ACA; the second consisting of the association module of the AI and PTLp; the third consisting of the primary sensory module of the supplemental somatosensory area (SSs), MOp, and SSp; and the last consisting of the VIS, AUD, PL, IL, and ORB, which exhibited various primary sensory modules, association modules, and prefrontal modules of cortical hierarchies (Fig. 3e). Interestingly, the first three groups shared the same cortical hierarchy, with the exception of the primary sensory module’s preference for projections to GABAergic neurons. All other modalities had general preferences for projections to glutamatergic neurons. These results reflect the fact that cortical regions with the same hierarchical modules share a common pattern of inputs to the RSP. Moreover, the primary sensory modules have very different patterns of network modulation of GABAergic neurons in the RSP compared with the effects of other modules on RSP glutamatergic neurons.
Most cortical regions have certain cortical inputs to both RSP subregions, but projection fibres from the RSP are distributed in only a few regions, mainly the ACA, MOs, MOp, SSp, VIS, and PTLp, with similar upstream regions (Fig. 3a, c, and Supplementary Fig. 3a). However, the connection strength and spatial pattern between the two RSP subregions or type-specific neurons in the downstream circuits were quite different. For example, the MOs (p = 0.0299) and PTLp (p = 0.001) had significantly greater proportions of inputs to RSPd GABAergic neurons than RSPv GABAergic neurons. On the other hand, the proportion of inputs to RSPv glutamatergic neurons was significantly greater than that to RSPd glutamatergic neurons, with a similar preference for the SSp and VIS (Fig. 3a). The efferent axons of the RSPd or RSPv were widely distributed in each layer of the downstream targets without layer specificity (Fig. 3d). Their projection patterns were classified into four groups via unsupervised hierarchical clustering (Fig. 3f).
The VIS was consistent with most cortical–RSP connectivity, with VIS inputs to the RSP visual signals far exceeding the reverse projections it received, and the former had a clear preference for inputs to the RSPd (Fig. 3a, g, and Supplementary Fig. 3a-b), which has been confirmed by multiple lines of evidence12. We found that glutamatergic neurons play a key role in both the RSPd and RSPv, receiving inputs preferentially from the VIS. Moreover, efferent fibres distributed in the VIS mainly originated from the glutamatergic neurons in the RSP subregions (Supplementary Fig. 3a, b).
Furthermore, to explore the connectivity between the RSPd and VIS, correlation coefficients were calculated via experiments within the same target (Fig. 3i, j). The mouse visual system was categorised into ventral streams [lateral visual area (VISl), laterointermediate area (VISli), posterolateral area (VISpl), and postrhinal area (VISpor)] and dorsal streams [anterolateral area (VISal), rostrolateral visual area (VISrl), anterior area (VISa), anteromedial visual area (VISam), and VISpm] based on the anatomical position28,29. This clustering suggested the possibility of two distinct subnetworks within the visual cortex: a dorsal stream comprising the VISam, the VISal, and the VISpm, and another stream (Fig. 3i). The output pattern of the RSPd to the VIS showed a similar subnetwork: a dorsal stream of the VISam and the VISpm, and the other regions (Fig. 3j). The striking similarity indicated a strict topological correspondence followed by subnetwork flows between RSPd and the dorsal VIS. On the other hand, neurons interacting with the RSPd were distributed mainly in the deeper layers of the VIS. For example, the inputs to both neuron types in the RSPd were distributed in VIS L5, followed by L2/3 and L6. This similar layer projection, however, diverged in the projecting circuits, with both glutamatergic and GABAergic neurons projecting predominantly to the deeper layer of the VIS (L6), whereas GABAergic neurons had a heavier projection towards the superficial layer of the VIS (L1) (Fig. 3h). These results highlight the special importance of GABAergic neurons in the RSPd for superficial VIS projections, revealing differences in the innervation patterns of the two types of glutamatergic and GABAergic neurons innervating projections from different layers of the cortico-cortical network.
In general, there is a strict topological correspondence between the bidirectional connectivity of the dorsal RSP and the dorsal VIS (Fig. 7c). The glutamatergic system plays a crucial role in both subregions of the RSP, which is capable of simultaneously receiving and projecting back to the VIS, whereas the GABAergic system in the RSPv only receives unidirectional modulation from the visual cortex.
Topological connectivity between RSP and thalamus
The thalamic nuclei have strong connectivity with the RSPd and RSPv and are potentially involved in the regulatory mechanisms of functions related to head direction and locomotion8. The input cells of the RSPd were found to have an abundant distribution in the anterior and lateral thalamus, including the AV, AM, AD, LD, and lateral posterior nucleus (LP), but were sparsely distributed in the ventral thalamus, such as the ventral anterior‒lateral complex (VAL), ventral medial nucleus (VM), and ventral posterior complex (VP) (Fig. 4a, c). Further analysis via one-way ANOVA revealed that, compared with RSPv glutamatergic neurons, neurons in the LP (p = 0.0061) or LD (p = 0.0353) provided a significantly greater proportion of inputs to RSPd glutamatergic neurons (Fig. 4d). This observation points to a distinct preference for RSPd inputs from the lateral thalamus. Additionally, we observed that RSPd efferent fibres labelled with eGFP were distributed mainly in the lateral and ventral thalamus, specifically in the VP, LP, LD, and reticular nucleus (RT), followed by the anterior thalamus (Fig. 4b, c). We also observed a significant difference in the proportion of RSPd outputs to the VP (p < 0.0001) and LP (p = 0.0389) compared with RSPv outputs, indicating a reverse preference for RSPd projections to the lateral and ventral thalamus (Fig. 4e). The input cells of the RSPv were primarily distributed in the anterior and lateral thalamus (such as the AV, AD, and LD, followed by the AM and LP) (Fig. 4a, c, d), which was more similar to the upstream inputs of the RSPd. However, only certain axonal fibres of RSPv glutamatergic neurons were distributed in the AV, LD, and RT (Fig. 4b, c, e). These results may imply unidirectional connectivity between RSPv GABAergic neurons and the anterior and lateral thalamus.
a, b Representative images of input cells (a) and projecting axons (b) from RSP subregions to the thalamus (100 μm thick coronal slice). Left: anatomical position of subregions. Right: RV-eGFP-labelled input cells in the thalamus. Scale bar: 100 μm. c Three-dimensional visualisation of the thalamus depicting input cells and axonal projection of both glutamatergic and GABAergic neurons in RSP subregions in representative samples. d, e The 11 subregions in thalamus input to RSP subregions (d) and project from RSP subregions (e). Different colours represent different RSP subregions and neuron types. One-way ANOVA followed by Tukey’s post hoc tests. *p < 0.05, **p < 0.01, ****p < 0.0001. Mean ± SEM. Input: RSPd GLU, n = 4; RSPd GABA, n = 4; RSPv GLU, n = 3; RSPv GABA, n = 4. Output: RSPd GLU, n = 4; RSPd GABA, n = 3; RSPv GLU, n = 3; RSPv GABA, n = 3. f, g Correlation and hierarchical clustering showing the variances in brain regions connected with inputs (f) and projections (g) in the thalamus. The heatmap represents Pearson’s correlation coefficient matrix.
On the basis of these findings, we hypothesised that there are two distinct groups of projection nuclei in the thalamus. The hierarchical clustering of the input strength of thalamic–RSP (Fig. 4f, g) revealed that the inputs of the thalamus were primarily grouped into two clusters: one consisting of the anterolateral thalamus of the AV and AD and one consisting of the other regions (Fig. 4f). This observation suggested the possibility of two subnetworks of inputs from the thalamus to the RSP. Similarly, the analysis of projection patterns from the RSP to the thalamus also indicated a consistent division between two distinct subnetworks: the anterolateral thalamus and the other regions (Fig. 4g).
In addition, we compared the connections of the thalamic–RSP subnetworks involving glutamatergic and GABAergic neurons in the RSP (Fig. 4d, e). Our analysis revealed that GABAergic neurons preferentially received inputs from the AV, AD and LD (p = 0.027), with a significant difference compared with glutamatergic neurons (Fig. 4d). On the other hand, compared with GABAergic neurons, significantly more projections came from the RSPd glutamatergic neurons in the medial, lateral, and ventral thalamus, such as the VAL (p = 0.0029), VM (p = 0.0026), VP (p < 0.0001), LP (p = 0.0351), and MD (p = 0.0414), as well as the LD and RT. Conversely, glutamatergic neurons in the RSPv preferentially received inputs from the AV and projected back to the AV, LD, and RT (Fig. 4d, e). Both glutamatergic and GABAergic neurons in the RSPd widely received strong inputs from the anterior and lateral thalamus as well as a small number of inputs from the ventral thalamus, whereas only glutamatergic neurons showed stronger outputs to the lateral thalamus and part of the ventral thalamus (Fig. 4d, e). Similarly, there was partial interconnection of glutamatergic neurons with the anterior, lateral, and ventral thalamus in the RSPv, whereas GABAergic neurons only unidirectionally received a large number of inputs from the anterior thalamus and a small number of inputs from the lateral thalamus (Fig. 4d, e).
In summary, although past studies have demonstrated strong reciprocal connectivity between the RSP and multiple thalamic nuclei, we suggest that this relationship may occur primarily between glutamatergic neurons in the RSPd and the lateral and part of the ventral thalamus and that the connectivity pattern follows strict topological correspondence. In contrast, GABAergic neurons in the RSP subregions were involved in unidirectional input circuits only (Fig. 7c).
Circuit connectivity between RSP and hippocampal formation
Although the hippocampal formation (HPF) exhibits strong connectivity with both the RSPd and RSPv, the HPF has been shown to have preferential inputs to the RSPv30, which has emerged as one of the most significant differences between the connectivity of the RSPd and RSPv in the rodent brain, providing robust support for the cognitive functions of the RSP8.
Our investigation revealed that the CA1, SUB, POST, and prosubiculum (ProS) regions served as the primary sources of inputs to the RSP (Fig. 5a, c). Both types of neurons in the RSP received large inputs from the SUB and CA1. Notably, the input cells in the RSP were all more distributed on the dorsal side of the HPF (e.g., CA1d, SUBd) (Fig. 5a), implying a topological preference of dorsal HPF projections to the RSP. Additionally, the ProS and POST also had certain inputs to the RSPv, confirming that the HPF is more closely connected to the RSPv, while the input cells of the RSPd were less distributed in other regions, except for the CA1 and SUB (Fig. 5c).
a, b Representative images of input cells (a) and projection axons (b) from RSP subregions to the HPF (100 μm thick coronal slice). Left: position of the images on the right. Right: RV-eGFP-labelled input cells in the HPF. Scale bar: 100 μm. c, d Mean proportion of HPF inputs (c) and axonal projections (d) to RSP subregions, where different colours represent connection strength. Input: RSPd GLU, n = 4; RSPd GABA, n = 4; RSPv GLU, n = 3; RSPv GABA, n = 4. Output: RSPd GLU, n = 4; RSPd GABA, n = 3; RSPv GLU, n = 3; RSPv GABA, n = 3.
In fact, the efferent fibre distribution in the HPF showed that type-specific neurons mediate major differences in the projection patterns of HPF-RSPv circuits. There was almost no reverse projection to the HPF from the RSPd, except for certain projections from GABAergic neurons in the RSPd to CA1. GABAergic neurons in the RSPv likewise had many projecting fibres to CA1, revealing bidirectional connectivity between GABAergic neurons in the two subregions of the RSP and CA1 (Fig. 5b, d). Specifically, the axonal fibres of both glutamatergic and GABAergic neurons in the RSPv were partially distributed in the SUB. In contrast, only a small number of glutamatergic neuron fibres from the RSPv were distributed in the POST (Fig. 5b, d).
Indeed, our results revealed the differential involvement of glutamatergic and GABAergic neuronal nuclei in the regulation of these circuits (Fig. 7c). Although we already know that the RSPv is more closely linked to the HPF, only GABAergic neurons actually play a key role, with a strong bidirectional connection to CA1 in the hippocampus, whereas glutamatergic neurons receive mainly unilateral inputs from the latter. The glutamatergic and GABAergic neurons in the RSPd also presented patterns similar to those of the HPF-RSPv circuits. The axonal fibres from the reverse projections of the RSPv were more distributed in the parahippocampal region, which is in accordance with findings reported previously31. In particular, both types of neurons in the RSPv exhibited bidirectional connections to the SUB, and the POST was also interconnected with the presence of glutamatergic neurons in the RSPv.
Neurochemical characterisation of the subcortical input cells
These findings support the existence of multiple types of GABAergic neurons in the HPF that project remote axonal projections to the RSP and other cortical regions. The eGFP-labelled input cells distributed in CA1 and SUB of the HPF were identified (Fig. 6a). To further characterise the neurochemical properties of these input cells, we performed double immunochemical staining against several biological markers. The results revealed the presence of PV+ input cells in both CA1 and SUB (Fig. 6a). Here, we confirmed that approximately 6% of the PV+ input cells in the CA1 region and 3% of the PV+ input cells in the SUB region were capable of forming synaptic connections with GABAergic neurons in the RSP (Fig. 6b). Inhibitory modulation of GABAergic neurons in the RSPv by these projection neurons in CA1 may be directly related to the modulation of social behaviours32.
a Immunohistochemical staining of PV+ neurons input from CA1 and SUB to RSPv, respectively. A three-panel present RV, anti-PV, and overlay. The arrows point out PV+ input cells. Scale bar: 200 μm (CA1, n = 3; SUB, n = 3). b Proportion of PV+ neurons among RV-eGFP-labelled neurons in CA1 and SUB. c Both PV+ neurons and cholinergic neurons in the NDB form monosynaptic inputs to GABAergic neurons in RSP. Scale bar: 200 μm (Chat, n = 3; PV+, n = 3). d Quantification of eGFP-labelled neurons that are cholinergic and PV+.
Axons of PV+ neurons from the basal forebrain mainly projected to the RSP, showing a layer-preferred pattern33. The eGFP-labelled input cells were mostly distributed in the NDB (128 eGFP-labelled neurons per mouse, n = 18), with fewer cells distributed in the MS and GP. Similarly, axons projecting from the RSP to the basal forebrain were predominantly distributed in the GP and NDB (Supplementary Fig. 4), indicating strong connectivity between the NDB and RSP. Interestingly, the connectivity between the two types of neurons in the NDB varied widely (Supplementary Fig. 4). Glutamatergic neurons in the RSP received only strong inputs from the NDB, and their reverse-projecting fibres accumulated outside the NDB in regions such as the GP. In our immunohistochemical double-labelling experiments, we discovered that GABAergic neurons in the RSPv were directly innervated by PV+ projection neurons and cholinergic neurons in the basal forebrain (Fig. 6c). Approximately 42% of the input cells in the basal forebrain were cholinergic, which is consistent with previous studies34. Additionally, approximately 10% of the inputs were PV+ projection neurons that did not express choline acetyltransferase (Fig. 6d).
Discussion
Here, we comprehensively mapped the input and output circuits of glutamatergic and GABAergic neurons in the RSP (Fig. 7a, b). Through systematic comparisons between the circuits of the RSPd and RSPv, we revealed the predominance of cell-type specificity in the topological connectivity structure between the two subregions and their major connectivity regions. Additionally, we emphasised the two distinct functional subnetworks related to visual stimulus modulation and spatial memory or navigation by the two RSP subregions.
a, b Whole-brain schematic of the inputs and projection of glutamatergic and GABAergic neurons in the RSPd (a) and RSPv (b). The colour of the outline of the point represents two targeted cell types. The points represent the inputs in each brain region, where colours reflect connectivity strength. The lines represent projection in each brain area, where the line thickness reflects the connectivity strength. The demarcations and annotations of brain regions are based on the Allen Reference Atlas. c Refined connectivity model of the RSPd and RSPv subnetworks. RSPd and RSPv mediated circuits are shown in different colours, the coprojections are shown in black, and the known circuits are shown in grey.
The RSPd and RSPv act as two separate projection nuclei that collaborate and follow the direction of the information processing flow35, acquiring various types of information from the primary sensory cortex for integration. Our study builds on the known strong connectivity of RSP subregions with the isocortex, the HPF, and thalamus36,37 and adds a more refined view of cell-type specificity and connectivity topologies. The retrograde labelling linkage group data showed highly consistent labelling results for Cre-dependent neuron types in the same target areas, indicating uniform upstream areas. Both the spatial distribution and strength of the projection fibres from the two RSP subregions in the downstream areas revealed significant differences between their respective preferred connectivity regions. Most of the samples used in these studies were from 3–8-month-old mice, but one sample was from an 18-month-old mouse. The whole-brain connectivity strength matrix in Fig. 2 shows that the results of this sample are consistent with the pattern of the overall results, and there is no significant difference between the quantised values and those of the other samples, indicating that the age of this sample does not affect the final experimental results. We can equally calculate the input fraction of the areas that project to the target area via the average number of input cells/starter cells per area. Since the input cells and starter cells exhibit a high correlation, the results of the input fraction calculated with these two characterisations are similar. In addition, in the viral labelling of this study, although the input and output starter cells covered all layers, some samples were not densely labelled in all layers. The starter cells covered the intermediate RSP on the A-P axis, but there were differences between the distribution ranges of the input and output starter cells, for example in the output samples. These limitations may affect the input or output intensity.
The input patterns from the upstream cortical regions to the RSP have been classified into four main groups, reflecting the rule that cortical projections of the same hierarchical grade follow similar input patterns. Notably, we found that the three modules of the primary sensory cortex with the prefrontal cortex module and the association cortex module have different projection preferences for GABAergic and glutamatergic neurons in the RSP and that the differences in the modules may provide completely opposite information to the RSP. In contrast, the nuclei of RSP projections to the cortex do not follow the same hierarchical organisation pattern, and the significant differences between their reverse projections are related to cortico-cortical information processing sequences, which reflect more differences in the functional regulatory subnetworks involved in the RSPd and RSPv. Additionally, we emphasised the crucial role of glutamatergic neurons in the VIS-RSP subnetworks. Locomotion signals can directly project to VISp L6 through glutamatergic neurons in RSP, and this visual-motion processing is multisensory-dependent on the animal’s head movement38,39. Our findings revealed a preference between the dorsal VIS and glutamatergic neurons in the RSPd and that these projections followed a strict topological organisation (Fig. 3a, Supplementary Fig. 3a, b). This functional subnetwork may participate in the modulation of visual, egocentric and motor feedback8. Cell-type specificity also allows the RSP to regulate specific layers within the VIS. Most of the visual nuclei that are closely connected to the RSP are located in the deeper layers, but GABAergic neurons also have preferential connections to neural nuclei in the superficial layers of the VIS. These differences in projection patterns reflect the unique functional contributions of these cell types in information processing and integration within the cortical circuits.
The two RSP subregions are involved in distinct subnetworks connecting the diverse thalamic nuclei, which have projective topologies for separate functions40. The strong connectivity of the anterior thalamus with both RSP subregions suggests the key importance of the RSP in spatial navigation and memory41,42, and the mediodorsal (MD) thalamus is involved in the modulation of mechanisms such as executive control, memory and reward processing43. Hierarchical clustering of RSP connectivity patterns revealed two groups of nuclei, anterior and lateral thalamic nuclei, which separately connected the RSPv and RSPd, indicating multiple thalamic–RSP subnetworks. Notably, except for the glutamatergic neurons in the RSPd that are interconnected with the lateral and ventral thalamus, the GABAergic neurons in both subregions may share thalamic inputs. Thalamic–RSP connections were much less common than cortical–RSP connections were, which was consistent with previous research44.
There are fewer projections from the RSPv to the HPF than from the HPF to the RSP, which is consistent with previous reports indicating that the RSP mainly indirectly projects through the parahippocampal and anterior thalamic nuclei to the HPF45. Moreover, HPF–RSP connectivity showed cell-type specificity. Both glutamatergic and GABAergic neurons in the RSPv are involved in the “memory circuit”16,32 from the SUBd to the RSPv. As one critical HPF subregion that projects to the RSP, CA1 has some GABAergic neurons projecting to both RSP subregions, which preferentially target RSPv L1 to regulate inputs from the anterior thalamic nucleus46. However, GABAergic neurons in the RSP have reverse projections to CA1. In addition, hippocampal spatial information also modulates the RSPd through the RSPv and the anterior thalamic nucleus47.
The circuit structure revealed that the RSPd and RSPv are involved in two distinct subnetworks (Fig. 7c), which may be related to different functions, such as visual accommodation and spatial navigation and memory. In the first subnetwork, the RSPd forms a topological connectome with the lateral thalamic nucleus (represented by LD and LP) and the dorsal VIS. The inputs of LP can provide both distributed information from visual scenes and locomotion signals48. On the other hand, the lateral thalamic nucleus receives visual inputs from V1 and other dorsal cortices, as well as subcortical inputs from the superior colliculus and dorsolateral geniculate nucleus, forming multiple visual spaces48,49. It also provides some modulation of GABAergic neurons and receives projections from glutamatergic neurons in the RSPv. In the second subnetwork, the RSPv links the hippocampus, anterior thalamic nuclei (represented by the AV and AD), medial mammillary (MM) and SC, which together form regulatory subnetworks. In particular, the direct inputs from the SUBd to the RSPv are accompanied by a bifurcation of nearly half of the projection neurons to the MM, which simultaneously inputs to the anterior thalamic nucleus along with another cluster of nuclei from the SUBd, which in turn project to the RSPv37. Recent studies have shown that the circuit formed by the RSP and SC is critical for accurate orientation to shelter during escape50. The RSPv is also associated with predicting upcoming objects51. Although the topological and cell-type specificity of the connectome enables the two RSP subregions to connect to separate circuits, forming distinct functional subnetworks, the two main subnetworks actually interact together in their functional mechanisms to support the complex cognitive functional mechanisms of the RSP.
In conclusion, our study comprehensively created and complemented whole-brain connectivity maps of type-specific neurons in the RSPd and RSPv, enabling us to more systematically reveal the subnetworks mediated by the two RSP subregions that were connected to multiple regions. The results emphasised a strong relationship between the topological organisation patterns and the connectivity patterns of type-specific neurons in the RSP. This connectivity atlas provides the groundwork for further exploration and a more refined understanding of the anatomical connectivity and circuit function of different cell types within the RSP.
Methods
Animals
For the RV-tracing experiments, C57BL/6J, Emx1-ires-Cre22, and Vgat-ires-Cre52 male mice (3–8 months old) were used. The input samples were primarily from 3–8-month-old mice (one sample was from an 18-month-old mouse, RSPd GABA4); the output samples were primarily from 3–8-month-old mice. All mice were housed at 22 ± 1 °C and 55 ± 5% humidity with food and water ad libitum. All animal experiments were conducted in accordance with the Institutional Animal Ethics Committee of Hainan University.
Virus
For the retrograde monosynaptic tracing, two AAV helpers [rAAV-EF1α-DIO-mCherry-F2A-TVA-WPRE-hGH and rAAV-EF1α-DIO-RG (both 2–5 × 1012 gcml–1)] and RV-EnvA-△G-eGFP (2 × 108 gcml–1) were used. For the anterograde axonal tracing, rAAV-EF1α-DIO-eGFP (2–5 × 1012 gcml–1) was used. All viruses were purchased from BrainVTA.
Stereotactic injections
For the retrograde monosynaptic tracing, 150 nl of AAV helper (1:2) containing rAAV-EF1α-DIO-mCherry-F2A-TVA-WPRE-hGH and rAAV-EF1α-DIO-RG (50 nl/min) was injected into the RSPd (−2.1, −0.7, −0.55) or RSPv (−2.1, −0.2, −1) in Emx1-ires-Cre and Vgat-ires-Cre mice. Since the RSP spans from −1.155 to −4.955 mm on the A‒P axis, the selected RSPd (−2.1, −0.7, −0.55) or RSPv (−2.1, −0.2, −1) sites were at the intermediate position of the RSP. Here, we refer to the main research area as the “intermediate retrosplenial cortex”. The avian-specific retroviral receptor (TVA), mCherry, and rabies glycoprotein (RG) occurred exclusively in the labelled cells. Three weeks later, 200 nl of RV-EnvA-△G-eGFP (30 nl/min) was injected into the same site, infecting only TVA-expressing cells with the gene encoding the enhanced green fluorescent protein (eGFP). One week later, the mice were perfused. The cells labelled with red fluorescence, as well as the red and green double-labelled cells, were confined to the injection site range (Fig. 1d), and the distribution of all labelled sites is shown in Fig. 1e, f and Supplementary Fig. 1d. Neurons colabelled with both red and green fluorescent proteins within the injection sites were designated starter cells24.
For the axonal AAV tracing, 100 nl of rAAV-EF1α-DIO-eGFP (40 nl/min) was injected into the RSPd (−2.1, −0.7, −0.55) or RSPv (−2.1, −0.2, −1) in Emx1-ires-Cre and Vgat-ires-Cre mice. Three weeks later, the mice were perfused.
Histology
The mice were intraperitoneally injected (100 g/ml) with sodium pentobarbital (2% chloral hydrate and 10% urlatan) and then perfused with 0.01 M PBS (phosphate-buffered saline, PBS, Sigma‒Aldrich) and 4% PFA (paraformaldehyde, PFA, Sigma‒Aldrich).
The mouse brains were removed and postfixed in 4% PFA solution at 4 °C for 12 h. For whole-brain imaging, the whole brain was embedded in glycidyl methacry-late (GMA) resin53. The embedding protocol has been previously described1. Briefly, the coronal brain sections were rinsed overnight with 0.01 M PBS solution and subsequently dehydrated in a graded ethanol series (50, 70 and 95% ethanol, changing from one concentration to the next every hour, at 4 °C). After dehydration, the whole brain was immersed in a graded GMA series, including 0.2% Sudan Black B (SBB) (70, 85 and 100% GMA for 2 h each and 100% GMA overnight at 4 °C). The samples were impregnated in a prepolymerization solution (GMA or SBB solution) for 3 days at 4 °C and embedded in a vacuum oven at 38 °C for 24 h. Each 100 g of GMA solution (100%) consisted of two resin components (A component, 67 g; B component, 29.4 g), 2.8 g of deionised water, 0.2 g of SBB and 0.6 g of 2,2′-azo-bis-butyronitrile (ABIN) as an initiator. The 70% and 85% GMA solutions (wt/wt) were prepared from 95% ethanol and 100% GMA.
For the immunohistochemistry, the fixed brains were embedded in oxidised agarose. Briefly, agarose type I (Sigma) was oxidised by stirring in 10 mM sodium periodate (NaIO4, Sigma) solution for 2 h at room temperature (20–25 °C); then, the oxidised agarose was washed three times in 0.01 M PBS (Sigma‒Aldrich Inc.) and resuspended in 0.01 M PBS to bring the final concentration to 5%. The mouse brain was pat-dried and embedded in melted oxidised agarose via a cube-shaped mould and placed in a 4 °C refrigerator for solidification. The mouse brain was subsequently sectioned at 50 µm on a vibrating microtome (Leica, VT1200S). The sections of interest were blocked with 5% (wt/vol) BSA containing 0.3% Triton-X 100 (vol/vol) in 0.01 M PBS for 1 h and then incubated with the following primary antibodies (12 h at 4 °C): anti-PV (1:500, mouse, Millipore, MAB1572) and anti-chat (1:800, goat, Millipore, AB144P). After rinsing, the sections were incubated with the following fluorophore-conjugated secondary antibodies for 2 h at room temperature (1:500; Invitrogen): Alexa Fluor 647, donkey-anti-mouse; and Alexa Fluor 594, donkey-anti-goat. The antibodies were diluted in the same blocking solution.
fMOST instrument
The fMOST system was used for precise whole-brain imaging of GMA-embedded mouse brains21. Briefly, the fMOST system uses a mercury lamp as a light source, a digital micromirror device for generating an illumination grid pattern and a water immersion objective for imaging. The sample was sliced at a thickness of 2 microns and imaged at each level with a diamond knife. After a surface was completed, the 3D translation stage moved the sample for mosaic scanning and sectioning. The section imaging process was repeated until whole-brain imaging data with a voxel resolution of 0.32 × 0.32 × 2 μm3 were obtained, and 3 days were needed to achieve the final 3D reconstruction.
Image preprocessing
The raw data obtained through the brain positioning system needed image preprocessing. Briefly, on the basis of accurate spatial orientation and adjacent overlap, the mosaics of each coronal section were stitched to obtain an entire section. Lateral illumination correction was then performed section by section. Image preprocessing was implemented in C++ and optimised by Intel MPI Library (v.3.2.2.006, Intel). The entire dataset was executed in 6 h on a computing server (72 cores, 2 GHz per core).
Registration
We extracted the regional features of the whole-brain anatomic invariant of interest. On the basis of the Allen Mouse Brain Common Coordinate Framework version 3 (Allen CCFv3) brain atlas, the dataset was rotated and resampled at a voxel resolution of 10 × 10 × 10 μm3, and the extracted feature regions were registered via the SyN algorithm54. To quantify and 3D display the connectivity patterns of the whole-brain neural circuits, the information of the neurons was extracted via NeuroGPS software55. The data were subsequently imported into Amira Software (v6.1.1, FEI), which was placed into the 3D outline of the corresponding brain regions to generate the results.
Quantification of whole-brain inputs and projections
To distinguish the projection signals from the background, we used a Gaussian filter to remove the background, and the images were binarised. These regions were subsequently segmented to obtain the conversion parameters for transforming the whole image stack to Allen CCFv3. To quantify the input and output connectivity, we transformed the coordinates of the somas of the input cells and image stacks of the labelled signals to Allen CCFv356.
For the cell counting, the number of input cells of different brain regions was quantified. For axon fibres, the projection signal in each area was quantified. To obtain the connection strength between the different samples, the proportions of connectivity in different regions were calculated.
Statistical analysis
The statistical graph results are derived from a 3D dataset, whereas the testing data are obtained through sampling. Amira software was used to visualise the inputs and outputs of the glutamatergic and GABAergic neurons in the RSP. To quantify the similarities of the input and output patterns, we used MATLAB (R2020b, MathWorks) to calculate Pearson’s correlation coefficients. Tukey’s honest significant difference test or t-test was used for the statistical comparisons. The confidence level was set to 0.05 (p-value), and all measurements are presented as the mean ± SEM. To compare the whole-brain inputs across all samples, hierarchical clustering and bootstrapping were performed via “heatmap”, which is a package of R. All histograms were generated via GraphPad Prism 8.0.2.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
Vann, S. D., Aggleton, J. P. & Maguire, E. A. What does the retrosplenial cortex do? Nat. Rev. Neurosci. 10, 792–802 (2009).
Valenstein, E. et al. Retrosplenial amnesia. Brain 110, 1631–1646 (1987).
Morris, R., Pandya, D. N. & Petrides, M. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J. Comp. Neurol. 407, 183-192 (1999).
Mitchell, A. S., Czajkowski, R., Zhang, N., Jeffery, K. & Nelson, A. J. D. Retrosplenial cortex and its role in spatial cognition. Brain Neurosci. Adv. 2, 2398212818757098 (2018).
Keshavarzi, S. et al. Multisensory coding of angular head velocity in the retrosplenial cortex. Neuron 110, 532–543.e539 (2022).
Fischer, L. F., Mojica Soto-Albors, R., Buck, F. & Harnett, M. T. Representation of visual landmarks in retrosplenial cortex. eLife https://doi.org/10.7554/eLife.51458 (2020).
Mao, D., Kandler, S., McNaughton, B. L. & Bonin, V. Sparse orthogonal population representation of spatial context in the retrosplenial cortex. Nat. Commun. 8, 243 (2017).
Aggleton, J. P., Yanakieva, S., Sengpiel, F. & Nelson, A. J. The separate and combined properties of the granular (area 29) and dysgranular (area 30) retrosplenial cortex. Neurobiol. Learn Mem. 185, 107516 (2021).
Vogt, B. A. & Miller, M. W. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J. Comp. Neurol. 216, 192–210 (1983).
Wyss, J. M. & Van Groen, T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus 2, 1–11 (1992).
Zheng, L. & Yao, H. Stimulus-entrained oscillatory activity propagates as waves from area 18 to 17 in cat visual cortex. PLoS ONE 7, e41960 (2012).
Van Groen, T. & Wyss, J. M. Connections of the retrosplenial granular b cortex in the rat. J. Comp. Neurol. 463, 249–263 (2003).
Brennan, E. K. W., Sudhakar, S. K., Jedrasiak-Cape, I., John, T. T. & Ahmed, O. J. Hyperexcitable neurons enable precise and persistent information encoding in the superficial retrosplenial cortex. Cell Rep. 30, 1598–1612.e1598 (2020).
Nixima, K., Okanoya, K., Ichinohe, N. & Kurotani, T. Fast voltage-sensitive dye imaging of excitatory and inhibitory synaptic transmission in the rat granular retrosplenial cortex. J. Neurophysiol. 118, 1784–1799 (2017).
Miyashita, T. & Rockland, K. S. GABAergic projections from the hippocampus to the retrosplenial cortex in the rat. Eur. J. Neurosci. 26, 1193–1204 (2007).
Yamawaki, N., Corcoran, K. A., Guedea, A. L., Shepherd, G. M. G. & Radulovic, J. Differential contributions of glutamatergic hippocampal->retrosplenial cortical projections to the formation and persistence of context memories. Cereb. Cortex 29, 2728–2736 (2019).
Zhang, S. et al. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345, 660–665 (2014).
Lee, S., Kruglikov, I., Huang, Z. J., Fishell, G. & Rudy, B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 16, 1662–1670 (2013).
Zhao, M. et al. Whole-brain direct inputs to and axonal projections from excitatory and inhibitory neurons in the mouse primary auditory area. Neurosci. Bull. 38, 576–590 (2022).
Ma, G. et al. Hierarchy in sensory processing reflected by innervation balance on cortical interneurons. Sci. Adv. https://doi.org/10.1126/sciadv.abf5676 (2021).
Gong, H. et al. High-throughput dual-colour precision imaging for brain-wide connectome with cytoarchitectonic landmarks at the cellular level. Nat. Commun. 7, 12142 (2016).
Chan, C. H. et al. Emx1 is a marker for pyramidal neurons of the cerebral cortex. Cereb. Cortex 11, 1191–1198 (2001).
Huang, Z. J. Toward a genetic dissection of cortical circuits in the mouse. Neuron 83, 1284–1302 (2014).
Watabe-Uchida, M., Zhu, L., Ogawa, S. K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Tran-Van-Minh, A., Ye, Z. & Rancz, E. Quantitative analysis of rabies virus-based synaptic connectivity tracing. PLoS ONE 18, e0278053 (2023).
Zingg, B. Input–output organization of the mouse claustrum. J. Comp. Neurol. 526, 2428–2443 (2018).
Brennan, E. K. et al. Thalamus and claustrum control parallel layer 1 circuits in retrosplenial cortex. eLife 10, e62207 (2021).
Wang, Q., Sporns, O. & Burkhalter, A. Network analysis of corticocortical connections reveals ventral and dorsal processing streams in mouse visual cortex. J. Neurosci. 32, 4386–4399 (2012).
Yao, S. et al. A whole-brain monosynaptic input connectome to neuron classes in mouse visual cortex. Nat. Neurosci. 26, 350–364 (2023).
Aggleton, J. P., Wright, N. F., Vann, S. D. & Saunders, R. C. Medial temporal lobe projections to the retrosplenial cortex of the macaque monkey. Hippocampus 22, 1883–1900 (2012).
Sugar, J., Witter, M. P., van Strien, N. M. & Cappaert, N. L. The retrosplenial cortex: intrinsic connectivity and connections with the (para) hippocampal region in the rat. An interactive connectome. Front Neuroinform 5, 7 (2011).
Shi, Y., Yan, J., Xu, X. & Qiu, Z. Gating of social behavior by inhibitory inputs from hippocampal CA1 to retrosplenial agranular cortex. Neurosci. Bull. 40, 1635–1648 (2024).
Tian, J. et al. Dissection of the long-range projections of specific neurons at the synaptic level in the whole mouse brain. Proc. Natl Acad. Sci. USA 119, e2202536119 (2022).
Sun, Q. et al. A whole-brain map of long-range inputs to GABAergic interneurons in the mouse medial prefrontal cortex. Nat. Neurosci. 22, 1357–1370 (2019).
Le Merre, P., Ahrlund-Richter, S. & Carlen, M. The mouse prefrontal cortex: unity in diversity. Neuron 109, 1925–1944 (2021).
Yamawaki, N., Radulovic, J. & Shepherd, G. M. A corticocortical circuit directly links retrosplenial cortex to M2 in the mouse. J. Neurosci. 36, 9365–9374 (2016).
Kinnavane, L., Vann, S. D., Nelson, A. J. D., O’Mara, S. M. & Aggleton, J. P. Collateral projections innervate the mammillary bodies and retrosplenial cortex: a new category of hippocampal cells. eNeuro 5, ENEURO.0383-17 (2018).
Chaplin, T. A. & Margrie, T. W. Cortical circuits for integration of self-motion and visual-motion signals. Curr. Opin. Neurobiol. 60, 122–128 (2020).
Velez-Fort, M. et al. A circuit for integration of head- and visual-motion signals in layer 6 of mouse primary visual cortex. Neuron 98, 179–191.e176 (2018).
Roy, D. S., Zhang, Y., Halassa, M. M. & Feng, G. Thalamic subnetworks as units of function. Nat. Neurosci. 25, 140–153 (2022).
Jankowski, M. M. et al. The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front. Syst. Neurosci. 7, 45 (2013).
Roy, D. S. et al. Anterior thalamic dysfunction underlies cognitive deficits in a subset of neuropsychiatric disease models. Neuron 109, 2590–2603.e2513 (2021).
Saalmann, Y. B. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front. Syst. Neurosci. 8, 83 (2014).
Zhang, S. et al. Organization of long-range inputs and outputs of frontal cortex for top-down control. Nat. Neurosci. 19, 1733–1742 (2016).
Prasad, J. A. & Chudasama, Y. Viral tracing identifies parallel disynaptic pathways to the hippocampus. J. Neurosci. 33, 8494–8503 (2013).
Yamawaki, N. et al. Long-range inhibitory intersection of a retrosplenial thalamocortical circuit by apical tuft-targeting CA1 neurons. Nat. Neurosci. 22, 618–626 (2019).
Mao, D. et al. Hippocampus-dependent emergence of spatial sequence coding in retrosplenial cortex. Proc. Natl Acad. Sci. USA 115, 8015–8018 (2018).
Roth, M. M. et al. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat. Neurosci. 19, 299–307 (2016).
Bennett, C. et al. Higher-order thalamic circuits channel parallel streams of visual information in mice. Neuron 102, 477–492.e475 (2019).
Campagner, D. et al. A cortico-collicular circuit for orienting to shelter during escape. Nature 613, 111–119 (2023).
Lande, A. S., Garvert, A. C., Ebbesen, N. C., Jordbraek, S. V. & Vervaeke, K. Representations of tactile object location in the retrosplenial cortex. Curr. Biol. 33, 4599–4610.e4597 (2023).
Chaudhry, F. A. et al. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J. Neurosci. 18, 9733–9750 (1998).
Ren, M. et al. Simultaneous acquisition of multicolor information from neural circuits in resin-embedded samples. Front. Neurosci. 12, 885 (2018).
Ni, H. et al. A robust image registration interface for large volume brain atlas. Sci. Rep. 10, 2139 (2020).
Quan, T. et al. NeuroGPS: automated localization of neurons for brain circuits using L1 minimization model. Sci. Rep. 3, 1414 (2013).
Xu, Z. et al. Whole-brain connectivity atlas of glutamatergic and GABAergic neurons in mouse dorsal and median raphe nucleus. eLife 10, e65502 (2021).
Acknowledgements
We thank Xiangning Li, Zhiyu Deng, Shuxin Wang, Jiayan Zhang, Jiamei Qi, Haiyang Du and Yuchao Bi for help with experiments and data analysis. This work was financially supported by the STI2030-Major Projects (Nos. 2021ZD0201004, 2021ZD0201001), the National Natural Science Foundation of China Grant (No. 32192412, 32400931), and the Key R&D Projects in Hainan Province (ZDYF2024SHFZ265).
Author information
Authors and Affiliations
Contributions
X.W. and H.G. conceived and designed the study. Y.L., M.R., B.L. and H.Z. optimised viral tracing, sample processing and data analysis. T.J. and X.J. performed the whole-brain imaging and processing. Y.L., M.R. and X.W. wrote and modified the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were approved by the Animal Ethics Committee of Hainan University.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Fereshteh Nugent and Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Ren, M., Liu, B. et al. Dissection of the long-range circuit of the mouse intermediate retrosplenial cortex. Commun Biol 8, 56 (2025). https://doi.org/10.1038/s42003-025-07463-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-07463-8