Abstract
Black corals, primarily deep-sea cnidarians (Anthozoa: Antipatharia), are inferred to have originated either in the Ediacaran or Cambrian based on molecular clock estimates. However, only the fossil family Sinopathidae, comprising Sinopathes and Sterictopathes, from the Early Ordovician of Hubei, China, has been recorded in the fossil record. The affinity of this family has been questioned because of morphological inconsistencies between fossil and extant species. Here we describe two transitional species of Sterictopathes from the Middle Ordovician of Shaanxi, China, bridging the fossil gaps and thereby elevating the genus Sterictopathes to a new family, Sterictopathidae fam. nov. The hypothesized evolutionary trend toward regularity in the axial skeleton from the Ordovician to modern Antipatharia is highlighted by reduced ridges and longitudinal fusion of networks. This discovery and confirmation of Ordovician black corals paves the way for future fossil findings and offers new insights into the early evolution of Hexacorallia.
Similar content being viewed by others
Introduction
Modern antipatharians are noncalcareous, colonial anthozoans distributed in oceans worldwide ranging from shallow to hadal depths (three to 8900 meters)1,2. Black corals have ecological and cultural importance3. To date, 301 extant black coral species belonging to eight families have been described4. Generally, it is believed that over 75% of known species occur in depths deeper than 50 m5. Many genera belonging to the families Antipathidae, Aphanipathidae, and Myriopathidae are reported primarily in the shallow and mesophotic depths6, while families like Schizopathidae and Cladopathidae are mainly distributed in the abyssal and hadal zones below 3500 m7. Black corals are characterized by a spiny, proteinaceous chitin-based skeleton comprised of multiple concentric layers surrounding a small central canal8. The skeletal spines can be triangular, conical, hooked, hemispherical, or blade-like; sometimes they are laterally compressed; they can be smooth or bearing protuberances or tubercles, with or without apical bifurcations or multi-lobed; they can be irregularly arranged or aligned in distinct longitudinal rows9.
Fossil records of black corals are quite sparse compared with those of calcareous corals10,11,12,13,14,15. Historically, the first fossil “black coral” Antipathes vetusta Michelotti, 183916 was described based on stem fragment fossils from the Miocene of Turin, Italy13. It has since been re-identified as a gorgonian17,18,19. Another Miocene fossil record of the extant species Leiopathes glaberrima appeared in several fossil black coral lists10,14,15,20 but could not be traced to any morphological illustrations or descriptions.
At present, only one black coral fossil family, the Sinopathidae, composed of three species (Sinopathes reptans, Sinopathes sp., and Sterictopathes radicatus), has been described exclusively from the same Early Ordovician Fenxiang Formation (470 Ma) in the Hubei, South China11,12. The antipatharian affinity of Sinopathes11 was contentious because of morphological differences between fossil and extant species10,21. The concerns mainly focused on the morphology of the spines of Sinopathes reptans, which are sculptured with distinct and continuous longitudinal costellae, as well as the presence of some tubular spines with a central canal10. This is inconsistent with the morphology of modern black corals. Brugler et al.10 even hypothesized Sinopathes reptans as a cnidarian closely related to the hydrozoan Hydractinia, as spine-like structures are also present in the hydrorhiza mat of some encrusting Hydractinia colonies22. Baliński and Sun12 argued that Sinopathes reptans and Hydractinia differ in colonial growth patterns: the former forming upright and branched colonies, while the latter generates mat-like or stoloniferous encrusting colonies12. Additionally, they also explained that the spines of Sinopathes reptans are multi-layered and solid. Moreover, the exceptionally rare tubular-like spine with a central canal was attributed to a preservation artifact12. But they did not directly address the concern on the delicate longitudinal costellae on the spines12.
Except for the abovementioned fine costellae on the spines, the arrangement pattern of spines, a diagnosis for antipatharian systematics, also varies significantly in the Sinopathidae. Spines of most extant antipatharians are rather uniform and are arranged in longitudinal rows23,24,25,26, albeit a few of species exhibit irregularly arranged spines27,28. Whereas spines of Sinopathes are distributed irregularly and vary in size and shape11,12. The affinity of Sterictopathes is also puzzling as its typical anastomosing networks formed by smooth spines buttressed by radiating, plate-like ridges12 are absent in extant black corals. Because of the huge morphological differences between these fossils and extant forms, both Sinopathes and Sterictopathes were excluded from two recent time-calibrated molecular phylogenies that point to Ediacaran or Cambrian origin of Antipatharia29,30.
Herein we report two intermediate species (Sterictopathes seira sp. nov., Sterictopathes sp.) based on 256 specimens from the Middle Ordovician Xiliangsi Formation of the Fanjiagou section in Shaanxi, China, providing evidence to bridge the fossil gaps between the early Ordovician genus Sterictopathes and extant relatives, and shedding new light on the evolution of skeleton regularity in black corals.
Results
Systematic paleontology
Phylum Cnidaria Hatschek, 1888
Sub-Phylum Anthozoa Ehrenberg, 1834
Class Hexacorallia Haeckel, 1896
Order Antipatharia Milne-Edwards & Haime, 1857
Family Sterictopathidae Hao, Han, Baliński, Brugler & Song fam. nov
LSIDurn:1sid:zoobank.org:act:8C4E500A-807D-4B40-B027-BE3DD0BBAA1D
Type genus
Sterictopathes Baliński & Sun, 2017.
Etymology
From the type genus Sterictopathes Baliński & Sun, 2017.
Diagnosis
Corallum consists of an encrusted basal plate and an upright, branching stem, both covered with spines and high ridges radiating from the spine bases, usually forming an anastomosing network. Branches thin-walled. Spines cylindrical to conical, blunt, irregularly distributed, occasionally arranged in short longitudinal or oblique rows.
Remarks
Initially, family Sinopathidae included Sinopathes, characterized by a smooth colony surface, and Sterictopathes, which has a strongly sculptured surface12. However, if taking into account the noticeable difference in their skeleton ornamentation, it seems more appropriate to erect a new family Sterictopathidae, and confine Sinopathidae to the type genus Sinopathes.
Genus
Sterictopathes Baliński & Sun, 2017
Type species
Sterictopathes radicatus Baliński & Sun, 2017
Sterictopathes seira Hao, Han, Baliński, Brugler & Song sp. nov.
LSIDurn:1sid:zoobank.org:act:C033876B-188B-4E31-B149-9884CB29DEB7
Etymology
The Greek word seira, meaning for line, masculine in gender, refers to the longitudinal arrangement pattern of spines and their basal ridges.
Material, locality, and horizon
A total of 253 specimens of Sterictopathes seira sp. nov. were recovered (Supplementary Table 1) from the Lower part of the Ordovician Xiliangsi Formation in Fanjiagou section, Ningqiang, Shaanxi, China (Fig. 1). These specimens show a three-dimensional preservation with slight compression, and high fragmentation suggesting potential transport by currents before burial. In addition to this black coral, the fossil assemblage indicates a mixed, allocative phase of fossil deposition. It also includes abundant specimens of the benthic trilobite Ningkianites sp. (Supplementary Fig. 1a, b, k), an indeterminate benthic brachiopod species (Supplementary Fig. 1c), the pelagic conodonts Lenodus antivariabilis and Scolopodus striatus (Supplementary Fig. 1m, n), and the graptolites Undulograptus austrodentatus (Supplementary Fig. 1i, j) and Azygograptus suecicus (Supplementary Fig. 1d–h, l, o).
a Geological maps showing the investigated Fanjiagou locality, the lower right thumbnail is adopted from a standard map of China (GS-2016-1549, Ministry of Natural Resources of China). b Stratigraphic column of the Xiliangsi Formation, Fanjiagou section. c, d Field images of the Fanjiagou section bearing nodule limestones (the yellow arrow in d) where the investigated black coral fossils of Sterictopathes seira sp. nov. were recovered. The index graptolite Unduloraptus austrodentatus indicating the bottom of the Darriwilian Stage, ELI-FJG01320 (e) ELI-FJG01285 (f). Scale bars: e, f = 500 μm.
The occurrence of Undulograptus austrodenfatus, an index graptolite for the Darriwilian stage of the Middle Ordovician31, dated to approximately 467 million years ago, confirms the co-occurrence of this species with the Xiliangsi Formation in Shaanxi. This correlation is crucial to understanding the geological context of black coral occurrence. According to Lai et al.32, the Xiliangsi Formation was deposited in a nearshore shallow marine environment characterized by good light transmittance and high oxygen content. The depositional environment resembled that of the Early Ordovician Fenxiang Formation33,34.
Type material
Holotype, ELI-FJG01001 (Fig. 2h); paratypes, ELI-FJG03118, ELI-FJG03595, ELI-FJG03113 (Fig. 2g, i, j).
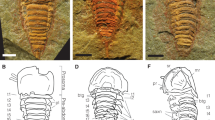
a–f Sterictopathes radicatus Baliński & Sun, 2017, Early Ordovician, Fenxiang Formation, Huanghua quarry, Hubei, China. g–n Sterictopathes seira sp. nov., Middle Ordovician, Xiliangsi Formations, Fanjiagou section, Ningqiang, Shaanxi, China. o–t Sterictopathes sp., Middle Ordovician, Xiliangsi Formations, Fanjiagou section, Ningqiang, Shaanxi, China. d, l, r Networks formed by complete and incomplete ridges. e, m, s Complete and incomplete ridges. f, n, t Multi-layered concentric structure of broken tips of branches. The arrows in (a, i) indicate junctions comprised by two (blue arrows), three (red arrows), or four (orange arrows) ridges that assembled at the base of different spines, respectively. CR, complete ridge, IR incomplete ridge, MC multi-layered concentrical structure, PP polyp pit, sb secondary branch, SP spine. Specimens: a, c–e ELI-HHC017, b, f ELI-HHC051, g ELI-FJG03118, h, k, l, n ELI-FJG01001 (holotype), i ELI-FJG03595, j, m ELI-FJG03113, o, q, r ELI-FJG02426. p, s, t ELI-FJG04013. Scale bars: a–c, h–k, o–q = 500 μm; d, e, l, m, r, s = 200 μm; f, n, t = 30 μm.
Diagnosis
Corallum with a branching angle of about 45°. The surface of branches with spines, ridges radiating from the spine bases, and networks of ridges forming frequent deep cavities bordered by 4–17 ridges. Spines narrowly conical to cylindrical, occasionally bifid or trifid, smooth-surfaced, arranged irregularly but tending to form rows.
Description and comparisons
The studied material of Sterictopathes seira sp. nov. consists of fragmented coralla, mainly branches, characterized by a dense coverage of spines, ridges and network of ridges (Fig. 2g–j and Supplementary Fig. 2). Branches approximately 0.07–0.34 mm in diameter, are mostly straight or, exceptionally rarely, slightly curved (Fig. 2j and Supplementary Fig. 2c). Notably, some specimens exhibit branching at an angle of approximately 45°, with short, bramble-like second-order structures oriented parallel or perpendicular to the plane of the first-order branches (Fig. 2g).
There are multiple networks of different sizes on the surface of the branches, and polygonal networks each variably consisted of 4–13 spines connected by 2–4 sub-transverse or oblique ridges, and 2–13 sub-longitudinal ridges with thickness ranging from 1.4 to 2.9 µm (Fig. 2l). The networks are deeply concaved and smooth surfaced, depending on the number of ridges. Larger networks with more longitudinal ridges, superficially long quadrilateral, extend longitudinally (Fig. 2h).
The spines on branches are smooth-surfaced, and narrowly conical to cylindrical (Fig. 2k–n). Some of the spines are bifid or trifid (Fig. 3), and the cross section of spines exhibits circular or sub-elliptic concentric layered rings (Fig. 3). The base of the spines has 2–5 ridges, but mostly 2–3. The bases of adjacent spines are completely connected by a long, prominent, complete ridge or a short, low, much narrow incomplete one (Fig. 2m). The complete ridges are gentle hill-like in cross section, symmetrical in two ends, whereas the incomplete ones (inconspicuous ridges connecting a single spine) decrease in height and disappear distally. In addition to many oblique or sub-transverse ridges, adjacent joined ridges often form a multiple of longitudinal, subparallel zigzag rows of ridges (Fig. 4a–f).
a–l multi-layered concentric structure of spines. m–p distal bifurcation of spines. Abbreviations: BI bifurcation, MC multi-layered concentrical structure. Specimens: a–c ELI-FJG03291, d, e ELI-FJG01257, f–h ELI-FJG03149, i, j ELI-FJG05092, k–n ELI-FJG01051, o, p ELI-FJG02201. Scale bars: a, d, f, i, k, o = 0.5 mm; b–c, e, g–h, j = 0.02 mm; l–n, p = 0.05 mm.
Abbreviations: CR complete ridge, IR incomplete ridge, LR longitudinal subparallel rows of ridges, OR oblige ridge, PP polyp pit, TR transversal ridge, Zr zigzag row of ridges. Specimens: a, b ELI-FJG01293, c, d ELI-FJG01273, e–g ELI-FJG02147, h, i ELI-FJG01089, j, k ELI-FJG03465, l, m ELI-FJG02398, n, o ELI-FJG02323, p, q ELI-FJG02189, r, s ELI-FJG02184. Scale bars, a, e, h, j, l, n, p, r = 0.5 mm; b–d, f, i, k, o, q = 0.2 mm; g = 0.05 mm; m, s = 0.1 mm.
The skeletal surface of S. seira, particularly in pits or in proximity to the spine base, often exhibits shallow polygonal imprints measuring 9–14 µm in diameter (Supplementary Fig. 3). Similar structures in S. radicatus have previously been interpreted as being the imprints of secretory epithelial cells11.
Micro-CT observations of virtual cross-sections of S. seira branches reveal a characteristic antipatharian pattern35, characterized by a central canal surrounded by a multi-layered concentric structure (Fig. 5a, b, d, e). Some branches reveal Sterictopathes seira (Fig. 5m–o) and Sterictopathes sp. (Fig. 5g–i) encrusting around the Sphenothallus tube12,36, which is characterized by longitudinal, radial, thin septa-like structures (up to nine) in cross section.
a–f Extant black corals with characteristic internal multi-layered concentrical structures. a–c Antipathidae, Stichopathes spinosa; d–f Antipathidae, Antipathes sarothrum. g–u Three fossil species of the family Sterictopathidae fam. nov.: g–i Sterictopathes sp. encrusting an interior tube of another Cnidaria Sphenothallus sp. with three characteristic internal septa (white arrows); j–o Sterictopathes seira sp. nov. with internal multi-layered concentrical structure of itself (j–l) or encrusting the tube of Sphenothallus sp; m–o with ten characteristic internal septa (white arrows); p–u Sterictopathes radicatus encrusting two interior tubes of Sphenothallus sp., only one tube (p–r) with four internal septa (white arrows). Specimens: a–c ZSM20030289, d–f ZSM20030284, g–i ELI-FJG04013, j–l ELI-FJG03118, m–o ELI-FJG02302, p–r ELI-HHC031, s–u ELI-HHC051. Abbreviations: BR branch, CG circular gap, CC central canal, IS irregular space, MC multi-layered concentrical structure, RI ridge, SE septa, SP spine, SPH Sphenothallus, STP Sterictopathes sp, STR Sterictopathes radicatus, STS Sterictopathes seira. Scale bars: a–u = 150 μm. The 3D construction data of the extant species Leiopathes bullosa (Leiopathidae) is available form Morphobank (accession number: M894365-M894377).
Chemical component
Spectral examination reveals notable D-band (disordered organic matter) and G-band (graphitic organic matter) signatures, confirming the identification of materials as organic carbon (Supplementary Fig. 4). Additionally, Energy Dispersive X-ray spectroscopy (EDS) mapping demonstrates the relative abundance of C, with minor amounts of P and Ca (Supplementary Fig. 4), originating from secondary phosphatation, as frequently observed in Cambrian small shelly fossils37. No instances of calcite or aragonite pseudocrystals replaced by apatite were detected in SEM.
Remarks
The skeletons of both Sterictopathes seira sp. nov. and S. radicatus are characterized by the presence of sculpture in the form of a high, anastomosing network of ridges and spines, as well as a multilayered, concentric structure (Fig. 2n, f and Supplementary Fig. 5p). They do, however, differ in several characters: (1) S. seira has larger, deeply concave cavities (approximately 139–689 µm long and 102–120 µm wide), delimited by 4–17 adjacent sub-longitudinal, oblique, and sub-transverse ridges and spines (Figs. 2l, 4), while S. radicatus has smaller, flat-bottomed cavities (124–254 µm in length and 85–128 µm in width) bordered by 3–4 irregularly arranged ridges (Fig. 2a–e); (2) S. seira exhibits multiple longitudinal rows of ridges parallel to each other (Fig. 4), while S. radicatus exhibits randomly oriented ridges (Supplementary Fig. 5); (3) spines of S. seira are buttressed by straight ridges (Figs. 2m, 4), while in S. radicatus there is occasionally no spine development at the junction of three ridges (Supplementary Fig. 5a–c); and (4) the spines of S. seira are mostly supported by 2–3 ridges (Fig. 2k–m), whereas those in S. radicatus are often supported by 3–4 ridges (Fig. 2c–e).
In the present Middle Ordovician black coral collection from the Xiliangsi Formation, three rare specimens have been provisionally identified as an undetermined species within the genus Sterictopathes, here referred to as Sterictopathes sp. (Fig. 2o–t). It apparently belongs to Sterictopathes, as the presence of networks of ridges. The simplified spine-ridge junctions and isolated spines in Sterictopathes sp. quite resemble those of extant antipatharians (Fig. 5c, f; Supplementary Table 2). Meanwhile, it differs from the remaining congeneric species in several characteristics: the cavities of networks are flat-bottomed and formed by 4–12 longitudinally arranged spines and shallow ridges (in lateral view); the spines are short and conical, buttressed mostly by 0–2 straight ridges (Fig. 2o–t); adjacent ridges joined together and arranged longitudinally or slightly obliquely (Fig. 2o–t).
Phylogeny
The topology of the phylogenetic tree built using four concatenated DNA barcoding genes (Supplementary Fig. 6b) is consistent with a recent mitochondrial genomic tree or phylogenomic trees composed of fewer black coral species9,38, supporting the separation of the seven extant black coral families with available barcoding data. On the morphological tree (Supplementary Fig. 6a), the fossil families Sterictopathidae and updated Sinopathidae (excluding Sterictopathes) form two separate monophyletic clades with Bayesian posterior probabilities (BPP) of 1.00 and 0.27, respectively. Furthermore, Sterictopathidae clusters with the paraphyletic clade of Leiopathidae, supported by a BPP value of 0.33. In the consensus tree integrating morphological and molecular data (Supplementary Fig. 6c), the family Sterictopathidae also constitutes a stable monophyletic clade (BPP value, 0.76) of Antipatharia. However, family Sinopathidae appears as a paraphyletic outgroup of extant black corals, probably due to the inclusion of unidentified Sinopathes specimens (Sinopathes sp., specimen Tianjialing T13; Sinopathes sp., specimen Gudongkou 115E)12. In summary, both the morphological and the consensus trees of cladistic analysis support the monophyletic clade of Sterictopathidae as a part of Antipatharia (Fig. 6a and Supplementary Fig. 6c).
a Bayesian analyzes concatenating morphology and molecular data for selected fossil and extant black corals (see detailed topology in Supplementary Fig. 6c); fossil families are shown in bold. b schematic construction of typical regular network types observed in eight extant black coral families. c–e Construction of irregular networks of three fossil species of the family Sterictopathidae fam. nov.: Sterictopathes sp. (c), S. seira (d) and S. radicatus (e). f Conceptual ancestor of the Sterictopathidae fam. nov. with fully-developed irregular networks. g–j Hypothetical evolutionary trend of black coral skeletal networks and ridges. Erected colonial black coral with (h) or without (g) ridges; i encrustation of ancestral colonial black corals by asexual reproduction; j ancestral solitary polypoid periderm of hexacorallian with a basal periderm. k–l Artist reconstructions of the black coral Sterictopathes seira sp. nov. Abbreviations: CO coenenchyme, BP basal plate, CC central canal, CR complete ridge, IR incomplete ridge, PO polyp, RI ridge, SP spine, TH theca.
Discussion
Black coral affinities
Both Sterictopathidae and Sinopathidae possess skeletal spines and multi-layered concentric structures comparable to extant black corals. The discovery of two intermediate species (Sterictopathes seira and Sterictopathes sp.) provides key evidence to bridge fossil gaps, in particular, between the Early Ordovician Sterictopathes reptans and extant species, supporting the antipatharian affinities of Sterictopathidae. The fully developed (Sterictopathes radicatus), simplified (S. seira), and reduced (Sterictopathes sp.) spiny networks represent morphological variations due to the disappearing tendency of the transversal spine-ridge junctions in Sterictopathidae (Fig. 6). Moreover, except for few partial transversal spines and ridges, the characteristic that one longitudinal ridge connecting one or two adjacent spines seen in Sterictopathes sp. closely resemble some of extant species (Supplementary Table 2). Notably, incomplete or complete longitudinal ridges are also present in five extant black coral families, including Leiopathidae, Antipathidae, Cladopathidae, Aphanipathidae, and Schizopathidae (Supplementary Table 2). Particularly, some primary spines (polypar and abpolypar spines) and secondary spines buttressed radially by 0-4 (mostly 2) inconspicuous, complete or incomplete, thin ridges are present in a specimen of extant Stichopathes spp. (Fig. 13j in ref. 39).
The only remaining concern challenging the antipatharian affinity of Sinopathidae10 is the presence of continuous fine longitudinal costellae in Sinopathes reptans. However, this fine sculpture is absent in extant black corals and specimens of Early Ordovician Fenxiang Formation identified as Sinopathes sp12. Longitudinal grooves and ridges (functioning as epidermal channels for water and nutrient transport)40 are also common on the surface of multi-layered skeleton of octocorallians like Corallium, Isidella, and Lepidisis40,41,42, but the size of these structures is up to about 200 times larger than that found on Sinopathes. The present morphological phylogenetic analyzes suggest the updated Sinopathidae as a stem group of Antipatharia (Fig. 6a and Supplementary Fig. 6c), but whether the spine networks of Sterictopathidae originated from a common ancestor shared by Sinopathidae and Sterictopathidae remains unresolved.
Evolution of spine-ridge junctions in the black coral skeleton
The irregular arrangement of skeletal spines in the Early Ordovician black coral Sinopathes reptans was proposed to be a plesiomorphic trait, suggesting a subsequent evolutionary trend towards regularity11,12. However, the previous evidence for this hypothesis is weak due to lacking of transitional fossils between Sinopathidae and extant black corals. Notably, Sterictopathes radicatus from the same formation, which exhibits irregular skeletal networks, does not represent a transitional fossil species between Sinopathidae and extant families.
Instead, the present study identifies transitional black coral species (Sterictopathes sp., Sterictopathes seira) between the Early Ordovician species S. radicatus and extant black corals, allowing us to hypothesize some of the major evolution of skeleton regularity in black corals, as listed chronologically below: (1) The hypothesized ancestor of Sterictopathidae is assumed to have irregular networks with complete, uniformed, straight, and short ridges (Fig. 6f). (2) S. radicatus from the Early Ordovician displays irregular networks with each spine buttressed mostly by 3–5 ridges (Fig. 6e). (3) S. seira from the Middle Ordovician exhibits degraded networks due to the vanishing tendency of transverse ridges, with each spine mainly comprising 2–3 ridges (Fig. 6d). (4) Sterictopathes sp. from the Middle Ordovician show simplified networks with almost complete disappearance of transverse ridges, with 0–2 ridges connecting each spine (Fig. 6c). (5) In modern black corals, networks are replaced either by isolated spines or spines connected by incomplete or complete, unnoticeable longitudinal ridges (Fig. 6b). The density of spines is lower in most modern black corals, which is probably due to more or less reduction of primary spines to secondary spines as seen in some extant forms, i.e., Stichopathes spp. (Fig. 13j in ref. 39), Antipathes griggi (Fig. 2e, f in ref. 43), Antipathes fruticosa (Fig. 9b, c in ref. 43), Stichopathes sp. SCBUCN-8850 (Fig. 3G–J in ref. 44). However, exceptions to this trend have been reported, including species such as Cirrhipathes anguina, Cirrhipathes cf. indica, Cirrhipathes rumphii, and Stichopathes cf. maldivensis (Figs. 14, 17, 19, 20, 23 in ref. 45). In summary, the number of spine-ridge junctions appears to decrease gradually from the Early Ordovician to modern black corals, and the arrangement of spines becomes more regular.
The rise and longitudinal fusion of networks
Due to lacking of soft-tissued preservation (i.e., polyps, mesenteries, and coenenchyme) in Ordovician black corals, the relationships between their soft-tissue and axial skeletons remain intriguing. We suggest that the deep network depressions in Sterictopathidae, although organic in composition, may find analogs in the polypoid pits or calcareous skeletal cups of colonial scleractinians, a group of colonial hexacorallians closely related to the Antipatharia46. The bottom of the scleractinian cup, in widely spaced polyps, consists of a basal plate and a separate theca47. But the scleractinian theca beneath tightly spaced polyps (i.e., Favia) appears as common walls, which are organized as polygonal networks48 resembling that of Sterictopathidae.
Additionally, the fusion of skeletal cups into deep and long rows48, seen in the derived clade of Hexacorallia (i.e., brain corals)46, is highly reminiscent of the apparent trend of fusion and expansion of organic networks in Ordovician Sterictopathidae. In this regard, we infer that the deep network depressions and ridges in Sterictopathidae could be interpretable as polyp pits and organic theca walls, respectively. These networks may have facilitated the firm adherence of colonial polyps and ambient coenenchyme to the axial skeletal surface, preventing exfoliation by varying water currents on the continental shelf. Networks with abundant prominent spines, most likely, function effectively for defense (i.e., to prevent gnawing of predators). Furthermore, the occurrence of spines increases the surface area for cementing one skeletal layer to the other, while also acting as continuous rivets to resist delamination of shear forces produced by skeletal bending and torsion49. If this statement is true, the occurrence of spines might eliminate or reduce the demand of small fibril biases between helically wound layers49. The expansion of the network in Sterictopathidae, most probably, was advantageous in accommodating growing polyps and nutrient sharing among adjacent polyps. Particularly, the longitudinal fusion may relate to upward growth via asexual reproduction and rapid branch growth.
Origin of the antipatharian erect axial skeleton
The independent evolution of upright colonial forms46 in Hydrozoa50 and Octocorallia51, taken together with molecular phylogeny of anthzooans29,30,46, may provide valuable hints into the rise of the upright axial skeleton of colonial antipatharians. The common ancestor of hexacorallians, likely soft-bodied and solitary in the Cryogenian–Ediacaran30, may have secreted a basal, organic exoskeleton for fixing in/on the substrate (Fig. 6j). Against the backdrop of the Cambrian arms race and macrophagous predation52,53, the asexual reproduction of polyps to increase body size (Fig. 6i) would lead to a sessile, sheet-like colony that may have independently arisen multiple times in different orders of cnidarians46. Their sheet-like basal skeleton can be represented by the encrustation of Sterictopathes radicatus12 (Fig. 5p–u) and S. seira (Fig. 5m–o) on the tube wall of Sphenothallus.
The escalated ecological tiering competition among colonial, sessile suspension feeders in the Cambrian–Ordovician marine environments54,55 presumably have facilitated the independent evolution of upward growth of modular colonies, as exemplified by Sterictopathidae enclosing the Sphenothallus tube (Fig. 5m–u); their concentric axial skeleton (Fig. 6h) can be regarded as a stacking of annually secreted basal sheets, in microscope-scale deposition comparable to the calcareous lamellate skeleton of other fossil coral orders such as Tabulata and Rugosa56.
Materials and Methods
Fossil and extant materials
In this study, 265 black corals fossils were examined (Supplementary Table 1). They were collected from the Middle Ordovician (Darriwilian Stage) Xiliangsi Formation at Fanjiagou section, Ningqiang county, Shaanxi, China (32°58´7.48˝N, 106°28´7.99˝E, specimen numbers with prefix ELI-FJG), and early Ordovician Fenxiang Formation in Huanghua quarry, Hubei, China (30° 51´36.94˝ N, 111° 21´49.64˝ E, with prefix ELI-HHC), respectively (Fig. 1). These fossils were recovered from limestone rocks through 5%–10% acetic acid leaching, and manually picked up from acid residues under a stereo microscope. The Middle Ordovician material from the Xiliangsi Formation comprised 253 specimens of Sterictopathes seira sp. nov. and three specimens provisionally identified as Sterictopathes sp. (Supplementary Table 1). Eight specimens of S. radicatus were isolated from its type locality (Baliński & Sun, 2017) in the Fenxiang Formation, Hubei (Supplementary Table 1). All fossil specimens are deposited in Shaanxi Key Laboratory of Early Life and Environments, Northwest University, Xi’an, China.
For comparative analysis, type material of six extant species (Antipathes sarothrum, Myriopathes stechowi, Stichopathes spinosa, Myriopathes bifaria, Leiopathes bullosa, Rhipidipathes colombiana), deposited in Zoologische Staatssammlung München (prefix ZSM), was examined. Additionally, recent material of two black coral species (Leiopathes sp., Leiopathidae; Tylopathes sp., Stylopathidae) collected by the manned submersible Shenhaiyongshi from the South China Sea was utilized to observe internal compositions distributed between concentric skeleton layers. In situ and laboratorial images of the black coral Bathypathes sp. (Schizopathidae) collected by the manned submersible Fendouzhe from the Diamantina Fracture Zone was used for reference for artist reconstruction of the fossil species Sterictopathes seira sp. nov. The specimens collected by the submersibles are deposited in Institute of Deep-Sea Science and Engineering, Chinese Academy of Sciences (with prefix IDSSE).
Scanning electron microscopy
All investigated black coral specimens as well as other organisms in the Xiliangsi Formation, including trilobites, brachiopods, bivalve mollusks, pelagic conodonts, and index graptolites (Fig. 1, Supplementary Fig. 1) were imaged with a FEI Quanta 400 FEG scanning electron microscope (SEM).
X-ray tomographic microscopy
Sixteen selected fossil specimens and six extant black corals were scanned using microcomputed tomography (Micro-CT) (Xradia520 Versa) at Northwest University. Micro-CT 3D images and videos were segmented and visualized using VG studio 2.2 Max and Dragonfly 4.1 (Fig. 5).
Energy-dispersive X-ray spectrometry analyzes
Four specimens of Sterictopathes seira sp. nov. and S. radicatus were analyzed under the environmental mode by Energy Dispersive X-ray spectrometry (EDS) system, with 20.0 kV, 60 Pa and WD 11.4 mm at Northwest University (Supplementary Fig. 4a–d).
Phylogenetic analysis
Three trees were generated by Bayesian inference-based phylogenetic analyzes: a morphological tree, a molecular tree, and a consensus tree combining morphological and molecular matrices (Supplementary Fig. 6; Supplementary Table 3, 4).
The morphological tree was constructed based on 26 characters extracted from 42 selected black coral species and the sea anemone outgroup Nematostella vectensis (Supplementary Fig. 6a, Supplementary Table 3, and Supplementary Date 1). The 36 extant black corals were selected from all eight extant black coral families. Priority was given to black coral species with molecular data deposited in GenBank for analyzes. Morphological data were obtained from original species descriptions, recent re-descriptions (where available), specific fossil records, or extant specimens explicitly linked to published molecular data (Supplementary Table 3). Molecular data consisted of concatenated partial sequences of the COI, COIII, 18S and 28S genes (Supplementary Fig. 6b, Supplementary Table 4 and Supplementary Date 2). The consensus dataset combined the above morphological and molecular matrices (Supplementary Fig. 6c and Supplementary Data 3). Bayesian analyzes were run using MrBayes (v.3.2.7) and the GTR model, with gamma-distributed rate variation and variable coding. Bayesian analyzes were run using MrBayes (v.3.2.7) with data matrices and scripts detailed in Supplementary Data 1–3.
Raman spectroscopy
Raman spectra of Sterictopathes seira were obtained using a JY Horiba LabRam Odyssey equipped with a 532 nm laser source at Northwest University. Each target was subjected to a 30-second acquisition time with one accumulation, aiming to minimize laser-induced heating and achieve a favorable signal-to-noise ratio. The laser beam was directly focused on the fossil surface, and up to four distinct regions were probed for each sample. The Raman spectra were processed using Labspec6 software (Supplementary Fig. 5e–h).
Comparative analysis of potential empty gaps in extant black coral skeleton layers
In this study, empty gaps were identified between fossil black coral skeleton layers. For comparative analysis, extant black corals were subjected to treatment with NaClO to eliminate proteins distributed between concentric skeleton layers. Branch pieces from Leiopathes sp. and Tylopathes sp., collected from the South China Sea, were immersed in 2 ml centrifuge tubes each containing 800 µl of sodium hypochlorite solution at 60 °C for 2 h. Subsequently, these samples were examined using micro-CT for 3D morphology (Supplementary Fig. 7) at Northwest University.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All original images and videos (3.42 G) that support this study have been deposited in Science Data Bank (DOI: 10.57760/sciencedb.12385) and MorphoBank (https://morphobank.org/permalink/?P4854).
References
Agarwal, M. et al. Distribution and ecology of shallow-water black corals across a depth gradient on Galápagos rocky reefs. Coral Reefs 43, 1–13 (2024).
Molodtsova, T. N. Black corals (Anthipatharia: Anthozoa: Cnidaria) of the north-eastern Atlantic. Adv. Marine Biol. 63, 141–151 (2006).
Wagner, D. et al. The biology and ecology of black corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia). Adv. Mar. Biol. 63, 67–132 (2012).
WoRMS. Antipatharia. Accessed at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=22549 (2024).
Cairns, S. Deep-water corals: an overview with special reference to diversity and distribution of deep-water Scleractinian corals. Bull. Mar. Sci. 81, 311–322 (2007).
Bo, M. et al. Antipatharians of the Mesophotic Zone: Four Case Studies. Mesophotic Coral Ecosystems, Vol. 12 (Springer, 2019).
Molodtsova, T. N. et al. One of the deepest genera of Antipatharia: taxonomic position revealed and revised. Diversity 15, 436 (2023).
Daly, M. et al. The phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after Linnaeus. Zootaxa 1668, 127–182 (2007).
Horowitz, J. et al. Ameripathidae, a new family of antipatharian corals (Cnidaria, Anthozoa, Hexacorallia, Antipatharia). ZooKeys 1203, 355 (2024).
Brugler, M., Opresko, D. & France, S. The evolutionary history of the order Antipatharia (Cnidaria: Anthozoa: Hexacorallia) as inferred from mitochondrial and nuclear DNA: implications for black coral taxonomy and systematics. Zool. J. Linn. Soc. 169, 312–361 (2013).
Baliński, A., Sun, Y.-L. & Dzik, J. 470-Million-year-old black corals from China. Naturwissenschaften 99, 645–653 (2012).
Baliński, A. & Sun, Y. -l. Early Ordovician black corals from China. Bull. Geosci. 92, 1–12 (2017).
Michelin, H. Iconographie Zoophytologique, Description Par Localités Et Terrains Des Polypiers Fossiles De France Et Pays Environnants etc (Bertrand, 1840–1848).
Milne-Edwards, H. H. Monographie des Polypiers fossiles des terrains paléozoïques. Archs Mus. Hist. nat. Paris 5, 1–502 (1851).
Wells J. W., H. D. Coelenterata. In: Moore RC (ed) Treatise on Invertebrate Paleontology; part F. F1–F166 (Lawrence, 1956).
Michelotti, G. Specimen Zoophytologiae Diluvianae (Turin, 1839).
Kölliker, R. A. Die bindesubstanz der coelenteraten. Icones histiologicae oder Atlas der vergleichenden Gewebelehre. 2, 87–181 (1865).
Zittel, K. A. Handbuch der Paläontologie. 1 Band. I Abtheilung Paläozoologie (München, 1876–1880).
Pax, F. Beitrag zur Kenntnis der japanischen Dörnchenkorallen. Zool 63, 407–450 (1932).
Edwards, H. M. & Haime, J. A monograph of the british fossil corals. First part. Introduction; Corals from the Tertiary and Cretaceous formations. Palaeontogr 3, 1–71 (1850).
Horowitz, J. The Taxonomy, Biodiversity, and Evolutionary History of Black Corals (Order Antipatharia) PhD thesis, (James Cook University, 2022).
Bouillon, J. et al. An Introduction to Hydrozoa (Scientifiques du Muséum, 2006)
Opresko, D. M. Revision of the Antipatharia (Cnidaria: Anthozoa). Part II. Schizopathidae. Zool. Med. Leiden. 76, 411–442 (2002).
Opresko, D. Revision of the Antipatharia (Cnidaria: Anthozoa). Part IV. Aphanipathidae. Zool. Med. Leiden. 78, 209–240 (2004).
Opresko, D. Revision of the Antipatharia (Cnidaria: Anthozoa). Part III. Cladopathidae. Zool. Med. Leiden. 77, 495–536 (2003).
Opresko, D. Revision of the Antipatharia (Cnidaria: Anthozoa). Part I. Establishment of a new family, Myriopathidae. Zool. Med. Leiden. 75, 343–370 (2001).
Opresko, D. & Molodtsova, T. N. New species of deep-sea antipatharians from the North Pacific (Cnidaria: Anthozoa: Antipatharia), Part 2. Zootaxa 4999, 401–422 (2021).
Chimienti et al. A new species of Bathypathes (Cnidaria, Anthozoa, Antipatharia, Schizopathidae) from the Red Sea and its phylogenetic position. ZooKeys 1116, 1 (2022).
Horowitz, J. et al. Bathymetric evolution of black corals through deep time. Proc. R. Soc. B 290, 20231107 (2023).
Quattrini, A. et al. Palaeoclimate ocean conditions shaped the evolution of corals and their skeletons through deep time. Nat. Ecol. Evol. 4, 1531–1538 (2020).
Zhang, Y. et al. Ordovician integrative stratigraphy and timescale of China. Sci. China Earth Sci. 62, 61–88 (2019).
Lai, C. G. et al. Restudy of the Ordovician of southern Shaanxi-northern Sichuan Areas. Profess. Pap. Stratigr. Palaeontol. 23, 1–36 (1990).
Feng, Z. Lithofacies Paleogeography of the Cambrian and Ordovician in South China (Geological Publishing House, 2004).
Balinski, A. & Sun, Y. Fenxiang biota: a new Early Ordovician shallow-water fauna with soft-part preservation from China. Sci. Bull. 60, 812–818 (2015).
Goldberg, W. Chemistry and structure of skeletal growth rings in the black coral Antipathes fiordensis (Cnidaria, Antipatharia). Hydrobiologia 216, 403–409 (1991).
Dzik, J., Baliński, A. & Sun, Y.-L. The origin of tetraradial symmetry in cnidarians. Lethaia. 50, 306–321 (2017).
Bengtson, S. Early Cambrian fossils from South Australia. Mem. Assoc. Australas. Palaeontol. 9, 1–364 (1990).
Barrett, N. et al. Phylogenetics and mitogenome organisation in black corals (Anthozoa: Hexacorallia: Antipatharia): an order-wide survey inferred from complete mitochondrial genomes. Front. Mar. Sci. 7, 440 (2020).
Terrana, L. et al. Whip black corals (Antipatharia: Antipathidae: Stichopathes) of the Mesophotic Coral Ecosystem of Mo’orea (French Polynesia), with the description of a new species. Zootaxa 5486, 182–212 (2024).
Grillo, M. C., Goldberg, W. M. & Allemand, D. Skeleton and sclerite formation in the precious red coral Corallium rubrum. Mar. Biol. 117, 119–128 (1993).
Tu, T.-H., Dai, C.-F. & Jeng, M.-S. Taxonomic revision of Coralliidae with descriptions of new species from New Caledonia and the Hawaiian Archipelago. Mar. Biol. Res. 12, 1003–1038 (2016).
Goedert, J., Guthrie, L. & Kiel, S. Octocorals (Alcyonacea and Pennatulacea) from Paleogene deep-water strata in western Washington State, USA. J. Paleontol. 96, 539–551 (2022).
Opresko, D. A new name for the Hawaiian antipatharian coral formerly known as Antipathes dichotoma (Cnidaria: Anthozoa: Antipatharia) 1. Pac. Sci. 63, 277–291 (2009).
Tapia-Guerra, J. et al. First ecological characterization of whip black coral assemblages (Hexacorallia: Antipatharia) in the Easter Island Ecoregion, Southeastern Pacific. Front. Mar. Sci. 8, 755898 (2021).
Terrana, L., Bo, M., Opresko, D. M. & Eeckhaut, I. Shallow-water black corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia) from SW Madagascar. Zootaxa 4826, 1–62 (2020).
McFadden, C. et al. Phylogenomics, Origin and Diversification of Anthozoans (Phylum Cnidaria). Syst. Biol. 70, 635–647 (2021).
Hyman, L. H. The Invertebrates: Protozoa through Ctenophora (Libbie Henrietta, 1940).
Ruppert, E. E., Fox, R. S. & Barnes, R. D. Invertebrate Zoology: A Functional Evolutionary Approach (Thomson-Brooks/Cole, 2004).
Kim, K., Goldberg, W. M. & Taylor, G. T. Architectural and mechanical properties of the black coral skeleton (Coelenterata: Antipatharia): a comparison of two species. Biol. Bull. 182, 195–209 (1992).
Cartwright, P. The development and evolution of hydrozoan polyp and colony form. Hydrobiologia 530, 309–317 (2004).
Fretter, V. & Graham, A. A Functional Anatomy of Invertebrates (Academic Press, 1976).
Ou, Q. et al. Dawn of complex animal food webs: a new predatory anthozoan (Cnidaria) from Cambrian. The Innovation. 3, 1 (2022).
Bengtson, S. Origins and early evolution of predation. Paleontol. Soc. Pap. 8, 289–318 (2002).
Ausich, W. I. & Bottjer, D. J. Tiering in suspension-feeding communities on soft substrata throughout the Phanerozoic. Science 216, 173–174 (1982).
Bottjer, D. J. & Ausich, W. I. Phanerozoic development of tiering in soft substrata suspension-feeding communities. Paleobiology 12, 400–420 (1986).
Wendt, J. Corals and coralline sponges. Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends (ed Joseph G. Carter) 5, 45–66 (Springer, 1989).
Acknowledgements
We thank Prof. Yuanlin Sun (Peking University) for insightful suggestions on field work, the assistance of all onboard staffs during the deep-sea cruises conducted by the R/Vs Tansuoyihao and Tanshuoerhao (IDSSE). We also thank Jingpeng Yuan for the artistic reconstructions, Hujun Gong, Juan Luo, and Na Liu (Northwest University) for their assistance with laboratory work, Prof. Glen Brock (Macquaeie University), Kun Liang (Nanjing Institute of Geology and Paleontology, CAS), Xiuchun Jing (China University of Geosciences, Beijing) and Qiang Ou (China University of Geosciences, Beijing) for discussions of the earlier draft, Lucas Terrana (Université Libre de Bruxelles) and two anonymous reviewers for their significant comments. This study was supported by the National Key Research and Development Program of China (2023YFF0803601), the National Natural Science Foundation of China (42372012, 42276090, 41720104002, 42202009), the International Partnership Program of Chinese Academy of Sciences (183446KYSB20210002), the project of IDSSE, Chinese Academy of Sciences (E371020101), Hainan Provincial Natural Science Foundation of China (324MS114), and the Programme of Introducing Talents of Discipline to Universities (D17013).
Author information
Authors and Affiliations
Contributions
J.H. and X.S. designed and organized the research, provided research funding; J.H., X.S., W.H., A.B., M.B., Y.Y., and T.K. wrote the paper; J.H., W.H., D.W, X.W., and Y.Y. collected the fossil material; W.H., J.H., and X.W. examined the fossil material; X.S., M.R.B. and B.R. examined the extant materials; W.H., J.H., X.S., and A.B. prepared the systematics; X.S., W.H., and D.W. ran the phylogenetic analyzes; J.S., W.H., T.K., and Y.Y. performed micro-CT 3D constructions. All authors revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Lucas Terrana and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Aylin Bircan, Ophelia Bu.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hao, W., Han, J., Baliński, A. et al. Unveiling the early evolution of black corals. Commun Biol 8, 579 (2025). https://doi.org/10.1038/s42003-025-08022-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08022-x