Abstract
How and to what extent mosquito-virus interaction is influenced by climate change is a complex question of ecological and epidemiological relevance. We worked at the intersection between thermal biology and vector immunology and studied shifts in tolerance and resistance to the cell fusing agent virus (CFAV), a prominent component of the mosquito virome known to contribute to shaping mosquito vector competence, in warm-acclimated and warm-evolved Aedes albopictus mosquitoes. We show that the length of the thermal challenge influences the outcome of the infection with warm-evolved mosquitoes being more tolerant to CFAV infection, while warm-acclimated mosquitoes being more resistant and suffering from extensive fitness costs. These results highlight the importance of considering fluctuations in vector immunity in relation to the length of a thermal challenge to understand natural variation in vector response to viruses and frame realistic transmission models.
Similar content being viewed by others
Introduction
Current anthropogenic climate change has profound and complex implications for the prevalence and the transmission dynamics of arboviruses such as dengue, Zika and chikungunya, which are an impending risk for 3.9 billion people in tropical and subtropical areas of the world1,2. Climate warming is expected to cause large shifts in the distributions of the main arboviral vectors, the mosquitoes Aedes aegypti and Aedes albopictus, and changes in their phenology, further expanding the risk of arboviral diseases in both subtropical and temperate areas3,4. Mosquitoes are ectotherms, and environmental temperature (Ta) impacts nearly all aspects of their life cycle5,6; increase in Ta is also known to accelerate viral replication in mosquitoes7, but whether mosquito response to viruses changes in relation to Ta remains largely unexplored.
Organisms can respond to a viral infection by actively trying to limit viral replication, a strategy called resistance, or by controlling the cost of infection without impeding viral replication, a strategy called tolerance8,9. Tolerance is not well-understood in mosquitoes because to date vector immunology has been biased by an anthropogenic perspective and focused on identifying pathways used by mosquitoes to resist viral infections to leverage them into transmission control strategies10,11. In the ’90 plant pathologists developed a biological and mathematical framework to distinguish effects of resistance and tolerance in an infection experiment12. This framework implies the use of reaction norms that measure host longevity across a range of pathogen doses, in which resistance is defined as “the inverse of the pathogen burden” and tolerance as “the rate of change in host fitness at increasing viral loads” (i.e. the slope of the reaction norm)13,14,15. Resistance and tolerance can act independently, synergistically or alternating, but have different ecological and evolutionary repercussions16,17,18,19. By directly limiting viral replication, resistance mechanisms impose a strong selective pressure on viruses20,21. In contrast, tolerance minimises cost of infection, resulting in neutral or positive effects on pathogens22. Understanding whether global climate change favours a shift between resistance and tolerance, or their synergistic interaction, is crucial because the efficiency of one immunological response over the other has immediate effects on viral transmission dynamics and long-term impacts on the evolution of both vectors and viruses23,24,25.
In this study we used Ae. albopictus and the orthoflavivirus cell fusing agent virus (CFAV, Flaviviridae family) to explore if Ta alters the dynamic response of mosquitoes to a viral infection. Aedes albopictus is an invasive species originally from southeast Asia, which established in tropical, subtropical and temperate areas of the word and emerged as the main arboviral vector in Europe and North America3. CFAV is the first-identified and, so far, the best-characterised Aedes insect-specific virus (ISV)26,27,28,29,30,31,32. ISVs are insect-restricted viruses, which are found with higher prevalence and frequency than arboviruses in field mosquitoes33,34,35. Additionally, ISV infection is known to trigger mosquito immunity36, thus conditioning vector competence to arboviruses, but the global distribution patters of ISVs in Ae. albopictus populations remains largely unexplored. These patterns, in conjunction with local impacts of climate change, could provide insights into the natural heterogeneity of arboviral transmission dynamics37.
We also reasoned that anthropogenic climate change manifests not only through the gradual and continuous Ta increase (i.e. global warming), but also through extensive Ta fluctuations, which can last few days or months (i.e. heat waves)38,39. To test if immunological mechanisms induced by a short-term thermal challenge differ from those resulting from a constant and prolonged exposure to heat38,39, we studied CFAV infection in mosquitoes exposed to 32 °C, a temperature above the optimum for Ae. albopictus5, for one or ten generations.
In the following, we show that exposure to heat increases overall Ae. albopictus tolerance to CFAV infection, but the length of the thermal challenge alters mosquito fitness and influences the immunological response to CFAV.
Results
We exposed Ae. albopictus mosquitoes to a hot thermal regime (32 °C for 14 h and 26 °C for 10 h) for one or ten generations generating warm-acclimated and warm-evolved mosquitoes, respectively (Fig. 1). We infected these mosquitoes with CFAV and studied immunological traits of tolerance and resistance, as well as the cost of infection in terms of mosquito longevity and its reproductive output in comparison to infection of mosquitoes maintained under our standard laboratory thermal conditions, hereon referred to as “standard”. All infected mosquitoes were kept at the same temperature at which they were raised.
We compared the tolerance and the resistance to CFAV and the fitness of CFAV infected mosquitoes maintained under a hot (32 °C for 14 h and 26 °C for 10 h) thermal regime or our standard laboratory conditions (constant 28 °C). Mosquitoes were exposed to the hot thermal regime for either one or ten generations resulting in warm-acclimated and warm-evolved mosquitoes, respectively. Representative results of resistance, tolerance and mosquito fitness are shown in the bold frame “Created in BioRender (Agreement number CF27LQ941M). “.
Exposure to heat increases viral tolerance in Ae. albopictus mosquitoes
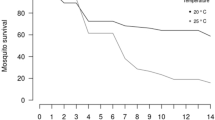
We measured tolerance as the rate of change in host fitness at increasing viral loads as recently proposed12,18. To do so, we infected Ae. albopictus mosquitoes with five doses of CFAV spanning 10 to 105 viral particles and followed mosquito survival after infection. We saw reduced longevity in mosquitoes maintained under standard conditions when we compared mock infected vs CFAV-infected mosquitoes, starting with 102 viral particles (Fig. 2A, Supplementary Data 1). We observed that the longevity of warm-acclimated mosquitoes was significantly reduced with respect to mock-infected mosquitoes only after infection with the highest CFAV dose (Media−105 viral particles, z.ratio = −3.493, Df=inf, p = 0.0063). We did not see significant changes in longevity of warm-evolved mosquitoes at any CFAV infecting doses (Fig. 2B, C, Supplementary Data 1). We found that the regimen at which mosquitoes were reared (Chisq=8.762, Df=2, p = 0.013), the injected viral dose (Chisq=63.381, Df=5, p = 2.43 ×10−12), and their interaction reduced survival (Chisq=21.851, Df=10, p = 0.016).
Survival probability of (A) mosquitoes kept at standard conditions (Std), (B) warm-acclimated (Acc) and (C) warm-evolved (Evo) mosquitoes after mock infection (media) or infection with five different concentrations of CFAV (105, 104, 103, 102, 10 viral particles). In all graphs, we used the Kaplan–Meier survival analysis with a cox proportional hazard test to determine the effect of CFAV infection on mosquito survival. D Tolerance curves of warm-acclimated (pink), warm-evolved (red) and mosquitoes kept at standard conditions (blue) were built by plotting mosquito survival time after infection versus the log10 scale of infecting dose. E Best fit values of vigour, severity and sensitivity, and their 95% confidence intervals (CI) were plotted for each thermal regime. Tolerance parameters were compared based on the non-overlapping 95% CI (Supplementary Data 1). Each point represents an individual mosquito, and lines are logistic fits of the data. Significant result as follows: **P-value < 0.01, ***P-value < 0.001, ****P-value < 0.0001.
Next, we plotted the survival time of each mosquito in relation to the infecting CFAV dose (Fig. 2D) and fitted a four-parameter logistic model with a Poisson regression to identify vigour, meaning the survival time of uninfected hosts; severity, meaning longevity of the host when infected with the highest viral load and sensitivity, or effective concentration 50 (EC50), which defines the viral load that reduces vigour by half40. The choice of the model was based on the goodness of fit (Supplementary Data 1) and previous studies40,41. To make comparisons across thermal conditions, we also tried to constrain the slope of the curves to -1, as described elsewhere41. The slope of the curve denotes the rate of loss of host health40. We could not fit the tolerance curve of warm-acclimated mosquitoes with a slope to −1 (Supplementary Data 1). This result indicates that, when summed to the challenge of infection, acclimation reduces mosquito health faster than what observed in both warm-evolved and mosquitoes maintained under standard conditions. We found that observed differences of warm-acclimated mosquitoes could be fitted with a slope of −4, which was then used also for tolerance curves of warm-evolved and mosquitoes maintained under standard conditions. Using a likelihood ratio test we found that the tolerance curves of warm-evolved, warm-acclimated and standard mosquitoes differed among each other (in all comparisons p < 0.0001). Then we compared vigour, sensitivity and severity of the tolerance curves using a non-overlapping 95% confidence internal (CI) (Fig. 2E, Supplementary Data 1). All tested parameters showed significant differences between at least two groups. Warm-acclimated mosquitoes had the lowest vigour (95% CI: 12.38 − 13.39) and highest severity (95% CI: 9.44 − 11.29). Vigour of mosquitoes maintained under standard conditions (95% CI: 16.23 − 17.43) and warm-evolved (95% CI: 15.85 − 17.06) mosquitoes was comparable. Warm-evolved mosquitoes also showed the lowest severity (95%CI: 12.95 − 14.35). Interestingly, both warm-acclimated (EC50 95%CI 4.272 to not determined) and warm-evolved mosquitoes showed higher sensitivity than those kept under standard conditions (EC50 95%CI 1.384 − 2.261) (Fig. 2D, E, Supplementary Data 1). These results support the conclusion that exposing mosquitoes to heat increases their overall tolerance to a viral infection, but the length of the thermal challenge impacts vigour, severity and longevity. Multi-generational exposure to heat imposes no cost on CFAV infected mosquitoes.
Warm-acclimated Ae. albopictus have higher resistance
Next, we studied if exposure to heat alters Ae. albopictus resistance to CFAV. We decoupled resistance into two different phenotypes: prevalence of infection and viral load42. We tested these traits 3- (Fig. 3A, B) and 5-days post infection (dpi) (Fig. 3 C, D) in mosquitoes injected with the five viral doses used to assess tolerance. Prevalence of CFAV was dose dependent. The two lower infecting titers, 10 and 102 viral particles, resulted in a significantly lower prevalence compared to the higher three (Supplementary Data 2). We also observed an effect of the length of the thermal challenge on the prevalence of infection. Warm-acclimated mosquitoes had lower prevalence than both warm-evolved mosquitoes and mosquitoes kept under standard conditions regardless of the injected dose and dpi (Fig. 3 A, C warm-acclimated vs. mosquitoes kept under standard conditions z.ratio=4.617, df=Inf, p < 0.0001; warm-acclimated vs. warm-evolved z.ratio=4.939, df=Inf, p < 0.0001). Additionally, warm-evolved mosquitoes showed lower prevalence of infection compared to mosquitoes kept under standard conditions 3 dpi (Fig. 3 A z.ratio=2.367, df=Inf, p = 0.021), but this difference was lost 5 dpi (Fig. 3 C z.ratio=1.425, df=Inf, p = 0.1542). The length of the thermal challenge also influenced viral loads, in a time-dependent manner (Table 1). Warm-acclimated mosquitoes constrained viral infection more efficiently than both warm-evolved mosquitoes and mosquitoes kept under standard conditions within 3 dpi, independently of the injected concentration (Fig. 3 B Supplementary Data 2, z.ratio=2.896, df=Inf, p = 0.0004). This was a transient effect since mosquitoes from all regimens showed comparable viral loads 5 dpi, indicating that neither 28° nor 32 °C are temperatures restrictive for CFAV replication (Fig. 3 D Supplementary Data 2).
A Bar plot showing prevalence of infection as percentage of infected mosquitoes in relation to CFAV doses 3 dpi. B Quantification of CFAV genomes in relation to doses of injected CFAV 3 dpi. C Bar plot showing prevalence of infection as percentage of infected mosquitoes in relation to CFAV doses 5 dpi. D Quantification of CFAV genomes in relation to doses of injected CFAV 5 dpi. Data for warm-acclimated mosquitoes are in pink, warm-evolved mosquitoes in red and mosquitoes kept under standard conditions in blue. In plots B and D, each point represents data from an individual mosquito; black solid lines and bars are the median of viral load and the 95% confidence intervals, respectively.
Warm-acclimated mosquitoes suffer extensive fitness costs from CFAV infection
Lastly, we examined the effect of heat exposure on Ae. albopictus and its influence on the fitness cost associated with CFAV infection. As a readout for the cost of infection, we used mosquito longevity and reproductive output, measured from five different parameters: percentage of sterile female, fecundity (i.e. number of eggs produced by each female), fertility (i.e. number of hatching eggs), percentage of non-viable eggs and progeny number per female (i.e. number of larvae/female). Linear and binomial generalized linear models were used to determine the effects of thermal regimens, CFAV infection, and their interaction on reproductive traits (Supplementary Data 3). To decouple the effects of temperature and CFAV infection, we first compared the fitness of warm-acclimated, warm-evolved and mosquitoes kept under standard conditions without infection (Gray comparison in Fig. 4). We observed a significant reduction in female longevity (Fig. 4A), but higher fecundity, fertility and progeny/female in warm-acclimated vs. both warm-evolved and mosquitoes kept under standard conditions (Fig. 4 B-D, Supplementary Data 3). We did not see significance differences in any of the tested parameters between warm-evolved and mosquitoes kept at standard conditions without infection (Fig. 4 A-D, Supplementary Data 3). We saw that CFAV infection reduces longevity of warm-acclimated and mosquitoes kept under standard conditions, but not that of warm-evolved mosquitoes (Fig. 4A). CFAV infection also reduces fecundity of warm-acclimated mosquitoes (Fig. 4B, Supplementary Data 3). Oviposition was tested for all mosquitoes for seven days post blood meal based on the observation that control mosquitoes do not oviposit eggs after this period43, nevertheless it is possible reduced fecundity of warm-acclimated mosquitoes is due egg retention. Only warm-acclimated mosquitoes showed an effect of injection on fertility and progeny (Fig. 4C, D, Supplementary Data 3). Mosquitoes kept under standard conditions also exhibited reduced fecundity, but this was due to the combined effect of the injection and CFAV infection (Fig. 4B, Supplementary Data 3). Warm-evolved mosquitoes showed no fitness effects due to either injection or CFAV infection (Fig. 4C-D, Supplementary Data 3).
A Longevity, B fecundity, C fertility and D number of larvae per female in warm-acclimated (pink), warm-evolved (red) and standard (blue) mosquitoes. Each point represents data from an individual mosquito; black solid lines are the mean and bars show the 95% confidence intervals (CI). In all the plots, control, medium and CFAV refer to data from non-injected, medium-injected, and mosquitoes infected with 105 viral particles, respectively. In longevity solid lines refer to medium injected mosquitoes and dashed lines represent CFAV infection. Grey lines are used for between regimen and black lines for within regimen comparisons. Ns represents not significant, *P-value < 0.05, **P-value < 0.01, ***P-value < 0.001, and ****P-value < 0.0001.
Discussion
Transmission heterogeneity is an inherent property of arboviral diseases2. Biotic and abiotic factors, including natural variation of vector competence across mosquito populations and current anthropogenic climate change effects on both vectors and arboviruses are known to contribute to differences in transmission dynamics44,45,46,47,48. The need of including these variables to frame more realistic models of transmission, especially at a local scale, is being increasingly appreciated49,50. Our results highlight important shifts between resistance and tolerance to viral infection in Ae. albopictus, shifts that depend on the length of a thermal challenge. One generational exposure to heat, which mimics a sudden thermal fluctuation51, results in fitness costs and increased resistance to viral infection. In contrast, mosquitoes conditioned to heat through several generations, which mimics current global warming, are as healthy as, if not healthier than, those not exposed to a thermal challenge and have increased viral tolerance.
Besides having important implications for understanding natural heterogeneity of mosquito vector competence, this immunological shift has also long-term impacts on both vectors and viruses because resistance and tolerance have drastically different ecological and evolutionary consequences23,24,25. By directly fighting infection, resistance implies the emergence of an arm-race between the virus infective strategies and mosquito immunity that could foster molecular evolution through continuous selection of adaptation and counter adaptation52. On the contrary, tolerance is not directed towards the infecting agent, but encompasses strategies used to ameliorate the damage caused by the infection53. As such, tolerance should reduce selective pressure on viruses, resulting in different evolutionary trajectories. Despite having opposite outcomes, tolerance and resistance mechanisms may be interlinked. For instance, some resistance effectors, such as antimicrobial peptides or reactive oxygen species (ROS), have direct or indirect toxic effects on the mosquitoes besides the pathogen, thus affecting mosquito tolerance8. Additionally, resistance mechanisms can be energetically demanding13,14,53,54,55 requiring appropriate energy management as opposed to tolerance strategies, which manage host homeostasis during an infection. As a consequence, virus and mosquito evolutionary trajectories ultimately will be influenced both by the extent of the physiological and genetic factors shared between resistance and tolerance mechanisms and the prevalence of one mechanism over the other.
The concept of tolerance is slowly breaching into vector biology based on the discovery of a plethora of ISVs that can persistently infect mosquitoes shaping their vector competence37 and the observations that also arboviral infections are persistent in mosquitoes, with apparent no, or limited, fitness consequences56,57,58. Additionally, recent studies in Drosophila melanogaster and Ae. aegypti are showing the advantages of addressing insect response to infections accounting for tolerance18,41,59,60,61. For instance, while in infection experiments using 1000 median tissue culture infectious dose (TCID50) of Drosophila C virus (DVC), Dr. melanogaster longevity appeared to depend on the presence of the G9a gene61, when the role of G9a was studied using a dose-response curve approach, G9a knockout was shown to reduce fly longevity with a lower amount of virus with respect to control flies, indicating that this gene controls sensitivity to infection41. Similarly, using a dose-response curve, blood meal was shown to have an immediate, but transient effect on resistance, and a more prolonged impact on tolerance to both bacterial and arboviral infections in Ae. aegypti59,60. Here, the application of a reaction norm approach allowed us to identify previously unappreciated fitness costs of CFAV infection31. We show that the longevity of mosquitoes reared under standard conditions is reduced starting with injection of 102 CFAV particles, a value lower than that found in wild-caught mosquitoes62. Our results further show that temperature modulates CFAV prevalence and load, with one generational exposure to heat reducing viral prevalence. These results are critical for potential applications of CFAV as a biological control agent to control arboviral infection in mosquitoes63,64. Moreover, our results could contribute to explaining the heterogenicity of CFAV detection in mosquito field populations by highlighting the interplay between abiotic (i.e. temperature) and biotic (i.e. mosquito immunity) factors shaping its prevalence and load28,29. The environmentally-conditioned presence of CFAV in mosquito populations also caution on its use to elucidate mosquito population structure and dynamics34,35,64.
Temperature is known to influence insect ability to mount an effective immune response against pathogens65. Orthoflavivirus infection prevalence increases in Ae. albopictus and Ae. aegypti at high temperatures, alongside with dissemination and transmission rates7,66,67. Exposure of adult mosquitoes to warmer temperatures, following development at temperate conditions, is also known to favour upregulation of heat shock proteins, which have pan-antiviral activity in mosquitoes68,69. However, heat-shock proteins can be toxic and result in cellular damages at high concentrations, suggesting their upregulation is limited during an acute thermal stress69. Owing to extensive cross-talks in the signalling cascades that regulates stress-related transcription factors70, modification of the expression profile of antimicrobial peptides and other immune-related genes was also observed in mosquitoes exposed to a heat challenge6,71,72. These results agree with our observation that one generational exposure to heat results in mosquitoes fighting viral infection, with lower tolerance with respect to warm-evolved mosquitoes. Because warm-acclimated mosquitoes had altered fitness compared to warm-evolved ones in the absence of infection, our results further support the conclusion that acclimation is a stressful condition, which conditions mosquito immunity, as previously shown5,69. In contrast, warm-evolved mosquitoes did not suffer fitness costs with respect to mosquitoes reared at constant conditions either in presence or absence of CFAV infection and showed less resistance to viral infection in comparison to warm-acclimated mosquitoes. These results support the conclusions that short vs long term thermal challenges evoke different physiological mechanisms in mosquitoes. These results also align with recent data from several mosquito species, including Ae. albopictus5, which support the existence of thermal adaptation in mosquitoes73,74. Thermal adaptation includes profound and lasting behavioural and physiological changes, which allow a species to develop, survive and reproduce under otherwise unfavourable conditions such as those associated with long-term global warming38. We observed that, after evolving for ten generations under a hot thermal regime Ae. albopictus mosquitoes showed lifespan and reproductive output comparable to those of mosquitoes maintained under control conditions, suggesting adaptation mechanisms to the imposed thermal challenge. This conclusion is further supported by the observation that warm-evolved mosquitoes displayed different fitness traits than those of warm-acclimated ones. The mechanisms of thermal acclimation and adaptation are still not completely understood in mosquitoes and require in depth investigations, which are beyond the scope of this work. Here, we start addressing the complexity of climate change effects on mosquito immunity, which contributes to modulating viral transmission dynamics. We show the importance of addressing the length of the thermal challenge, besides temperature when studying the impact of climate change on mosquito biology. Considering the potential application of CFAV as a biological control agent, our results support the conclusion that global warming could favour CFAV persistence, but unexpected thermal fluctuations could negatively impact its prevalence in mosquito populations. Our results also have important implications on arboviral transmission dynamics because while it has been amply demonstrated that there is mosquito-population and viral-species variation in the detailed profile of genes and small RNA molecules triggered upon infection, immunity pathways and the trend of their activation following infection are conserved across viral and mosquito species75,76,77. Increased viral tolerance raises the likelihood of viral transmission in warm-evolved mosquitoes.
Materials and methods
Mosquito rearing
We used the Foshan laboratory population78,79, which we maintain in a Binder KBWF climatic chamber under a constant temperature of 28 °C, 70 ± 5% relative humidity and a 12:12 hours light/dark photoperiod. We rear larvae at the density of approximately 200 individuals in one litre of distilled water in BugDorm plastic pans (19×19×6 cm). We feed larvae daily with 10 pellets of Tetra Goldfish Gold Colour fish food (Tetra Werke, Germany) until pupation. We keep adult mosquitoes in 30 cm³ BugDorm cages and give them ad libitum access to 20% sucrose solution. We offer defibrinated mutton blood (Biolife Italiana) to females 7 − 10 days post emergence using a Hemotek feeding apparatus.
Thermal challenge
We exposed Ae. albopictus mosquitoes throughout their life-cycle to a thermal challenge consisting of 14 h light (3AM-5PM) at 32 °C and 10 h dark (5PM-3AM) at 26 °C. We chose 32 °C because it corresponds to the average Ta registered in the summer (August) of 2021 in northern Italy80 and was previously shown to be a thermal condition above the optimum for Ae. albopictus5. We designed three experimental groups exposed to two different thermal conditions: 1. standard: laboratory population of mosquitoes that has been reared under standard conditions for more than ten generations and represents the control; 2. warm-acclimated: mosquitoes reared under thermal challenge for one generation; 3. warm-evolved: mosquitoes reared under thermal challenge for ten generations. We established three replicates for each experimental group starting each with 1000 eggs. This number of eggs was maintained across generations for the warm-evolved mosquitoes.
Viral preparation
We obtained CFAV (Rio Piedras 2002, Ref-SKU: 001v-EVA68) from Ronald Van Rij (Radboud University, Nijmegen, NL). We propagated the virus in C6/36 cells maintained at 28°C on Leibovitz’s L-15 Medium (Gibco, REF:11415-049) with 2% FBS (Gibco, REF: 16000-044 Lot: 2421078RP). Three T75 tissue cultured flasks at an 80% confluency were used to start viral propagation. We used one of the three flasks to determine the number of cells present at the time of infection to calculate the multiplicity of infection (MOI). A second flask was infected at MOI of 0.1 (i.e. 1 viral particle per 10 cells). After five days, we clarified the media by centrifugation at 4000 × g for ten minutes at 4°C. Viral stock was concentrated with an AMICON Ultra-15 100k centrifugal filter (Millipore, UFC910008) using a swing bucket rotor and centrifugation at 4000 × g for 30 minutes. We quantified the viral stock by a focus forming assay and stored it at −80 °C. A third flask was kept at the same conditions of the infected one, including collection and storage of its media, which was used to inject mock infected mosquitoes.
Viral infection
Three to eight days old females were used in infection experiments. All infections were performed between 2 to 5 PM (11 hours after the start of the light cycle) to reduce biases due to the circadian rhythm81. We injected mosquitoes intrathoracically with 50 nL of CFAV or medium from C6/36 cells using the Nanoject III (Durmond). The volume of the injected virus was kept constant through viral concentrations, which were generated in RPMI-1640 media (2% FBS) when needed. Prior the injection, we cold anesthetized mosquitoes and separated them in three groups. First group is the control (Ctr); these females were not injected but were kept on ice for the same time as injected mosquitoes. The second group was mock infection (Med), including females injected with media from C6/36 cells. The third group was CFAV infected females, meaning females injected with CFAV. After infection, we returned all mosquitoes to the same rearing condition where they were raised until needed for further experiments.
Tolerance
We quantified tolerance by measuring longevity of mosquitoes infected with five increasing viral concentrations, such as 1.32 × 10, 1.32 × 102, 1.32 × 103, 1.32 × 104, 1.32 × 105. For simplicity we use the notation 10, 102, 103, 104, 105 viral particles in the rest of the manuscript12. For each concentration we used 20 females, along with a group of 20 mosquitoes mock infected with media and a group of 20 mosquitoes that were not injected. After infection, we kept mosquitoes in 266 mL cardboard cups, added a cotton ball with water and a cotton ball with 20% sucrose solution. Mosquitoes that died within 3 dpi were not included in survival analyses because we assumed their death was caused by injection-related injuries. This experiment was repeated three times for mosquitoes kept at standard condition or exposed to thermal challenge for one or ten generations.
Resistance
We infected mosquitoes with the same viral concentrations used to determine tolerance and we measured viral genomes in mosquitoes three- and five- dpi, through quantitative polymerase chain reaction (qPCR).
We extracted RNA using 500 µL of TRIzol Reagent (Invitrogen, Ref: 15596018) per mosquito, followed the manufacturers’ manual and resuspended the final pellet in 20 μL of Ultra-pure water. Then, 10 μL of the total RNA was used to produce cDNA with the GoScript Reverse Transcription Mix (Promega) following manufacturer’s recommendation. We used the QuantiNova SYBR Green PCR kit (Qiagen) for qPCR and primers targeting the NS3 viral gene30. The 2-ΔΔCt method was used to calculate viral loads relative to the housekeeping Rpl34 Ae. albopictus gene82. We infected 12 mosquitoes per concentration and collected 6 mosquitoes per timepoint; we repeated the experiment three times for mosquitoes kept under standard condition or exposed to the thermal challenge for one or ten generations.
Mosquito fitness
Fitness assessment was conducted in parallel for mosquitoes kept under standard conditions, warm-acclimated and warm-evolved mosquitoes. We chose to measure mosquito longevity and the female reproductive output, given their contribution for population maintenance across generations and their dependence on Ta83. We infected three to eight old females with 105 viral particles. After infection, females were transferred to separate cages in groups of 50 and 10 three-to-eight-day old males were added to each cage for mating. Seven days post injection, we offered a blood meal to the females. We transferred engorged females to cardboard cups in groups of 12-20 for two days and then we transferred them to individual plastic cups. We added filter paper and water at the bottom of the cup as oviposition site, mesh on top and cotton balls to provide sucrose solution. Seven days after blood feeding, we counted the number of eggs laid per female, as a measure of their fecundity. We dried eggs for two days before hatching them. The percentage of hatched eggs over the total number of eggs laid per female was measured as fertility. We also counted the number of larvae resulting from eggs laid by each female as progeny production. We further recorded the percentage of females that did not oviposit egg and the number of females that produce non-fertile eggs as sterility and infertility, respectively.
Statistics and reproducibility
Analyses of resistance, survival and reproductive output were done with R version 4.3.3 (The R Foundation for Statistical Computing, 2024). They all included the temperature treatment (standard, warm-acclimated and warm-evolved), injected dose (10 to 105 CFAV viral particles) and their interaction as fixed factors. Analysis of resistance included days post injection (3 dpi and 5 dpi) and its interaction with all other variable as an additional fixed factor. Significance of each factor was calculated with the function ANOVA from the car package version 3.1-284. To estimate the correlation coefficients between viral dose and treatment, each model was reduced to a minimal model by removing non-significant interactions. Whenever a significant interaction remained, we used a type III ANOVA; otherwise, we used a type II. We used the functions emmeans and pairs from the emmeans package version 1.10.085 for post-hoc analysis, adjusting p-values with the Tukey method.
To assess tolerance, mosquito survival post injection was analyzed using a Cox proportional hazards model (function coxph from the survival package version 3.5-8). Model assumptions were tested using the cox.zph function (survival package version 3.5-8)86.
We used GraphPad Prism (Version 10.2.0) to fit the four parameter logistic model as described by the following equation: Y=Bottom + (Top-Bottom)/(1 + 10^((LogEC50-X)×HillSlope)), where EC50 is the concentration of CFAV that reduces mosquito lifespan by half (between Bottom and Top). HillSlope is the steepness of the dose-response curve, and Top and Bottom are plateaus in the units of the Y axis.
Prevalence of viral infection was analyzed with a Bayesian generalized linear model using default priors (function bayesglm from the arm package version 1.13-1)87 with binomial distribution of errors. This approach was chosen to account for the observed quasi-separation of data for prevalence. Non-zero viral load were analyzed with a linear mixed-effect model with a normal distribution of errors (function Lmer from the lme4 package version 1.1-35.1)88. To reach normality of the residuals, we performed a boxcox transformation on the log10(viral titre) values using the function boxcox from the MASS package version 7.3-60.0.189.
The proportion of female that laid did not laid eggs (sterile female) and the ensuing proportion of laid eggs that did not hatched (non-viable eggs) were analyzed respectively with a generalized linear mixed-effect model (function Glmer from the lme4 package version 1.1-35.1)88 and Bayesian generalized linear model using default priors (function bayesglm from the arm package version 1.13-1)87 with a binomial distribution of errors. Number of eggs laid, percentage of eggs hatched and number of progeny per female were analyzed with linear mixed-effect models with a normal distribution of errors (function Lmer from the lme4 package version 1.1-35.1)88. Whenever relevant, model assumptions were assessed using the Dharma package (version 0.4.6)90. All graphs were generated using GraphPad Prism (Version 10.2.0).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The experimental data used to support our findings are available in zenodo with the https://doi.org/10.5281/zenodo.1375249991.
References
de Souza, W. M. & Weaver, S. C. Effects of climate change and human activities on vector-borne diseases. Nat. Rev. Microbiol. 22, 476–491 (2024).
Organization, W. H. Global Arbovirus Initiative, <https://www.who.int/initiatives/global-arbovirus-initiative#:~:text=Arthropod%2Dborne%20viruses%20(arboviruses),approximately%203.9%20billion%20people%20live.> (2024).
Kraemer, M. U. G. et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 4, 854–863 (2019).
Laporta, G. Z. et al. Global Distribution of Aedes aegypti and Aedes albopictus in a Climate Change Scenario of Regional Rivalry. Insects 14, https://doi.org/10.3390/insects14010049 (2023).
Carlassara, M. et al. Population-specific responses to developmental temperature in the arboviral vector Aedes albopictus: Implications for climate change. Glob Chang Biol. 30, e17226 (2024).
Murdock, C. C., Paaijmans, K. P., Cox-Foster, D., Read, A. F. & Thomas, M. B. Rethinking vector immunology: the role of environmental temperature in shaping resistance. Nat. Rev. Microbiol. 10, 869–876 (2012).
Terradas, G. et al. Temperature affects viral kinetics and vectorial capacity of Aedes aegypti mosquitoes co-infected with Mayaro and Dengue viruses. Parasit Vectors 17, 73 (2024).
Schneider, D. S. & Ayres, J. S. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 8, 889–895 (2008).
Prince, B. C., Walsh, E., Torres, T. Z. B. & Ruckert, C. Recognition of Arboviruses by the Mosquito Immune System. Biomolecules 13, https://doi.org/10.3390/biom13071159 (2023).
Tng, P. Y. L. et al. Cas13b-dependent and Cas13b-independent RNA knockdown of viral sequences in mosquito cells following guide RNA expression. Commun Biol. 3, 413 (2020).
Weng, S.-C., Masri, R. A. & Akbari, O. S. Advances and challenges in synthetic biology for mosquito control. Trends Parasitol. 40, 75–88 (2024).
Simms, E. L. Defining tolerance as a norm of reaction. Evol. Ecol. 14, 563–570 (2000).
Antonovics, J. & Thrall, P. H. The Cost of Resistance and the Maintenance of Genetic Polymorphism in Host-Pathogen Systems. Proc. Biol. Sci. 257, 105–110 (1994).
Brown, J. K. Yield penalties of disease resistance in crops. Curr. Opin. Plant Biol. 5, 339–344 (2002).
Simms, E. L. & Triplett, J. Costs and Benefits of Plant Responses to Disease: Resistance and Tolerance. Evolution 48, 1973–1985 (1994).
Ayres, J. S. & Schneider, D. S. Tolerance of infections. Annu. Rev. Immunol. 30, 271–294 (2012).
Medzhitov, R., Schneider, D. S. & Soares, M. P. Disease tolerance as a defense strategy. Science 335, 936–941 (2012).
Raberg, L., Graham, A. L. & Read, A. F. Decomposing health: tolerance and resistance to parasites in animals. Philos. Transac. R. Soc. B 364, 37–49 (2009).
Vale, P. F., Fenton, A. & Brown, S. P. Limiting damage during infection: lessons from infection tolerance for novel therapeutics. PLoS Biol. 12, e1001769 (2014).
Gandon, S. & Michalakis, Y. Evolution of parasite virulence against qualitative or quantitative host resistance. Proc. Biol. Sci. 267, 985–990 (2000).
Roy, B. A. & Kirchner, J. W. Evolutionary dynamics of pathogen resistance and tolerance. Evolution 54, 51–63 (2000).
Miller, C. V. L. & Cotter, S. C. Resistance and tolerance: The role of nutrients on pathogen dynamics and infection outcomes in an insect host. J. Animal Ecol. 87, 500–510 (2018).
Henschen, A. E. & Adelman, J. S. What Does Tolerance Mean for Animal Disease Dynamics When Pathology Enhances Transmission? Integr. Compar. Biol. 59, 1220–1230 (2019).
Miller, M. R., White, A. & Boots, M. The evolution of host resistance: tolerance and control as distinct strategies. J. Theor. Biol. 236, 198–207 (2005).
Restif, O. & Koella, J. C. Concurrent evolution of resistance and tolerance to pathogens. Am. Nat. 164, E90–102 (2004).
Logan, R. A. E. et al. Vertical and Horizontal Transmission of Cell Fusing Agent Virus in Aedes aegypti. Appl. Environ. Microbiol. 88, e0106222 (2022).
Ma, Q. et al. A mosquito small RNA genomics resource reveals dynamic evolution and host responses to viruses and transposons. Genome Res. 31, 512–528 (2021).
Nag, D. K. & Efner, K. Cell fusing agent virus rarely transmits vertically in artificially infected laboratory-colonized Aedes aegypti mosquitoes. Parasit Vectors 17, 177 (2024).
Scott, J. C. et al. Comparison of dengue virus type 2-specific small RNAs from RNA interference-competent and -incompetent mosquito cells. PLoS Negl. Trop. Dis. 4, e848 (2010).
Suzuki, Y. et al. Non-retroviral Endogenous Viral Element Limits Cognate Virus Replication in Aedes aegypti Ovaries. Curr. Biol. 30, 3495–3506.e3496 (2020).
Suzuki, Y. et al. No detectable fitness cost of infection by cell-fusing agent virus in Aedes aegypti mosquitoes. R. Soc. Open Sci. 11, 231373 (2024).
Zhou, N. et al. Cell fusing agent virus isolated from Aag2 cells does not vertically transmit in Aedes aegypti via artificial infection. Parasit Vectors 16, 402 (2023).
Kirstein, O. D. et al. Natural arbovirus infection rate and detectability of indoor female Aedes aegypti from Mérida, Yucatán, Mexico. PLoS Negl. Trop. Dis. 15, e0008972 (2021).
Parry, R., James, M. E. & Asgari, S. Uncovering the Worldwide Diversity and Evolution of the Virome of the Mosquitoes Aedes aegypti and Aedes albopictus. Microorganisms 9, https://doi.org/10.3390/microorganisms9081653 (2021).
Pan, Y. F. et al. Metagenomic analysis of individual mosquito viromes reveals the geographical patterns and drivers of viral diversity. Nat. Ecol. Evol. 8, 947–959 (2024).
Besson, B., Overheul, G. J., Wolfinger, M. T., Junglen, S. & Rij, R. P. v. Pan-flavivirus analysis reveals sfRNA-independent, 3′ UTR-biased siRNA production from an insect-specific flavivirus. J. Virol. 98, e01215–e01224 (2024).
de Faria, I. J. S., de Almeida, J. P. P. & Marques, J. T. Impact of symbiotic insect-specific viruses on mosquito vector competence for arboviruses. Curr. Opin. Insect Sci. 63, 101194 (2024).
IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. (2023).
Waidelich, P., Batibeniz, F., Rising, J., Kikstra, J. S. & Seneviratne, S. I. Climate damage projections beyond annual temperature. Nat. Clim. Chang. 14, 592–599 (2024).
Louie, A., Song, K. H., Hotson, A., Thomas Tate, A. & Schneider, D. S. How Many Parameters Does It Take to Describe Disease Tolerance? PLoS Biol. 14, e1002435 (2016).
Gupta, V. & Vale, P. F. Nonlinear disease tolerance curves reveal distinct components of host responses to viral infection. R. Soc. Open Sci. 4, 170342 (2017).
Dong, S. & Dimopoulos, G. Aedes aegypti Argonaute 2 controls arbovirus infection and host mortality. Nat. Commun. 14, 5773 (2023).
Khorramnejad, A. et al. Reproductive resource allocation correlates with successful global invasion of a mosquito species. bioRxiv, 2024.2007.2018.604133, https://doi.org/10.1101/2024.07.18.604133 (2024).
Bellone, R. et al. Climate change and vector-borne diseases: a multi-omics approach of temperature-induced changes in the mosquito. J. Travel Med. 30, https://doi.org/10.1093/jtm/taad062 (2023).
Morrison, A. C. et al. Quantifying heterogeneities in arbovirus transmission: Description of the rationale and methodology for a prospective longitudinal study of dengue and Zika virus transmission in Iquitos, Peru (2014-2019). PLoS One 18, e0273798 (2023).
Vazquez-Prokopec, G. M. et al. Inapparent infections shape the transmission heterogeneity of dengue. PNAS Nexus 2, pgad024 (2023).
Zardini, A. et al. Estimating the potential risk of transmission of arboviruses in the Americas and Europe: a modelling study. Lancet Planet Health 8, e30–e40 (2024).
Mackay, A. J., Yan, J., Kim, C. H., Barreaux, A. M. G. & Stone, C. M. Larval diet and temperature alter mosquito immunity and development: using body size and developmental traits to track carry-over effects on longevity. Parasit Vectors 16, 434 (2023).
Mordecai, E. A. et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 22, 1690–1708 (2019).
Thomas, M. B. Epidemics on the move: Climate change and infectious disease. PLoS Biol. 18, e3001013 (2020).
Coumou, D. & Rahmstorf, S. A decade of weather extremes. Nat. Clim. Chang. 2, 491–496 (2012).
Penteado, A. B. et al. Binding Evolution of the Dengue Virus Envelope Against DC-SIGN: A Combined Approach of Phylogenetics and Molecular Dynamics Analyses Over 30 Years of Dengue Virus in Brazil. J. Mol. Biol. 436, 168577 (2024).
Best, A., White, A. & Boots, M. Maintenance of host variation in tolerance to pathogens and parasites. Proc. Natl Acad. Sci. USA 105, 20786–20791 (2008).
Schwenke, R. A., Lazzaro, B. P. & Wolfner, M. F. Reproduction-Immunity Trade-Offs in Insects. Annu. Rev. Entomol. 61, 239–256 (2016).
Graham, A. L., Allen, J. E. & Read, A. F. Evolutionary Causes and Consequences of Immunopathology. Annu. Rev. Ecol. Evol. Syst. 36, 373–397 (2005).
da Silveira, I. D. et al. Zika Virus Infection Produces a Reduction on Aedes aegypti Lifespan but No Effects on Mosquito Fecundity and Oviposition Success. Front. Microbiol. 9, 3011 (2018).
Lambrechts, L. & Scott, T. W. Mode of transmission and the evolution of arbovirus virulence in mosquito vectors. Proc. Biol. Sci. 276, 1369–1378 (2009).
Styer, L. M., Meola, M. A. & Kramer, L. D. West Nile virus infection decreases fecundity of Culex tarsalis females. J. Med. Entomol. 44, 1074–1085 (2007).
Magistrado, D., El-Dougdoug, N. K. & Short, S. M. Sugar restriction and blood ingestion shape divergent immune defense trajectories in the mosquito Aedes aegypti. Sci. Rep. 13, 12368 (2023).
Maraschin, M. et al. Exploring dose-response relationships in Aedes aegypti survival upon bacteria and arbovirus infection. J. Insect Physiol. 151, 104573 (2023).
Merkling, S. H. et al. The epigenetic regulator G9a mediates tolerance to RNA virus infection in Drosophila. PLoS Pathog. 11, e1004692 (2015).
Martin, E. et al. Cell fusing agent virus (Flavivirus) infection in Aedes aegypti in Texas: seasonality, comparison by trap type, and individual viral loads. Arch. Virol. 165, 1769–1776 (2020).
Chen, J., Deng, S. & Peng, H. Insect-specific viruses used in biocontrol of mosquito-borne diseases. Interdisciplinary Med. 1, e20220001 (2023).
Hollingsworth, B. D., Grubaugh, N. D., Lazzaro, B. P. & Murdock, C. C. Leveraging insect-specific viruses to elucidate mosquito population structure and dynamics. PLoS Pathog. 19, e1011588 (2023).
Wojda, I. Temperature stress and insect immunity. J. Therm. Biol. 68, 96–103 (2017).
Delrieu, M. et al. Temperature and transmission of chikungunya, dengue, and Zika viruses: A systematic review of experimental studies on Aedes aegypti and Aedes albopictus. Curr. Res. Parasitol. Vector Borne Dis. 4, 100139 (2023).
Mercier, A. et al. Impact of temperature on dengue and chikungunya transmission by the mosquito Aedes albopictus. Sci. Rep. 12, 6973 (2022).
Qu, J. et al. The Hsf1-sHsp cascade has pan-antiviral activity in mosquitoes. bioRxiv, 2023.2002.2021.529413, https://doi.org/10.1101/2023.02.21.529413 (2023).
Ware-Gilmore, F., Novelo, M., Sgro, C. M., Hall, M. D. & McGraw, E. A. Assessing the role of family level variation and heat shock gene expression in the thermal stress response of the mosquito Aedes aegypti. Philos. Trans. R Soc. Lond B Biol. Sci. 378, 20220011 (2023).
Condé, R. et al. Heat Shock Causes Lower Plasmodium Infection Rates in Anopheles albimanus. Front. Immunol. 12, 584660 (2021).
Mack, L. K. & Attardo, G. M. Heat shock proteins, thermotolerance, and insecticide resistance in mosquitoes. Front. Insect Sci. 4, 1309941 (2024).
Sivan, A., Shriram, A. N., Muruganandam, N. & Thamizhmani, R. Expression of heat shock proteins (HSPs) in Aedes aegypti (L) and Aedes albopictus (Skuse) (Diptera: Culicidae) larvae in response to thermal stress. Acta Trop 167, 121–127 (2017).
Couper, L. I., Farner, J. E., Lyberger, K. P., Lee, A. S. & Mordecai, E. A. Mosquito thermal tolerance is remarkably constrained across a large climatic range. Proc. Biol. Sci. 291, 20232457 (2024).
Dennington, N. L. et al. Phenotypic adaptation to temperature in the mosquito vector, Aedes aegypti. Glob. Chang. Biol. 30, e17041 (2024).
Bonizzoni, M. et al. Complex modulation of the Aedes aegypti transcriptome in response to dengue virus infection. PLoS One 7, e50512 (2012).
Palmer, W. H., Varghese, F. S. & van Rij, R. P. Natural Variation in Resistance to Virus Infection in Dipteran Insects. Viruses 10, https://doi.org/10.3390/v10030118 (2018).
Tikhe, C. V. & Dimopoulos, G. Mosquito antiviral immune pathways. Dev. Comp. Immunol. 116, 103964 (2021).
Chen, X. G. et al. Genome sequence of the Asian Tiger mosquito, Aedes albopictus, reveals insights into its biology, genetics, and evolution. Proc. Natl Acad. Sci. USA 112, E5907–E5915 (2015).
Palatini, U. et al. Improved reference genome of the arboviral vector Aedes albopictus. Genome Biol. 21, 215 (2020).
Weather, W. <https://world-weather.info/forecast/italy/rome/august-2021/> (2021).
Shetty, V., Adelman, Z. N. & Slotman, M. A. Effects of circadian clock disruption on gene expression and biological processes in Aedes aegypti. BMC Genom. 25, 170 (2024).
Reynolds, J. A., Poelchau, M. F., Rahman, Z., Armbruster, P. A. & Denlinger, D. L. Transcript profiling reveals mechanisms for lipid conservation during diapause in the mosquito, Aedes albopictus. J. Insect Physiol. 58, 966–973 (2012).
Pawar, S. et al. Variation in temperature of peak trait performance constrains adaptation of arthropod populations to climatic warming. Nat. Ecol. Evol. 8, 500–510 (2024).
Fox, J. & Weisberg, S. An R companion to applied regression. (Sage publications, 2018).
Searle, S. R., Speed, F. M. & Milliken, G. A. Population Marginal Means in the Linear Model: An Alternative to Least Squares Means. Am. Stat. 34, 216–221 (1980).
Therneau, T. A package for survival analysis in S. R Package Version 2, 2014 (2015).
Gelman, A. et al. arm: Data analysis using regression and multilevel/hierarchical models. (2007).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 67, 1–48 (2015).
Ripley, B. MASS: support functions and datasets for Venables and Ripley’s MASS. R package version 7, 3–29 (2011).
Hartig, F. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R Packag version 020 (2018).
Perdomo, H. D. et al. Prolonged exposure to heat enhances mosquito tolerance to viral infection [Data set]. Zenodo, https://doi.org/10.5281/zenodo.13752499 (2024).
Acknowledgements
We are grateful to Dr. Chloe Lahondere from the Virginia Tech and all members of the Bonizzoni’s lab. for fruitful discussion. Authors would like to thank the following institutions for their financial support of research: Italian Ministry of Education, Universities and Research (MIUR) PRIN project 2022J45MLL; EU funding within the NextGeneration EU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT). A.Kh. was recipient of the Early-Career Award funded by the INF-ACT Foundation (INF-ACT/ECA/2024).
Author information
Authors and Affiliations
Contributions
H.D.P. designed and performed the experiments, analysed the results, designed the figures and wrote the manuscript. A.Kh. designed and performed the experiments and revised the manuscript. N.M.C. performed the molecular lab work. A.Kr. analysed the results. D.S. performed the experiments. MB. acquired the funding for the research, analysed the results and wrote the manuscript with contribution from all authors. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Michele Repetto. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Perdomo, H.D., Khorramnejad, A., Cham, N.M. et al. Prolonged exposure to heat enhances mosquito tolerance to viral infection. Commun Biol 8, 168 (2025). https://doi.org/10.1038/s42003-025-07617-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-07617-8