Abstract
Transmarine distribution and gigantism in the Late Cretaceous North American crocodyliform Deinosuchus has been difficult to reconcile with consistently inferred phylogenetic relationships to alligatorids, an otherwise freshwater and smaller-bodied group. We present an expanded phylogeny with increased spatiotemporally coherence that reinterprets species of Deinosuchus as stem-group crocodylians together with further putative alligatoroids, Leidyosuchus canadensis and the European Diplocynodon spp. (closely related to North American Borealosuchus). The novel topology elucidates the evolution of osmoregulation in Crocodylia and its close relatives by inferring plesiomorphic saltwater tolerance for Deinosuchus and the crown-group as well as secondary loss already in stem-group alligatorids. Divergence of Alligatoroidea coincided with extreme mid-Cretaceous sea level highs and the distribution of Deinosuchus across the Western Interior Seaway can be best explained by marine dispersal. Phylogenetic body-length analysis using a head-width proxy reveals phyletic dwarfism early in alligatoroid evolution and a reasonable total length estimate for the most complete specimen of Deinosuchus riograndensis. We find that gigantism in crocodyliforms is correlated with high-productive extensive aquatic ecosystems in the present and the past.
Similar content being viewed by others
Introduction
The history of Alligatoroidea, the total (stem + crown) group of extant alligators and caimans (Alligatoridae), can be traced back to the Late Cretaceous of North America. Previous phylogenies of extinct taxa implicitly suggest that the early evolution of the group was already characterised by high morphological disparity and complex biogeographic histories, implying rapid rates of evolution1,2,3,4. Several early alligatoroids from the Late Cretaceous (e.g., Brachychampsa spp., Albertochampsa langstoni, Stangerochampsa mccabei) overall fit an expected ancestral body-plan for the group and were characterised by a relatively small size, short and blunt snout, overbite dental occlusion, enlarged 4th maxillary tooth, molariform posterior dentition, and an initial distribution restricted to Laramidia, the western part of North America once bisected by the extensive epicontinental Western Interior Seaway (WIS)1,5. A putative alligatoroid has been reported from the Atlantic coast but its age postdates the existence of the WIS6. The absence of unambiguous alligatoroids in Appalachia, together with depositional environments7,8,9, imply a shared lack of saltwater tolerance with extant alligatorids10. In contrast, other species recovered as early diverging members of the group, like Deinosuchus spp. and Diplocynodon spp. resembled crocodyloids or stem-group crocodylians in having partly interfingering dental occlusion, an occlusal notch between the premaxilla and maxilla for the 4th dentary tooth, enlarged 4th and 5th maxillary teeth, narrower and longer snout, larger or even gigantic body-size, and transmarine distribution4,11. In addition to its extremely large body-size, the ‘terror-crocodile’ Deinosuchus12 furthermore possessed highly derived morphological specializations3 and, together with Diplocynodon, have also utilised coastal marine habitats13,14,15. Moreover, the earliest alligatoroid record (~82 Ma) already includes both of these highly divergent morphotypes (Deinosuchus and Brachychampsa sealyei16,17) potentially implying a significantly earlier origin of the group. Most molecular divergence age estimates, however, do not suggest an earlier age than ∼90 Ma18,19. Diplocynodon further complicates the picture with its early branching position within the group that is in turn conflicting with an endemic European distribution and comparatively late first appearance date (late Paleocene)1,4,20. Morphology, body size, stratigraphic age, biogeography, and physiology are therefore markedly difficult to reconcile with alligatoroid phylogeny.
We present an expanded molecular-informed morphological phylogeny and find that character states previously diagnosing Alligatoroidea have a broader taxonomic distribution, thereby recovering both Deinosuchus and Diplocynodon as stem-crocodylians. The novel topology explains the geographic distribution of Deinosuchus with inferred salt tolerance and resolves the biogeographic history of Diplocynodon. In light of the resulting simpler biogeographic pattern, we hypothesise that the basal split of crown-group crocodylians, involving caiman and alligator ancestors, was triggered by extreme mid-Cretaceous sea level rise. Finally, body-size analysis combined with the new phylogeny elucidates the body size evolution of Deinosuchus, alligatoroids, and other crocodyliforms.
Results and discussion
Phylogenetic analysis
Expansions and modifications implemented in the present morphological taxon-character matrix included the merging of published data subsets, addition of characters and character states, rigorous redefinitions of select characters, homology reassessments, update of select character state scores, addition of taxa, and the inclusion of a molecular scaffold in the phylogenetic analysis (see Supplementary information and Supplementary Data 1). All the datasets combined here are expansions of Brochu [1999]1. Our maximum parsimony analysis recovered 506 most parsimonious trees (strict consensus tree reported in Figs. 1, 2 and Supplementary Fig. 1). The most unstable taxa (Eocaiman spp. and Necrosuchus ionensis) were pruned from the strict consensus tree to increase resolution (see Supplementary Fig. 1 for their respective placement). Alligatoroidea is formed by Orientalosuchina and its sister-group Alligatoridae (total group of caimans and alligators). Globidonta, the stem-based lineage comprising Alligator mississippiensis and all crocodylians more closely related to it than to Diplocynodon ratelii1, is here redundant with Alligatoroidea because Diplocynodon is recovered as a non-alligatoroid stem-crocodylian. In contrast to previous phylogenies (Brochu [1999]1 and all subsequent expansions), Leidyosuchus canadensis, Deinosuchus spp., and Diplocynodon spp. form the stem-lineage of crown-group crocodyliforms instead of Alligatoridae (Fig. 1). This is in part due to the addition of two key Paleocene taxa to the dataset, Diplocynodon remensis and Borealosuchus griffithi. Diplocynodon spp. is recovered as a monophyletic clade, nested in a polytomic Borealosuchus from North America. This polytomy is caused by the occasional recovery of Borealosuchus griffithi as the sister taxon to Diplocynodon spp. in some trees, sharing a hypapophyseal keel present up to the 12th vertebra and a greatly reduced quadratojugal spine.
Borealosuchus griffithi has two alternative positions, either as sister to Diplocynodon spp. or an early diverging placement within Borealosuchus spp. (Supplementary Data 1 “Walter et al_[TNT]”). Purple diamonds and silhouettes correspond to known occurrences of very large to giant (≥7 m) crocodyliforms. Each of the illustrated taxa is associated with high-productivity wetland or marine habitats (see Supplementary information Table S1 for a list of taxa and sources). Ages are in Ma.
Purple diamonds and silhouettes correspond to known occurrences of very large to giant (≥7 m) crocodyliforms. Each of the illustrated taxa is associated with high-productivity wetland or marine habitats (see Supplementary information Table S1 for a list of taxa and sources). Ages are in Ma.
The strict consensus tree recovers a basal polytomy within Alligatoridae formed by Alligatorinae, Caimaninae (including Bottosaurus harlani), and the North American Late Cretaceous taxa Brachychampsa spp., Stangerochampsa mccabei, and Albertochampsa langstoni. The latter have two alternative positions either in Alligatorinae or along stem-Alligatoridae.
Phylogenetic body-size estimation
In light of the novel topology, with Deinosuchus spp., Leidyosuchus canadensis, and Diplocynodon spp. removed from Alligatoroidea, all early representatives of this clade were relatively small-sized and we therefore wanted to test whether the origin of alligatoroids was characterised by phyletic dwarfing. In addition, we wanted to test the impact of the phylogenetic correction and the current topology on body-size estimates of Deinosuchus spp. relative to previous studies. Previous works addressing Deinosuchus body-length did not employ phylogenetic correction and instead included it in a regression of distantly related extant taxa (Crocodylus porosus, Alligator mississippiensis12) with likely different body proportions5. Phylogenetic body-size estimates provide results that take into account the known or reconstructed proportions of close extant relatives21. We here used the same individual of D. riograndensis as previous non-phylogenetic work (TMM 43620-111,12 see Supplementary Data 2.3, and Supplementary information for the list of sources) but a skull width proxy21 instead of skull/mandible length, making the comparison only partly appropriate. However, skull width has been argued to be more reliable as it is less influenced by differences in body proportions caused by a long snout, a trait present in species of Deinosuchus3,21. Mean values of estimated body-length together with lowest and highest quantiles are provided in Supplementary Data 2.3, and a parsimony ancestral state reconstruction of size bins is shown in Fig. 3. We here divide body-sizes according to the following categories based on extant species: total length (TL) estimations of ca. 1.5 m and lower are considered small size, representing the general body-size of some fossil species and exceptionally small individuals of extant species. Medium size category includes TL estimations between 1.5 and 4.0 m, and comprises all extant species. Large size category includes TL estimations between 4.0 and 7 m and includes large to maximal body length of extant species22 (e.g. Crocodylus porosus, Gavialis gangeticus). TL estimations above 7 m are considered gigantic and are only known in extinct species23,24. The divergence of Alligatoroidea was coupled with size reduction and an ancestral body-length of 150–200 cm compared to 250–300 cm of the outgroup. Most Paleogene alligatoroids of North America retained a medium to small size or went through further shrinking including some taxa that are inferred to be relatively more terrestrial25. Larger size independently evolved in the lineage containing extant Alligator mississippiensis and its extinct Miocene relatives, as well as extant Melanosuchus niger and a clade of South American Miocene caimanines, involving independent gigantism in Purussaurus spp. and Mourasuchus amazonensis according to the present topology (Fig. 3). Species of the stem-crocodylian Deinosuchus acquired giant sizes although our estimates give shorter, and possibly more realistic, total body length compared to previous work12. The detailed results of the analysis are available in Supplementary Data 2.3.
Stem-crocodylian affinities of Deinosuchus can explain transmarine distribution through saltwater tolerance
Our maximum parsimony analysis resulted in a topology where several taxa previously considered to represent stem-alligatorids (i.e., all studies descending from that of Brochu1: e.g. refs. 3,4,20,21,22,23,26,27), such as Deinosuchus spp., Leidyosuchus canadensis, and Diplocynodon spp., are reinterpreted as stem-group crocodylians, regardless of the addition of the molecular scaffold (Fig. 1). The placement of these taxa along stem-crocodylians is more consistent with their plesiomorphic morphology relative to other alligatoroids1, their stratigraphic and geographic distribution, and the fact that Diplocynodon shares a number of remarkable derived traits with the stem-crocodylian Borealosuchus. Some of our results are congruent with recent published analyses using different datasets: some but not all analyses of Groh et al.28 employing quantitative characters recovered Diplocynodon spp. and Leidyosuchus canadensis as stem-crocodylians28 and Rio and Mannion4 recovered a paraphyletic Diplocynodon sister to the lineage of Longirostres also using quantitative characters in some of their analyses. Muscioni et al.29, based on a more similar dataset to that of the present study, found Diplocynodon, Leidyosuchus canadensis, as well as Deinosuchus riograndensis in a polytomy with Crocodylia.
Species of Deinosuchus from the Late Cretaceous (Campanian) coastlines of the North American Western Interior Seaway (WIS) and Atlantic have been considered among the largest crocodyliforms of all time with a body length previously estimated around 10 m3,12,30,31. Bite mark evidence suggests their diet even included large dinosaurs11,32,33. The first phylogeny including Deinosuchus1 found this taxon as an early diverging member of total-group Alligatoridae. All subsequent works, including a recent comprehensive revision of Deinosuchus3, confirmed this placement despite marked morphological contrast relative to contemporaneous early alligatoroids, such as Brachychampsa16,17.
The phylogeny herein, on the other hand, finds Deinosuchus schwimmeri and D. riograndensis outside Alligatoroidea, along the stem-lineage of crown-group crocodyliforms (Crocodylia). In other words, Deinosuchus was neither a ‘greater alligator’34 or a ‘terror crocodile’12. Our expanded dataset is overlapping in taxon and character sample with previous studies including D. schwimmeri and D. riograndensis1,23,26 and our character state scorings follow the latest work updating this taxon3. The more stemward position in our study is largely due to the addition of two key Paleocene taxa to the dataset, Diplocynodon remensis and Borealosuchus griffithi, which resulted in the placement of Diplocynodon spp., Deinosuchus spp. and Leidyosuchus canadensis as stem-crocodylians in our analysis. These three taxa share the above listed differences from true early alligatoroids (except large body size) and their stem-crocodylian placement is retained even with the removal of the molecular scaffold from our analysis. Deinosuchus is excluded from Crocodylia by lacking the following traits among others: an incisive foramen that abuts the toothrow, a single largest maxillary alveolus that is the 5th, and a frontoparietal suture that makes a modest entry into the supratemporal fenestrae. Some previous alligatoroid synapomorphies are now optimised as crocodylian plesiomorphies (Supplementary information, 2.1). This novel stem-crocodylian position of Deinosuchus spp. is consistent with its early stratigraphic age (early Campanian), plesiomorphic morphology3, and would also imply less homoplasies3 (e.g. character 71:0 was convergent with Borealosuchus but here optimised as a plesiomorphy for Crocodylia). Species of Deinosuchus nevertheless still share convergent characters with long-snouted taxa3 even in the current topology. Scoring Deinosuchus riograndensis in a different dataset (Rio & Mannion4) resulted in a relatively deeply nested position within Alligatoroidea, but we nevertheless find this highly doubtful due to the particularly poor stratigraphic fit of this topology and the ambiguous synapomorphies uniting Alligatoroidea, some of which are present only in a few of the ingroup taxa whereas others are present in several of the outgroup taxa as well (Supplementary information, 2.2).
Deinosuchus as a stem-crocodylian is furthermore more consistent with its biogeographic distribution on both sides of the Western Interior Seaway (WIS) in contrast to early members of true early alligatoroids restricted to the West until the retreat of the seaway6. Cossette & Brochu3 recently proposed that species of Deinosuchus were allopatric, with D. riograndensis distributed along the western coast of the WIS (Laramidia) and D. schwimmeri along the eastern and Atlantic coasts (Appalachia). The authors suggested that speciation in the clade occurred through vicariance, during the opening phase of the WIS, separating Deinosuchus ancestral populations. The main rationale behind this was due to the supposed alligatoroid affinity of Deinosuchus with extant relatives lacking lingual salt glands, which would render them incapable of osmoregulation and prolonged saltwater exposure required for crossing the extensive WIS11,35,36,37,38,39. The herein proposed stem-crocodylian position, however, no longer infers lack of osmoregulation and may explain the distribution of Deinosuchus through dispersal across the WIS. Saltwater tolerance is inferred to be plesiomorphic for Longirostres4,40 and may well have been plesiomorphic for Crocodylia as many stem-group taxa close to the crown appear to be euryhaline13. These include marine thoracosaurs (recovered as stem-crocodylians in tip-dated phylogenies)41, potentially Diplocynodon, occasionally recovered from marginal marine settings14,15, and Deinosuchus itself, which is mostly recorded from estuarine or nearshore habitats such as coastal plains, deltas or platform contexts11. Moreover, stable isotope analysis of carbon and oxygen from eastern Deinosuchus tooth enamel samples suggest consumption of seawater or marine prey13, the latter also supported by bite mark evidence of predation on nearshore marine turtles11. The simultaneous disappearance of Deinosuchus from the fossil record (supposed extinction) with the draining of megawetlands along the WIS and Atlantic coasts (including complete retreat of the former) later during the Cretaceous is furthermore consistent with a lifestyle linked to coastal habitats42,43. Borealosuchus may serve as an additional example for salt-tolerant stem-crocodylians as it is known to co-occur with Deinosuchus in the Moorville Chalk of Alabama, a marginal marine setting44. Taking this data together, our parsimony ancestral state reconstruction, including data from this study, implies that the presence of saltwater tolerance (osmoregulation) may have been plesiomorphic for Crocodylia (Fig. 4) and simply retained in species of Deinosuchus. Nevertheless, this does not mean that osmoregulation was necessarily achieved through the presence of lingual salt glands. Saltwater tolerance, possibly including lingual salt glands, were subsequently lost in alligatoroids and Gavialis45. Previous phylogenies left it ambiguous whether salt glands (with no known osteological correlates) were already lost in stem-group alligatoroids only1 and recent work proposed that salt tolerance may have been only lost in the crown-group 46. The topology of the present study, however, implies the loss of effective osmoregulation (possibly including lingual salt glands) in the stem-lineage as all early true alligatoroids in the new phylogeny come from freshwater deposits7,8,9 (Fig. 4).
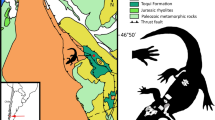
(A) Distribution of Deinosuchus riograndensis and D. schwimmeri3 during the Campanian around the Western Interior Seaway (WIS). (B) parsimony ancestral state reconstruction (equal branch length) of osmoregulation in Crocodylia and close relatives using presence/absence of salt glands, stable isotopes, and coastal marine occurrences as proxies11,13,14,15,17,35,36,37,38,39,40,42,44,120. The topology is from the present study except for ‘thoracosaurs’ for which we follow a more appropriate tip-dated work41. The analysis suggests potential plesiomorphic saltwater-tolerance for Deinosuchus and Crocodylia with early loss in Alligatoroidea. The distribution of Deinosuchus may be explained by dispersal across the WIS. Map is redrawn from118, early to late Campanian. Distribution of Deinosuchus spp. follows3,11,17 and references therein.
Morphological differences in western Deinosuchus riograndensis and eastern D. schwimmeri are relatively minor except for body size, with known specimens of the western taxon being considerably larger3. If speciation took place, dispersal is more consistent with the novel phylogeny than vicariance. Isolation would have been maintained through the episodic nature of the dispersal due to the significant width of the seaway. A literal reading of the fossil record would imply an east to west dispersal as eastern records are so far somewhat older11, but this simply may be a sampling bias in the fossil record of Deinosuchus.
Body-size estimation of Deinosuchus and evolution of gigantism in crocodyliforms
Previous work estimated the total body-length of Deinosuchus spp. between ca. 8 and 12 m (up to 12 m11; 7.67 and 10.640 m31; and 7.73 to 8.13 m47). Total body-length has been shown to more strongly correlate with head-width than with cranial length given the variability of rostral proportions among crocodylians48. Because Deinosuchus has a relatively long snout3, previous approaches11,12 may have overestimated the total length of this taxon, as they based their regression on shorter-snouted taxa, Alligator mississippiensis and Crocodylus spp. Our method of estimation differs from that of previous studies in employing a skull width proxy21 instead of femur31, mandible11,12 or vertebra11,47 and furthermore includes a phylogenetic correction to bypass the use of a unique extant proxy (e.g., Alligator mississippiensis, Crocodylus porosus) as body size proportions show strong phylogenetic structuring in crocodylians21. The phylogenetic approach, however, is still only sampling living crocodylians, a fraction of past morphological diversity, and body proportions of extinct taxa, particularly those (but not only) outside the clade may have significantly differed (including Deinosuchus). This implies that, following O’Brien et al.21, outer quartile estimates should be considered for taxa showing “sufficient biological evidence to presume that body size should be meaningfully greater or lesser than the mean estimate (e.g., terrestrial versus fully aquatic denizens, tail, or head size atypically large or small in a given taxon)”. Concerning the present estimate, we consider the 97.5 percentile estimate (7.66 m total length for Deinosuchus riograndensis and 6.37 m for Deinosuchus schwimmeri; see Supplementary Data 2.3) more realistic than the mean (5.80 m for Deinosuchus riograndensis and 4.83 m for Deinosuchus schwimmeri). Our reasoning is that the 97.5 percentile estimates lie between the conservative mean of our estimates and previous non-phylogenetic estimations using cranial length. Our estimate of D. riograndensis is based on the skull of the same individual as in Schwimmer11 (9.8 m) and Erickson and Brochu12 (8.43 to 9.10 m) who, however, both used the length of the lower jaw of the specimen. Furthermore, previous applications of phylogenetic body-size estimations systematically found lower mean estimates compared to non-phylogenetic methods21,48,49. The maximal size of D. riograndensis was, nevertheless, likely larger than even our 97.5 percentile estimates as evidenced by the larger size of the holotype specimen (AMNH 3073) compared to the specimen used in our study (TMM 43620-1).
Very large to gigantic body size (here defined as ≥ 7 m total length) has repeatedly evolved during the history of crocodyliforms and represents a wide range of taxa across the phylogenetic tree known from the Cretaceous to the present50,51,52,53,54 (Figs. 1 and 2). Previous work has underlined the importance of aquatic to semi-aquatic lifestyle52 and temperature50,55,56 in driving large body-size in crocodyliforms, but the triggers of extreme sizes across clades have not been explicitly addressed. We propose that Deinosuchus exemplifies an ecological pattern that has been universally characteristic of giant crocodyliforms and their ecosystems. Species of Deinosuchus were inhabitants of a marginal mega-wetland along the WIS and the Atlantic east coast, sustaining other extremely large megafauna species during highly favourable mean annual temperatures for growth11,42. Other species of giant crocodyliforms (e.g. Rhamphosuchus, Phosphatosaurus, Sarcosuchus, Crocodylus porosus; for a complete list, see Supplementary information Table S1) are/were likewise associated with extraordinarily productive and spatially extensive warm marine or wetland ecosystems including other megafauna. A proposed relationship of extreme body-size and ecosystem productivity is well in accordance with a global analysis of phanerozoic animals, excluding crocodyliforms, which found that the critical factor for gigantism is an unusually highly structured ecosystem in which productivity imposes only exceptionally low limits to sustain extraordinary body-size57. Favourably constant warm temperatures42, an evolutionary shift to rapid growth rates early in ontogeny 51, and elevated long-term ecosystem productivity can be therefore considered key for the evolution of gigantism in crocodyliforms. The existence of very large, ~ 7 m long crocodylians in the present and Pleistocene icehouse conditions (Crocodylus porosus51, C. thorbjarnarsoni23, Crocodylus sp.58) suggests that, contrary to what the literal reading of the fossil record implies, a world with enormous crocodyliforms may have been rather the norm than the exception in the last ~ 130 million years.
Systematics of Diplocynodon and implications for Euramerican paleobiogeography
Another novel aspect of the phylogeny presented in our study is the placement of the European Cenozoic Diplocynodon outside Crocodylia in a monophyletic group with species of North American Borealosuchus (Fig. 1; B. griffithi has two alternative positions within the clade). This novel result is largely the impact of the addition of the geologically earliest known species of Diplocynodon, D. remensis (late Paleocene) as well as the early Paleocene Borealosuchs griffithi to our dataset. Pre-cladistic work has long acknowledged the high morphological similarities between Diplocynodon and Borealosuchus59,60,61,62 but this signal was never recovered in phylogenetic analyses (e.g. refs. 1,4,20,23,26,63,64,65,66). Several plesiomorphies of Diplocynodon are shared with Borealosuchus and Deinosuchus but are absent in typical alligatoroids (e.g., long snout, confluent 3rd and 4th dentary alveoli, 4th and 5th maxillary alveoli equal in size, notch between premaxilla and maxilla in adults). A key character previously placing Diplocynodon in Alligatoroidea is the presence of a premaxillary-maxillary pit (instead of a notch) for the reception of the dentary fang early in ontogeny. The notch seen in adult Diplocynodon (the inferred plesiomorphic condition for Crocodylia) is secondary, developed later in ontogeny due to abrading occlusion1. However, the early ontogenetic pit is not confirmed for all species of Diplocynodon and more importantly, the condition remains unknown for Borealosuchus spp. and other stem-crocodylians1,64. The taxonomic distribution of the early ontogenetic premaxillary-maxillary pit is therefore ambiguous and might diagnose a more inclusive clade. On the other hand, some of the shared traits between Diplocynodon and Borealosuchus are derived and include the presence of ventral armour made of bipartite osteoderms (otherwise only known in Tsoabichi greenriverensis and extant caimanines), the exclusion of the nasals from the external naris, unequal anterior processes of the surangular, and the presence of occlusion pits between the 7th and 8th maxillary alveoli. Indeed, our phylogeny optimises these three character states as synapomorphies uniting the clade Diplocynodon + Borealosuchus.
This topology has far better stratigraphic fit for species of Diplocynodon and Borealosuchus compared to previous phylogenies: for the first time, we recover the oldest species (i.e. the late Paleocene D. remensis and the Late Cretaceous B. sternbergii) of each clade as also the earliest branching taxa. Although the early Paleocene Borealosuchus griffithi has an unresolved position in our phylogeny, several of our most parsimonious trees place this species as the sister taxon of Diplocynodon spp. Under this particular topology, Borealosuchus is paraphyletic and the ghost lineage of nearly 20 Myrs inferred by previous phylogenies (with Diplocynodon spp. as early branching alligatoroids) are reduced to ca. 6 Myrs. Notably, Diplocynodon remensis and Borealosuchus griffithi both share the derived trait of a shallow recess on the medial wall of the premaxillary-maxillary notch20, a character yet to be included in a phylogeny and explored for other species of Diplocynodon and Borealosuchus. A clade of (Borealosuchus spp. (B. griffithi + Diplocynodon spp.)) is implying a single dispersal from North America to Europe during the Paleocene. The earliest known occurrence of Diplocynodon in the late Paleocene of Europe (Diplocynodon remensis20) may underestimate the timing of the dispersal since a high number of North American species immigrated via Greenland and Scandinavia to Europe already during the early and middle Paleocene using the De Geer route67,68,69,70,71,72,73,74,75. In light of the herein recovered stem-crocodylian status of Diplocynodon (Fig. 4), a dispersal through a marine route cannot be excluded. A comprehensive revision of Paleogene Borealosuchus may contribute to testing or further refining these hypotheses.
Implications for crocodyliform extinction across the Eocene/Oligocene cooling
Our topology has implications for phylogenetic patterning of high crocodyliform extinction rates across the cooling climate of the Eocene/Oligocene transition in North America and Europe42. Previous phylogenies implied that all crocodyliform survivors in terrestrial ecosystems were alligatoroids, including Diplocynodon1,76,77,78,79,80. In contrast, the topology herein suggests a survival pattern less structured by phylogeny: in Europe, the stem-crocodylian Diplocynodon spp., whereas in North America, the alligatorine lineage leading to Alligator spp. crossed the transition63,81. On the other hand, the herein proposed sister-taxon of Diplocynodon, the North American Borealosuchus, did not survive into the Oligocene (with the last occurrence known from the middle Eocene; Borealosuchus wilsoni4,64). This divergent survival pattern may be best explained by independent cold adaptation in Diplocynodon and the lineage leading to Alligator. It has been previously proposed that following global cooling, shrinking habitats led to increased competition between large and small-bodied crocodylians and selective extinction of small-sized taxa52. An alternative explanation consistent with our body-size analysis, at least for alligatoroids, is that small-sized lineages evolved large body-sizes during the Neogene without selective extinction of small taxa.
Early alligatoroid evolution
In contrast to previous global phylogenies (refs. 1,3,4,21,23,26,27,63,82,83), the analysis herein advocates a less inclusive alligatoroid clade (Fig. 2). Under this topology, previously recovered synapomorphies for Alligatoroidea, including Deinosuchus (e.g. foramen aëreum set in from the margin of the retroarticular process, occlusion of anterior dentary teeth lingual to maxillary teeth, quadratojugal spine located between the posterior and superior angles of the infratemporal fenestra; see Cossette and Brochu3) are reoptimized to diagnose a more inclusive clade (Supplementary information 2.1). The earliest representatives of Alligatoroidea are herein restricted to only a few taxa from the Late Cretaceous of North America (Brachychampsa spp., Stangerochampsa mccabei, and Albertochampsa langstoni8,9,16), here recovered either as representatives of stem Alligatoridae or the early branching Alligatorinae (total group of Alligator spp.). Both alternatives would make the name Globidonta1 redundant with Alligatoroidea. This restricted taxonomic composition has a better stratigraphic fit owing to the removal of the stratigraphically old and morphologically specialised Deinosuchus. It also implies less homoplasy and eliminates the phenetic contrast with taxa previously inferred as early branching alligatoroids. In turn, taxa replaced as stem-crocodylians are arranged in a topology with a better stratigraphic fit, such as Diplocynodon and Borealosuchus (see above).
Almost all Cretaceous alligatoroids, under the novel topology, share a relatively reduced body-size compared to other non-alligatoroid crocodylians, suggesting phyletic dwarfism84 early during the evolution of the group (Fig. 3). An exception is Brachychampsa montana, which retains a body-size comparable to the ancestral condition of Crocodylia. Bottosaurus harlani is another large-sized early putative alligatoroid6 but its affinity with the group has been questioned63. Additionally, all Cretaceous alligatoroids share a short and blunt snout, full overbite dental occlusion, a caniniform 4th maxillary tooth, crushing posterior dentition, a North American Laramidian distribution, and freshwater habitat. The only exceptions in our topology are representatives of Late Cretaceous–Paleogene Orientalosuchina that are here recovered as the earliest diverging alligatoroids and are characterised by plesiomorphies including a 5th maxillary caniniform tooth, a notch between the premaxilla-maxilla for the reception of the 4th dentary tooth, as well as a strictly Asian distribution26,83,85,86,87. The global phylogenetic relationships of Orientalosuchina, however, has been unstable and studies variously placed them in stem-group Alligatoridae26,63,83,88, Crocodyloidea86,87,88,89, Caimaninae (Walter et al.63 under equal weighting), and Australian Mekosuchinae90. The alligatoroid position of Orientalosuchina in our phylogeny is not well supported since most synapomorphies uniting the two groups are unknown in most orientalosuchines and the outgroup (Supplementary information 2.1 and Supplementary Data 1). Additionally, their endemic Asian distribution is in contrast with that of all other early alligatoroids and would imply an early dispersal to Asia during the Late Cretaceous, a route otherwise poorly supported26.
Except for Orientalosuchina, the simplified paleobiogeographic pattern inferred by our topology is consistent with a vicariant divergence between Alligatoroidea and its sister-clade, Longirostres (Crocodylidae + Gavialidae18). Most early and living representatives of Longirostres have an Asian origin and/or distribution4, whereas all definite early alligatoroids are North American. The age of this divergence has been estimated into the early Late Cretaceous (ca. 90–100 Mya)18,19,41,91 coinciding with a period of extreme sea level increase culminating in the highest sea level during the entire Mesozoic and Cenozoic eras (90–94 Mya, Turonian)92. Exceptionally high sea level may have isolated North American and Asian ancestral stem-crocodylians by posing a wide marine barrier, even for saltwater tolerant species, across Beringia. In contrast, warm climate would have instead favoured high latitude faunal connections during the Turonian (Cretaceous thermal maximum93) and is therefore unlikely to have driven divergence. Based on our topology, we infer that alligatoroids, as a freshwater clade in the interior of the continent, secondarily lost osmoregulation ability (and possibly lingual salt glands) early during their evolution (Fig. 4). Our parsimony body-size analysis recovers a minimum of 20% reduction in total body length (TL) at the root of Alligatoroidea, involving a shrinkage from 200–250 cm to 150–200 cm. This reduction reaches up to 40% (from 200–250 cm to <150 cm) when early alligatorines such as Ceratosuchus burdoshi are considered (Fig. 3). Low body-size disparity and shrinking early in the evolution of the group is a novel finding of this study as previous body-size analyses employed different topologies (i.e. not accommodating molecular topologies in the phylogenetic framework, placing Diplocynodon and Leidyosuchus as early alligatoroids, and excluding Deinosuchus from the sample52,54,94). Small body size was broadly retained during the Paleogene and gigantic forms only evolved in the Neogene among caimanines (Purussaurus and Mourasuchus from South America). In addition, large size (3–4 m) independently evolved in the lineage of extant Alligator mississippiensis. Godoy et al.52 proposed that Cenozoic Crocodylia body-size progressively increased in response to selective extinction of smaller-bodied taxa due to global cooling-induced habitat loss and associated increased competition. However, as we demonstrate here, in alligatoroids at least, there were no large-bodied taxa before the Neogene and instead, small-bodied taxa may have simply evolved into large-bodied ones. In line with this, Brochu & Camp95 suggested that small-sized Paleogene specialists with crushing dentition evolved into larger-sized generalists in the Neogene, although we note that a specialised morphology may not be necessarily associated with narrow niche96. Under our topology, we detect a minor body-size increase in Alligator following the Eocene/Oligocene extinction of all other North American crocodylians79,97.
Methods
Phylogenetic analysis
We expanded and combined previous morphological taxon-character datasets3,26,27,63,83,98,99,100, that are themselves expand on previous work1,23,76,82. Our character/taxon dataset consists of 219 discrete morphological characters and 128 taxa, including taxa absent from other recent global datasets (e.g. Deinosuchus spp., Orientalosuchina, Diplocynodon remensis, and Borealosuchus griffithi). Character definitions and scorings were managed in Mesquite version 3.7101. Multistate characters forming a morphocline were treated as ordered. Ordering, however, does not impact the position of Diplocynodon, Leidyosuchus or Deinosuchus, with the exception that Deinosuchus is retrieved as the earliest diverging alligatoroid in few of the trees, a position inconsistent with circumstantcial evidence (see Discussion). In total, 19 new taxa were added, 20 additional characters, and over 50 character scores were updated relative to the parent dataset26. For details of the dataset and analysis see Supplementary information. The dataset is available in Supplementary Data 1.
The maximum parsimony analysis was performed in TNT 1.6102 using a manually implemented molecular scaffold91 based on the topology recovered by Oaks18 (see Supplementary information for topology; the constraints are embedded in the tnt file): the scaffold constrains extant species relationships on the basis of molecular topology and allows fossil taxa to be placed within this topology based on morphological characters. Enforcing constraints enables the recovery of Longirostres, the consensual clade uniting Gavialis gangeticus, Tomistoma schlegelli and Crocodylus niloticus in accordance with molecular18,41,103 and some recent morphological topologies4. The parent datasets here combined and expanded, however, are unable to recover this clade and therefore some previous studies employed a molecular scaffold26,63,91. It has been recently demonstrated that molecular scaffolds represent an appropriate alternative of total-evidence approaches for fossil crocodylian phylogenetic inference91. Nevertheless, the scaffold has apparently no impact on the stem-crocodylian placement of Deinosuchus, Diplocynodon or Leidyosuchus in our analyses, unless the key taxa, D. remensis and B. griffithi, are removed from the dataset.
A first round of New Technology Search was performed as advised for large datasets104, enabling all search algorithms (Sectorial search, XSS enabled; Ratchet; Drift; Tree fusing) and stabilising the consensus 5 times. A second round of New Technology Search was then conducted, but using the trees saved from RAM, disabling Sectorial searches. The consensus tree was obtained from trees recovered by the second round of calculation. Figures 1 and 2 were created using the R package strap developed by Bell and Lloyd105, using 1 Ma as minimum branch length and using taxon ages from Darlim et al. 91 and sources reported in Supplementary information (see Supplementary Data 1 for the complete list of ages).
Phylogenetic body-size analysis
The estimation of body sizes of extinct species was undertaken using a Bayesian phylogenetic approach and the application of regressions based on head width (HW) and total body length (TL) measurements from extant crocodylians21,48. We expanded previous regression datasets21,48 by adding the extant Osteolaemus osborni and thus including a total of 25 species and 207 specimens. Head width, measured as the distance between the extremes of the quadrates, was collected using ImageJ106 for 91 fossil and 16 extant taxa in our phylogenetic analysis (Supplementary Data 2.3, Table S1). For topological structure, we used the consensus tree obtained in the present study (Figs. 1, 2; Fig. S1), including time calibration. This involved adding age information for all tips sourced from Darlim et al.91 and other references (Supplementary information). The calibration employed 5 million years as minimum branch lengths (mbl method107) in the timePaleoPhy() function of the paleotree package108 in R 4.3.1109.
Total body length was estimated through the BayesModelS method110 for phylogenetic predictions, which adopts a Brownian motion model and employs a Monte-Carlo Markov-Chain (MCMC21,48,110,111). The phylogenetic signal values utilised by BayesModelS method were extracted from the phytools package112 through phylosig() function. The entire protocol, data sources, along with additional details are available in Supplementary Data 2, including packages such as car113, MASS114, caper115, evomap116, and rms117. A parsimony reconstruction of ancestral states was used to plot the discretised continuous values of mean total length in Mesquite98 on the strict consensus tree (see Supplementary Data 2.3). Temporal and taxic distribution of body size were visualised using ggplot2, deeptime and jpeg R packages.
Figures
All figures were produced using the free image editor GIMP and free vector graphics editor Inkscape (https://www.inkscape.org). The silhouette used for Deinosuchus in Figs. 1, 2 and 3 was created based on the artwork of Andrey Atuchin under the Creative Common BY-SA 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/). Remaining silhouettes used to illustrate clades and taxa in Figs. 1 to 4 were sourced from PhyloPic (https://www.phylopic.org/) and are in the Public Domain except for Gryposuchus (https://www.phylopic.org/images/d4225b65-a520-42ae-b3ab-8725778a8403/gryposuchus-pachakamue); Paleosuchus (https://www.phylopic.org/images/9289a813-73ad-4644-b738-d9be619d8219/paleosuchus), and Purussaurus (https://www.phylopic.org/images/b7fedb04-759e-4f1a-b8bb-d0faefc64e75/purussaurus-neivensis) by Armin Reindl and are accessible for reuse under the Creative Commons BY-NC 3.0 license (https://creativecommons.org/licenses/by-nc/3.0/deed.en); and Euthecodon, by Smokeybjb (https://www.phylopic.org/images/a1e916c4-e020-4657-932b-d74ec6c08e0a/euthecodon-brumpti); Crocodylus anthropophagus by Nobu Tamura (vectorised by Julian Bayona, https://www.phylopic.org/images/c60b0e39-1437-4bb4-8940-f6da3d943adf/crocodylinae-anthropophagus); Stomatosuchus by Stanton F. Fink (vectorised by Julian Bayona, https://www.phylopic.org/images/f7d45c6d-e506-4826-8ffe-3f75d588d378/stomatosuchus-inermis); Phosphatosaurus by Nobu Tamura (vectorised by Julian Bayona, https://www.phylopic.org/images/13ff6eb0-a671-44d8-8a51-8b9f95d49403/dyrosaurus-phosphaticus) accessible for reuse under the Creative Commons BY-SA 3.0 Unported license (https://creativecommons.org/licenses/by-sa/3.0/). Crocodylian skull silhouettes in Figs. 1 and 3 are original creations. Map in Fig. 4 was modified after118. All other elements presented in Figs. 1 to 4 are original creations119.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All supporting data, supplementary information and supplementary data are available in the following open access repository (Figshare): https://doi.org/10.6084/m9.figshare.27901317.
References
Brochu, C. A. Phylogenetics, taxonomy, and historical biogeography of Alligatoroidea. SVP Memoir. 6, 9–100 (1999).
Brochu, C. A. Phylogenetic approaches toward crocodylian history. Annu. Rev. Earth Planet. 31, 357–397 (2003).
Cossette, A. P. & Brochu, C. A. A systematic review of the giant alligatoroid Deinosuchus from the campanian of north America and its implications for the relationships at the root of Crocodylia. J. Vertebr. Paleontol. 40, e1767638 (2020).
Rio, J. P. & Mannion, P. D. Phylogenetic analysis of a new morphological dataset elucidates the evolutionary history of Crocodylia and resolves the long-standing gharial problem. PeerJ. 9, e12094 (2021).
Brochu, C. A. Crocodylian snouts in space and time: phylogenetic approaches toward adaptive radiation. Amer. Zool. 41, 564–585 (2001).
Cossette, A. P. & Brochu, C. A. A new specimen of the alligatoroid Bottosaurus harlani and the early history of character evolution in alligatorids. J. Vertebr. Paleontol. 38, 1–22 (2018).
Erickson, B. R. Albertochampsa langstoni, gen. et sp. nov. A new Alligator from the Cretaceous of Alberta. Sci. Publ. Sci. Mus. Minnesota 2, 1–13 (1972).
Norell, M., Clark, J. M. & Hutchison, J. H. The Late Cretaceous alligatoroid Brachychampsa montana (Crocodylia): new material and putative relationships. Am. Mus. Novit. 3116, 1–26 (1994).
Wu, X. C., Brinkman, D. B. & Russell, A. P. A new alligator from the Upper Cretaceous of Canada and the relationship of early eusuchians. Palaeontology 39, 351–376 (1996).
Taplin, L. E. Osmoregulation in crocodilians. Biol. Rev. 63, 333–377 (1988).
Schwimmer, D. R. King Of The Crocodylians: The Paleobiology Of Deinosuchus. 1st edn, Vol. 240 (Indiana University Press, 2002).
Erickson, G. M. & Brochu, C. A. How the ‘terror crocodile’ grew so big. Nature 398, 205 (1999).
Wheatley, P. V. Understanding Saltwater Tolerance and Marine Resource Use in the Crocodylia: a Stable Isotope Approach. Vol. 334 (University of California, Santa Cruz, 2010).
Venczel, M. & Codrea, V. A. A new late Eocene alligatoroid crocodyliform from transylvania. C. R. - Palevol. 21, 411–429 (2022).
Kocsis, L. et al. Geochemical investigation of the mixed Máriahalom vertebrate fauna at the Paleogene–Neogene boundary in the central paratethys: environmental conditions and age constrain. Swiss J. Palaeontol. 142, 17 (2023).
Williamson, T. E. Brachychampsa Sealeyi, sp. nov., (Crocodylia, Alligatoroidea) From the Upper Cretaceous (Lower Campanian) Menefee Formation, Northwestern New Mexico. J. Vertebr. Paleontol. 16, 421–431 (1996).
Mohler, B. F., McDonald, A. T. & Wolfe, D. G. First remains of the enormous alligatoroid Deinosuchus from the Upper Cretaceous Menefee formation, New Mexico. PeerJ. 9, e11302 (2021).
Oaks, J. R. A time-calibrated species tree of Crocodylia reveals a recent radiation of the true crocodiles. Evolution 65, 3285–3297 (2011).
Pan, T. et al. Near-complete phylogeny of extant Crocodylia (Reptilia) using mitogenome-based data. Zool. J. Linn. Soc. 191, 1075–1089 (2021).
Martin, J. E., Smith, T., Lapparent de Broin, F., Escuillié, F. & Delfino, M. Late Palaeocene eusuchian remains from Mont de Berru, France, and the origin of the alligatoroid Diplocynodon. Zool. J. Linn. Soc. 172, 867–891 (2014).
O’Brien, H. D. et al. Crocodylian head width allometry and phylogenetic prediction of body size in extinct crocodyliforms. Integr. Org. Biol. 1, obz006 (2019).
Whitaker, R. & Whitaker, N. Who’s got the biggest? Crocodile Specialist Group Newsletter 27, 26–30 (2008).
Brochu, C. A. & Storrs, G. W. A giant crocodile from the Plio-Pleistocene of Kenya, the phylogenetic relationships of Neogene African crocodylines, and the antiquity of Crocodylus in Africa. J. Vertebr. Paleontol. 32, 587–602 (2012).
Aureliano, T. et al. Morphometry, bite-force, and paleobiology of the Late Miocene Caiman Purussaurus brasiliensis. PloS one 10, e0117944 (2015).
Rauhe, M. Die lebensweise und okologie der Geiseltal-krokodilier - abschied von traditionellen Lehrmeinungen. Hallesches Jahrbuch für Geowissenschaften 17, 65–80 (1995).
Massonne, T., Vasilyan, D., Rabi, M. & Böhme, M. A new alligatoroid from the Eocene of Vietnam highlights an extinct Asian clade independent from extant Alligator sinensis. PeerJ 7, e7562 (2019).
Stocker, M. R., Brochu, C. A. & Kirk, E. C. A new caimanine alligatorid from the middle Eocene of Southwest Texas and implications for spatial and temporal shifts in Paleogene crocodyliform diversity. PeerJ 9, e10665 (2021).
Groh, S. S., Upchurch, P., Barrett, P. M. & Day, J. J. The phylogenetic relationships of neosuchian crocodiles and their implications for the convergent evolution of the longirostrine condition. Zool. J. Linn. Soc. 188, 473–506 (2020).
Muscioni, M. et al. Acynodon adriaticus from Villaggio del Pescatore (Campanian of Italy): anatomical and chronostratigraphic integration improves phylogenetic resolution in Hylaeochampsidae (Eusuchia). Cretac. Res. 151, 105631 (2023).
Colbert, E. H., Bird, R. T. & Brown, B. A gigantic crocodile from the Upper Cretaceous beds of Texas. Am. Mus. Novit. 1688, 1–24 (1954).
Farlow, J. O., Hurlburt, G. R., Elsey, R. M., Britton, A. R. & Langston, W. Jr Femoral dimensions and body size of Alligator mississippiensis: estimating the size of extinct mesoeucrocodylians. J. Vertebr. Paleontol. 25, 354–369 (2005).
Rivera-Sylva, H. E., Frey, E. & Guzman-Gutierrez, J. R. Evidence of predation on the vertebra of a hadrosaurid dinosaur from the Upper Cretaceous (Campanian) of Coahuila, Mexico. Carnets Geol. Letter 2, (2009).
Schwimmer, D. R. & Harrell, S. D. Trace fossils from both ends of Deinosuchus: Late Cretaceous estuarine crocodylian bite marks and coprolites from West Georgia. Geol. Soc. Am. Abstracts Progr. 42, 104 (2010).
Schilthuizen, M. A. Greater Alligator. https://www.science.org/content/article/greater-alligator (1999).
Jackson, K., Butler, D. G. & Brooks, D. R. Habitat and phylogeny influence salinity discrimination in crocodilians: implications for osmoregulatory physiology and historical biogeography. Biol. J. Linn. Soc. 58, 371–383 (1996).
Grigg, G. C., Beard, L. A., Moulton, T., Queirol Melo, M. T. & Taplin, L. E. Osmoregulation by the broad-snouted caiman, Caiman latirostris, in estuarine habitat in southern Brazil. J. Comp. Physiol. B: Biochem. Syst. Environ. Physiol. 168, 445–452 (1998).
Elsey, R. M. Unusual offshore occurrence of an American alligator. Southeast. Nat. 4, 533–536 (2005).
Lehman, T. M. et al. Stratigraphy and depositional history of the Aguja formation (Upper Cretaceous, Campanian) of West Texas, southwestern USA. Geosphere 20, 825–879 (2024).
Roberts, L. N. R. & Kirschbaum, M. A. Paleogeography And The Late Cretaceous of the Western Interior of Middle North America; Coal Distribution and Sediment Accumulation. https://pubs.usgs.gov/publication/pp1561 (1995).
Vélez-Juarbe, J., Brochu, C. A. & Santos, H. A gharial from the Oligocene of Puerto Rico: transoceanic dispersal in the history of a non-marine reptile. Proc. R. Soc. B: Biol. Sci. 274, 1245–1254 (2007).
Lee, M. S. Y. & Yates, A. M. Tip dating and homoplasy: reconciling the shallow molecular divergences of modern gharials with their long fossil record. Proc. R. Soc. B. 285, 20181071 (2018).
Markwick, P. J. Fossil crocodilians as indicators of Late Cretaceous and Cenozoic climates: implications for using palaeontological data in reconstructing palaeoclimate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 137, 205–271 (1998).
Dennis, K. J., Cochran, J. K., Landman, N. H. & Schrag, D. P. The climate of the Late Cretaceous: new insights from the application of the carbonate clumped isotope thermometer to Western Interior Seaway macrofossil. Earth Planet. Sci. Lett. 362, 51–65 (2013).
McCormack, L. Borealosuchus (Crocodylia) From the Early Campanian Mooreville Chalk Reveals New Insights Into the Late Cretaceous Fauna of Alabama and the Origin of Crocodylian Lineages https://iro.uiowa.edu/ (2019).
Taplin, L. E. & Grigg, G. C. Historical zoogeography of the eusuchian crocodilians: a physiological perspective. Amer. Zool. 29, 885–901 (1989).
Stout, J. B. Osmoregulation in alligatoroidea: shifting the paradigm untethers biogeographic questions. Hist. Biol. https://doi.org/10.1080/08912963.2024.2379029 (2024).
Iijima, M., Takahashi, K. & Kobayashi, Y. The oldest record of Alligator sinensis from the late pliocene of western Japan, and its biogeographic implication. J. Asian Earth Sci. 124, 94–101 (2016).
Paiva, A. L. S., Godoy, P. L., Souza, R. B., Klein, W. & Hsiou, A. S. Body size estimation of caimaninae specimens from the Miocene of South America. J. S. Am. Earth Sci. 118, 103970 (2022).
Engelman, R. K. A Devonian fish tale: a new method of body length estimation suggests much smaller sizes for Dunkleosteus terrelli (Placodermi: Arthrodira). Diversity 15, 318 (2023).
Grigg, G. & Seebacher, F. Crocodilian thermal relations. In Crocodilian Biology and Evolution. (eds. Grigg, G. C., Seebacher, F. & Franklin, C. E.) 672 (Comstock Publishing Associates, 2000).
Britton, A. R., Whitaker, R. & Whitaker, N. Here be a dragon: exceptional size in a saltwater crocodile (Crocodylus porosus) from the Philippines. Herpetol. Rev. 43, 541–546 (2012).
Godoy, P. L., Benson, R. B., Bronzati, M. & Butler, R. J. The multi-peak adaptive landscape of crocodylomorph body size evolution. BMC Evol. Biol. 19, 1–29 (2019).
Martin, J. E. et al. A large crocodyloid from the Oligocene of the Bugti Hills, Pakistan. J. Vertebr. Paleontol. 39, e1671427 (2019).
Gearty, W. & Payne, J. L. Physiological constraints on body size distributions in Crocodyliformes. Evolution 74, 245–255 (2020).
Lakin, R. J., Barrett, P. M., Stevenson, C., Thomas, R. J. & Wills, M. A. First evidence for a latitudinal body mass effect in extant Crocodylia and the relationships of their reproductive characters. Biol. J. Linn. Soc. 129, 875–887 (2020).
Stockdale, M. T. & Benton, M. J. Environmental drivers of body size evolution in crocodile-line archosaurs. Commun. Biol. 4, 38 (2021).
Vermeij, G. J. Gigantism and its implications for the history of life. PLoS One 11, e0146092 (2016).
Delfino, M. & de Vos, J. A giant crocodile in the Dubois collection from the Pleistocene of Kali Gedeh (Java). Integr. Zool. 9, 141–147 (2014).
Gilmore, C. W. Leidyosuchus sternbergii, a new species of crocodile from the Ceratops Beds of Wyoming. Proceedings U. S National Museum. 38, 485–502 (1910).
Mook, C. C. Diplocynodon remains from the bridger Beds of Wyoming. Am. Mus. Novit. 2007, 1–4 (1960).
Erickson, B. R. Osteology of the early eusuchian crocodile Leidyosuchus formidabilis, sp. nov. Monograph, Science Museum Minnesota. 2, 1–61 (1976).
Rauhe, M. & Rossmann, T. News about fossil crocodiles from the middle Eocene of Messel and Geiseltal, Germany. Hallesches Jahrbuch für Geowissenschaften 17, 81–92 (1995).
Walter, J. et al. On the origin of Caimaninae: insights from new fossils of Tsoabichi greenriverensis and a review of the evidence. Hist. Biol. 34, 580–595 (2022).
Brochu, C. A. A review of “Leidyosuchus” (Crocodyliformes, Eusuchia) from the Cretaceous through Eocene of North America. J. Vertebr. Paleontol. 17, 679–697 (1997).
Wu, X.-C., Brinkman, D. B. & Fox, R. C. A new crocodilian (Archosauria) from the basal Paleocene of the Red Deer River Valley, southern Alberta. Can. J. Earth Sci. 38, 1689–1704 (2001).
Rio, J. P., Mannion, P. D., Tschopp, E., Martin, J. E. & Delfino, M. Reappraisal of the morphology and phylogenetic relationships of the alligatoroid crocodylian Diplocynodon hantoniensis from the late Eocene of the United Kingdom. Zool. J. Linn. Soc. 188, 579–629 (2020).
West, R. M. & Dawson, M. R. Vertebrate paleontology and the Cenozoic history of the north Atlantic region. Polarforschung 48, 103–119 (1978).
Smith, T., Rose, K. D. & Gingerich, P. D. Rapid Asia-Europe-North America geographic dispersal of earliest Eocene primate Teilhardina during the Paleocene–Eocene thermal Maximum. Proc. Natl. Acad. Sci USA. 103, 11223–11227 (2006).
Mayr, G. Paleogene Fossil Birds 2nd edn, Vol. 262 (Springer Cham, 2009).
Boyer, D. M., Costeur, L. & Lipman, Y. Earliest record of Platychoerops (Primates, Plesiadapidae), a new species from Mouras quarry, Mont de Berru, France. Am. J. Phys. Anthropol. 149, 329–346 (2012).
Rose, K. D. The importance of Messel for interpreting Eocene Holarctic mammalian faunas. Palaeobiodivers. Palaeoenviron. 92, 631–647 (2012).
De Bast, E. & Smith, T. The oldest Cenozoic mammal fauna of Europe: implication of the Hainin reference fauna for mammalian evolution and dispersals during the Paleocene. J. Syst. Palaeontol. 15, 741–785 (2017).
Macaluso, L. et al. Biogeographic history of Palearctic caudates revealed by a critical appraisal of their fossil record quality and spatio-temporal distribution. R. Soc. Open Sci. 9, 220935 (2022).
Legendre, S. & Lévêque, F. Etalonnage de l'échelle biochronologique mammalienne du Paléogène d’Europe occidentale: vers une intégration à l'échelle globale. Mémoires et travaux de l’Institut de Montpellier 21, 461–473 (1997).
Brikiatis, L. The De Geer, Thulean and Beringia routes: key concepts for understanding early Cenozoic biogeography. J. Biogeogr. 41, 1036–1054 (2014).
Brochu, C. A. Alligatorine phylogeny and the status of Allognathosuchus Mook, 1921. J. Vertebr. Paleontol. 24, 857–873 (2004).
Kotsakis, T., Delfino, M. & Piras, P. Italian Cenozoic crocodilians: taxa, timing and palaeobiogeographic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 210, 67–87 (2004).
Delfino, M. & Smith, T. Reappraisal of the morphology and phylogenetic relationships of the middle Eocene alligatoroid Diplocynodon deponiae (Frey, Laemmert, and Riess, 1987) based on a three-dimensional specimen. J. Vertebr. Paleontol. 32, 1358–1369 (2012).
De Celis, A., Narváez, I. & Ortega, F. Spatiotemporal palaeodiversity patterns of modern crocodiles (Crocodyliformes: Eusuchia). Zool. J. Linn. Soc. 189, 635–656 (2020).
Seghetti, S. M., Georgalis, G. L., Tschopp, E. & Delfino, M. A historical overview of the reptile fauna from the Eocene Bolca fossil-Lagerstätte (Italy). Boll. Soc. Paleontol. Ital. 61, 119–143 (2022).
Brochu, C. A. A new alligatorid from the lower Eocene Green River formation of Wyoming and the origin of caimans. J. Vertebr. Paleontol. 30, 1109–1126 (2010).
Brochu, C. A. Phylogenetic relationships of Necrosuchus ionensis Simpson, 1937 and the early history of caimanines. Zool. J. Linn. Soc. 163, S228–S256 (2011).
Shan, H. Y., Wu, X.-C., Sato, T., Cheng, Y. N. & Rufolo, S. A new alligatoroid (Eusuchia, Crocodylia) from the Eocene of China and its implications for the relationships of Orientalosuchina. J. Paleontol. 95, 1321–1339 (2021).
Gould, G. C. & MacFadden, B. J. Gigantism, dwarfism, and Cope’s rule: “nothing in evolution makes sense without a phylogeny”. Bull. Am. Mus. Nat. Hist. 285, 219–237 (2004).
Martin, J. E. & Lauprasert, K. A new primitive alligatorine from the Eocene of Thailand: relevance of Asiatic members to the radiation of the group. Zool. J. Linn. Soc. 158, 608–628 (2011).
Wang, Y. Y., Sullivan, C. & Liu, J. Taxonomic revision of Eoalligator (Crocodylia, Brevirostres) and the paleogeographic origins of the Chinese alligatoroids. PeerJ 4, e2356 (2016).
Li, C., Wu, X.-C. & Rufolo, S. J. A new crocodyloid (Eusuchia: Crocodylia) from the Upper Cretaceous of China. Cretac. Res. 94, 25–39 (2019).
Wu, X.-C., Wang, Y. C., You, H. L., Zhang, Y. Q. & Yi, L. P. New brevirostrines (Crocodylia, Brevirostres) from the upper Cretaceous of China. Cretac. Res. 144, 105450 (2023).
Wu, X.-C., Li, C. & Wang, Y.-Y. Taxonomic reassessment and phylogenetic test of Asiatosuchus nanlingensis Young, 1964 and Eoalligator chunyii Young, 1964. Vertebrata PalAsiatica 56, 137 (2018).
Ristevski, J. et al. Migrations, diversifications and extinctions: the evolutionary history of crocodyliforms in Australasia. Alcheringa: Australasian Journal Palaeontology 47, 1–46 (2023).
Darlim, G., Lee, M. S. Y., Walter, J. D. & Rabi, M. The impact of molecular data on the phylogenetic position of the putative oldest crocodilian and the age of the clade. Biol. Lett. 18, 20210603 (2022).
Olde, K. et al. Geochemical and palynological sea-level proxies in hemipelagic sediments: a critical assessment from the Upper Cretaceous of the Czech republic. Palaeogeogr. Palaeoclimatol. Palaeoecol. 435, 222–243 (2015).
Moriya, K. Development of the Cretaceous greenhouse climate and the oceanic thermal structure. Paleontol. Res. 15, 77–88 (2011).
Godoy, P. L., & Turner, A. H. Body size evolution in crocodylians and their extinct relatives. Els. https://doi.org/10.1002/9780470015902.a0029089 (2020)
Miller-Camp, J. & Brochu, C. A. Crocodyliforms: large-bodied carnivores. In Messel: An Ancient Greenhouse Ecosystem. (eds. Smith, K. T. et al.) (Schweizerbart Sche Vlgsb., 2018).
Darlim, G. et al. An extinct deep-snouted alligator species from the quaternary of Thailand and comments on the evolution of crushing dentition in alligatorids. Sci. Rep. 13, 10406 (2023).
Hutchison, J. H. Western North American reptile and amphibian record across the Eocene-Oligocene boundary and its climatic implications. In Eocene-Oligocene Climatic and Biotic Evolution (eds. Prothero, D. R. & Berggren W. A.) 584 (Princeton Univ. Press, 1992).
Salas-Gismondi, R. et al. A Miocene hyperdiverse crocodylian community reveals peculiar trophic dynamics in proto-amazonian mega-wetlands. Proc. R. Soc. B Biol. Sci. 282, 20142490 (2015).
Bona, P., Ezcurra, M. D., Barrios, F. & Fernandez Blanco, M. V. A new Palaeocene crocodylian from southern Argentina sheds light on the early history of caimanines. Proc. R. Soc. B Biol. Sci. 285, 20180843 (2018).
Cossette, A. P. A new species of Bottosaurus (Alligatoroidea: Caimaninae) from the black peaks formation (Palaeocene) of Texas indicates an early radiation of North American caimanines. Zool. J. Linnean Soc. 191, 276–301 (2021).
Maddison, W. P. & Maddison, D. R. Mesquite: A Modular System For Evolutionary Analysis. Version 3.81. http://www.mesquiteproject.org (2023).
Goloboff, P. A. & Morales, M. E. TNT version 1.6, with a graphical interface for MacOS and Linux, including new routines in parallel. Cladistics 39, 144–153 (2023).
Janke, A., Gullberg, A., Hughes, S., Aggarwal, R. K. & Arnason, U. Mitogenomic analyses place the Gharial (Gavialis gangeticus) on the Crocodile tree and provide Pre-K/T divergence times for most Crocodilians. J. Mol. Evol. 61, 620–626 (2005).
Goloboff, P. A., Farris, J. S. & Nixon, K. C. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786 (2008).
Bell, M. A. & Lloyd, G. T. strap: an R package for plotting phylogenies against stratigraphy and assessing their stratigraphic congruence. Palaeontology 58, 379–389 (2015).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Laurin, M. The evolution of body size, cope’s rule and the origin of amniotes. Syst. Biol. 53, 594–622 (2004).
Bapst, D. W. paleotree: an R package for paleontological and phylogenetic analyses of evolution. Methods Ecol. Evol. 3, 803–807 (2012).
R. Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. https://www.R-project.org/ (2023).
Nunn, C. L. & Zhu, L. Phylogenetic prediction to identify “evolutionary singularities. In Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology: Concepts and Practice (Eds. Garamszegi, L. Z.) 552 (Springer Berlin, 2014).
Garland, T. Jr & Ives, A. R. Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am. Nat. 155, 346–364 (2000).
Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2, 217–223 (2012).
Fox, J. & Weisberg, S. An R Companion To Applied Regression 3rs edn, Vol. 600 (Sage publications, 2018).
Venables, W. N., Ripley, B. D. Modern Applied Statistics With S 2nd edn, (Springer-Verlag New York Inc, 2002).
Orme, D. Caper: Comparative Analyses of Phylogenetics and Evolution in R. https://cran.r-project.org/web/packages/caper/vignettes/caper.pdf (2018).
Smaers, J. B. & Mongle, C. S. evomap: R Package for the Evolutionary Mapping of Continuous Traits. https://github.com/JeroenSmaers/evomap (2014).
Harrell, F. E. rms: Regression Modeling Strategies. R Package Version 6.2-0. https://CRAN.R-project.org/package=rms (2017).
Scotese, C. R. The PALEOMAP Project PaleoAtlas for ArcGIS, Version 1, Volume 2, Cretaceous Paleogeographic, Paleoclimatic, and Plate Tectonic Reconstructions. (2008).
Walter J. D. et al. Files - Expanded phylogeny elucidates Deinosuchus relationships, crocodylian osmoregulation and body-size evolution. Figshare. https://doi.org/10.6084/m9.figshare.27901317.v1 (2025).
Burke, P. M. et al. Endocranial anatomy and phylogenetic position of the crocodylian Eosuchus lerichei from the late Paleocene of northwestern Europe and potential adaptations for transoceanic dispersal in gavialoids. Anat. Rec. 308, 636–670 (2024).
Acknowledgements
For access to specimens and their assistance in the collections, the authors thank Damien Germain and Nour-Eddine Jalil from the Muséum National d’Histoire Naturelle (Paris, France); Torsten Wappler, Marisa Blume and Lukardis Wencker from the Hessiches Landesmuseum Darmstadt (Darmstadt, Germany); Krister Smith, Anika Vogel and Thomas Lehmann from Senckenberg Museum Frankfurt (Frankfurt, Germany); Roberto Rozzi, Michael Stache, Oliver Wings, and Frank Steinheimer from the Zentralmagazin Naturwissenschaftlicher Sammlungen—Geiseltalsammlung (Halle (Saale), Germany); Loic Costeur from the Naturhistoriches Museum Basel (Basel, Switzerland), Josep Maria Robles from the Institut Català de Paleontologia Miquel Crusafont (Sabadell, Spain). We thank Christopher Brochu, Adam Cossette, Jonathan Rio, Philip Mannion, Loredana Macaluso, Lukardis Wencker, Gustavo Darlim, Yohan Pochat-Cottilloux, Ornella Bertrand, Hervé Bocherens, and France de Lapparent de Broin for discussions. We finally thank two anonymous reviewers whose comments and suggestions greatly improved the quality of the manuscript. This work was supported by Grants (ex-60% 2021–2024) from the Università degli Studi di Torino. The funder had no role in project design, data collection, analyses, or preparation of the manuscript. The following grants information are disclosed by the authors: Deutsche Forschungsgemeinschaft grant 417629144 awarded to M.R.; German Research Foundation [417629144]. Volkswagen Stiftung, ‘Research in Museums’ grant Az. 90 978 awarded to M.R. Synthesys+ Transnational Access grant (part of European Union’s Horizon 2020 research and innovation programme) awarded to JD.W. (2022). A.L.S.P. is funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant number: 2022/02249- 5). We acknowledge support from the Open Access Publication Fund of the University of Tübingen.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.D.W.: funding acquisition—data collection—analysis—investigation—figures—writing (original draft, review and editing); T.M.: data collection—analysis—investigation—writing (supplementary); A.L.S.P.: data collection—analysis—figures; J.E.M. data collection—writing (review and editing); M.D. supervision—project administration—data collection—investigation—writing (review and editing); M.R. conceptualization—funding acquisition—supervision—project administration—analysis—investigation—figures—writing (original draft, review and editing).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Luke Grinham, Dario Ummarino, and Borja Figueirido.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Walter, J.D., Massonne, T., Paiva, A.L.S. et al. Expanded phylogeny elucidates Deinosuchus relationships, crocodylian osmoregulation and body-size evolution. Commun Biol 8, 611 (2025). https://doi.org/10.1038/s42003-025-07653-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-07653-4